Ultrasound-Verified Peripheral Arthritis in Patients with HLA-B*35 Positive Spondyloarthritis
Abstract
1. Introduction
2. Materials and Methods
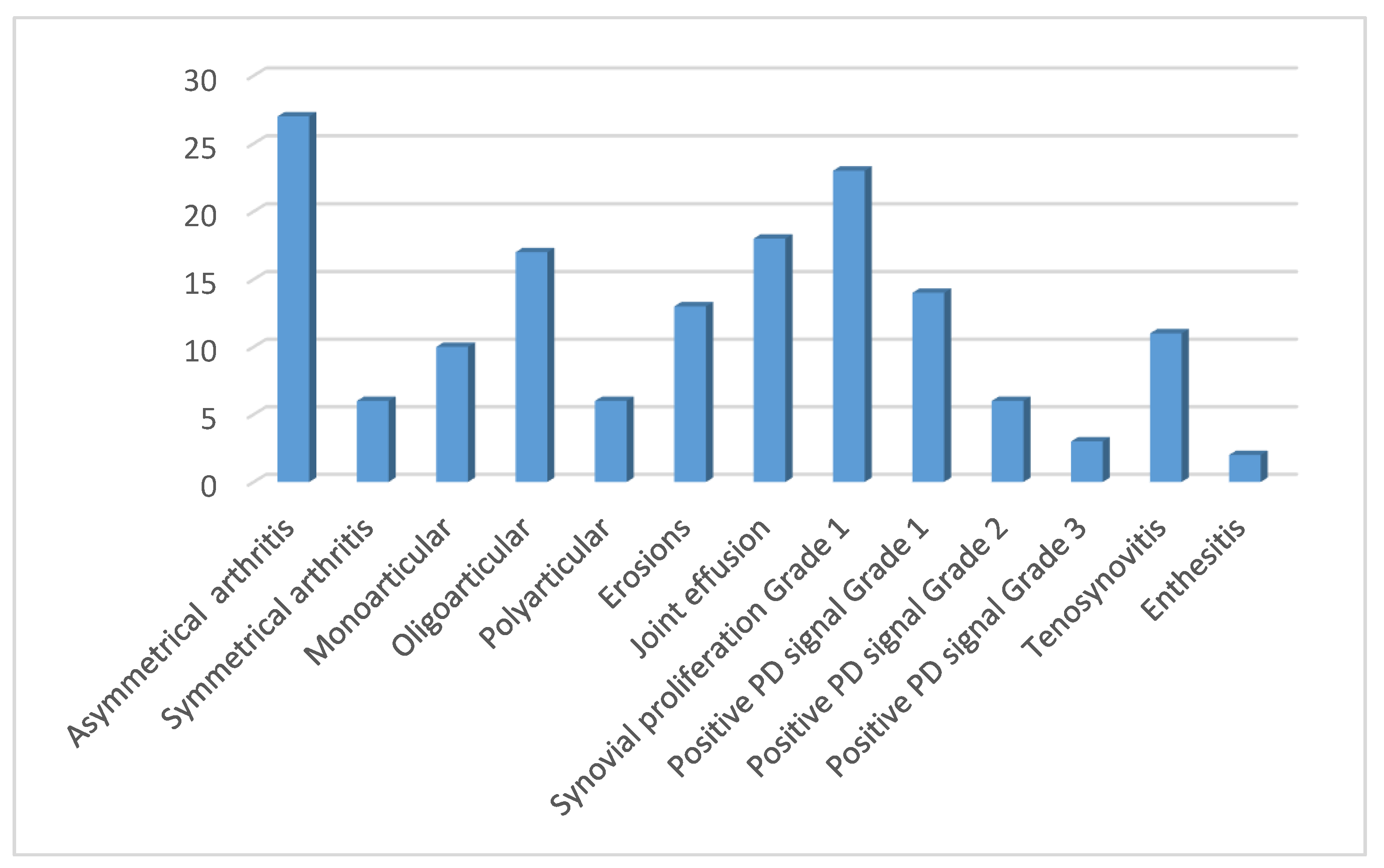
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dougados, M.; Hochberg, M.C. Why is the concept of spondyloarthropathies important? Best Pract. Res. Clin. Rheumatol. 2002, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.P.; Deodhar, A. The classification and diagnostic criteria of ankylosing spondylitis. J. Autoimmun. 2014, 48, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Spondyloarthritis in over 16 s: Diagnosis and Management; National Institute for Health and Care: London, UK, 2017.
- Braun, J.; Sieper, J. Building consensus on nomenclature and disease classification for ankylosing spondylitis: Results and discussion of a questionnaire prepared for the International Workshop on New Treatment Strategies in Ankylosing Spondylitis, Berlin, Germany, 18–19 January 2002. Ann. Rheum. Dis. 2002, 61 (Suppl. 3), iii61–iii67. [Google Scholar] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Estévez, E.C.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Landewe, R.; van der Heijde, D.; Listing, J.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Estévez, E.C.; Davis, J.; Dijkmans, B.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 2009, 68, 770–776. [Google Scholar]
- Poddubnyy, D.; Rudwaleit, M. Early spondyloarthritis. Rheum. Dis. Clin. N. Am. 2012, 38, 387–403. [Google Scholar] [CrossRef]
- Schett, G.; Lories, R.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, J.; He, C.; Li, D.; Tong, W.; Zou, Y.; Xu, W. Role of HLA-B27 in the pathogenesis of ankylosing spondylitis (Review). Mol. Med. Rep. 2017, 15, 1943–1951. [Google Scholar] [CrossRef]
- Dashti, N.; Mahmoudi, M.; Aslani, S.; Jamshidi, A. HLA-B*27 subtypes and their implications in the pathogenesis of ankylosing spondylitis. Gene 2018, 670, 15–21. [Google Scholar] [CrossRef]
- Brewerton, D.A.; Hart, F.D.; Nicholls, A.; Caffrey, M.; James, D.C.; Sturrock, R.D. Ankylosing spondylitis and HL-A 27. Lancet 1973, 1, 904–907. [Google Scholar] [CrossRef]
- Colbert, R.A.; DeLay, M.L.; Layh-Schmitt, G.; Sowders, D.P. HLA-B27 misfolding and spondyloarthropathies. Adv. Exp. Med. Biol. 2009, 649, 217–234. [Google Scholar] [PubMed]
- Tran, T.M.; Dorris, M.L.; Satumtira, N.; Richardson, J.A.; Hammer, R.E.; Shang, J.; Taurog, J.D. Additional human beta2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 2006, 54, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Rath, H.C.; Herfarth, H.H.; Ikeda, J.S.; Grenther, W.B.; Hamm, T.E., Jr.; Balish, E.; Taurog, J.D.; E Hammer, R.; Wilson, K.H.; Sartor, R.B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Investig. 1996, 98, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Taurog, J.D.; A Richardson, J.; Croft, J.T.; A Simmons, W.; Zhou, M.; Fernández-Sueiro, J.L.; Balish, E.; E Hammer, R. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1994, 180, 2359–2364. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Aslani, S.; Nicknam, M.H.; Karami, J.; Jamshidi, A.R. New insights toward the pathogenesis of ankylosing spondylitis; genetic variations and epigenetic modifications. Mod. Rheumatol. 2017, 27, 198–209. [Google Scholar] [CrossRef]
- Paine, A.; Ritchlin, C.T. Targeting the interleukin-23/17 axis in axial spondyloarthritis. Curr. Opin. Rheumatol. 2016, 28, 359–367. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.G.; McInnes, I.B.; Kirkham, B.W.; Sherlock, J.; Moots, R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: Recent advances and controversies. Ann. Rheum. Dis. 2019, 78, 1167–1178. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar]
- Wagener, P.; Zeidler, H.; Eckert, G.; Deicher, H. Increased frequency of HLA-Bw62 and Bw35 CREG antigens in HLA-B27 negative ankylosing spondylitis. Z. Rheumatol. 1984, 43, 253–257. [Google Scholar]
- Yamaguchi, A.; Tsuchiya, N.; Mitsui, H.; Shiota, M.; Ogawa, A.; Tokunaga, K.; Yoshinoya, S.; Juji, T.; Ito, K. Association of HLA-B39 with HLA-B27-negative ankylosing spondylitis and pauciarticular juvenile rheumatoid arthritis in Japanese patients. Evidence for a role of the peptide-anchoring B pocket. Arthritis Rheum. 1995, 38, 1672–1677. [Google Scholar] [CrossRef]
- Khan, M.A.; Kushner, I.; Braun, W.E. A subgroup of ankylosing spondylitis associated with HLA-B7 in American blacks. Arthritis Rheum. 1978, 21, 528–530. [Google Scholar] [CrossRef]
- Khan, M.A.; Kushner, I.; Braun, W.E. B27-negative HLA-BW16 in ankylosing spondylitis. Lancet 1978, 1, 1370–1371. [Google Scholar] [CrossRef]
- Kamanli, A.; Ardicoglu, O.; Godekmerdan, A. HLA-b27 subtypes in patients with spondylarthropathies, IgE levels against some allergens and their relationship to the disease parameters. Bratisl. Lek. Listy 2009, 110, 480–485. [Google Scholar]
- Said-Nahal, R.; Miceli-Richard, C.; Berthelot, J.; Duché, A.; Dernis-Labous, E.; Le Blévec, G.; Saraux, A.; Perdriger, A.; Guis, S.; Claudepierre, P.; et al. The familial form of spondylarthropathy: A clinical study of 115 multiplex families. Groupe Francais d’Etude Genetique des Spondylarthropathies. Arthritis Rheum. 2000, 43, 1356–1365. [Google Scholar] [CrossRef]
- Laza, I.M.; Ventades, N.G.; Hervella, M.; de-la-Rua, C. Contribution of ancient human remains analysis to the understanding of the variability in HLA-B gene variants in relation to the diagnosis of spondyloarthropathies. J. Autoimmun. 2018, 94, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Khmelinskii, N.; Regel, A.; Baraliakos, X. The Role of Imaging in Diagnosing Axial Spondyloarthritis. Front. Med. 2018, 5, 106. [Google Scholar] [CrossRef]
- Van Tubergen, A.; Heuft-Dorenbosch, L.; Schulpen, G.; Landewe, R.; Wijers, R.; van der Heijde, D.; van Engelshoven, J.; van der Linden, S. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: Does training improve quality? Ann. Rheum. Dis. 2003, 62, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Mandl, P.; Navarro-Compán, V.; Terslev, L.; Aegerter, P.; Van Der Heijde, D.; D’Agostino, M.A.; Baraliakos, X.; Pedersen, S.J.; Jurik, A.G.; Naredo, E.; et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Soso, D.; Aljinovic, J.; Marinovic, I.; Kojundzic, S.L.; Jelicic, E.C.; Krstulovic, D.M. The occurrence of sacroiliitis in HLA-B*35-positive patients with undifferentiated spondyloarthritis. A cross sectional MRI study. Clin. Rheumatol. 2020, 39, 2299–2306. [Google Scholar] [CrossRef]
- Dubost, J.J.; Demarquilly, F.; Soubrier, M.; Coussediere, C.; Ristori, J.M.; Sauvezie, B.J. HLA and self-limiting, unclassified rheumatism. A role for HLA-B35? J. Rheumatol. 1999, 26, 2400–2403. [Google Scholar] [PubMed]
- Moroldo, M.B.; Donnelly, P.; Saunders, J.; Glass, D.N.; Giannini, E.H. Transmission disequilibrium as a test of linkage and association between HLA alleles and pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 1998, 41, 1620–1624. [Google Scholar] [CrossRef]
- Orchard, T.R.; Thiyagaraja, S.; Welsh, K.I.; Wordsworth, B.P.; Hill Gaston, J.S.; Jewell, D.P. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology 2000, 118, 274–278. [Google Scholar] [CrossRef]
- Van Riel, P.L. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin. Exp. Rheumatol. 2014, 32.5 (Suppl. 85), S65–S74. [Google Scholar]
- Maska, L.; Anderson, J.; Michaud, K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res. 2011, 63 (Suppl. 11), S4–S13. [Google Scholar]
- Grubic, Z.; Burek Kamenaric, M.; Mikulic, M.; Stingl Jankovic, K.; Maskalan, M.; Zunec, R. HLA-A, HLA-B and HLA-DRB1 allele and haplotype diversity among volunteer bone marrow donors from Croatia. Int. J. Immunogenet. 2014, 41, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Möller, I.; Janta, I.; Backhaus, M.; Ohrndorf, S.; A Bong, D.; Martinoli, C.; Filippucci, E.; Sconfienza, L.M.; Terslev, L.; Damjanov, N.; et al. The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann. Rheum. Dis. 2017, 76, 1974–1979. [Google Scholar] [CrossRef]
- Naredo, E.; Bijlsma, J.W.J.; Conaghan, P.G.; Acebes, C.; Balint, P.; Berner-Hammer, H.; Bruyn, G.A.W.; Collado, P.; D’Agostino, M.A.; De Agustin, J.J.; et al. Recommendations for the content and conduct of European League Against Rheumatism (EULAR) musculoskeletal ultrasound courses. Ann. Rheum. Dis. 2008, 67, 1017–1022. [Google Scholar] [CrossRef]
- Stephens, M.A. EDF Statistics for Goodness of Fit and Some Comparisons. J. Am. Stat. Assoc. 1974, 69, 730–737. [Google Scholar] [CrossRef]
- Epis, O.; Paoletti, F.; D’Errico, T.; Favalli, E.; Garau, P.; Mancarella, L.; Pomponio, G.; Sandri, G.; Scioscia, C.; Selvi, E.; et al. Ultrasonography in the diagnosis and management of patients with inflammatory arthritides. Eur. J. Intern. Med. 2014, 25, 103–111. [Google Scholar] [CrossRef]
- Kaeley, G.S.; Bakewell, C.; Deodhar, A. The importance of ultrasound in identifying and differentiating patients with early inflammatory arthritis: A narrative review. Arthritis Res. Ther. 2020, 22, 1. [Google Scholar] [CrossRef]
- Veale, D.J.; Fearon, U. What makes psoriatic and rheumatoid arthritis so different? RMD Open 2015, 1, e000025. [Google Scholar] [CrossRef]
- Zochling, J.; Brandt, J.; Braun, J. The current concept of spondyloarthritis with special emphasis on undifferentiated spondyloarthritis. Rheumatology (Oxford) 2005, 44, 1483–1491. [Google Scholar] [CrossRef]
- Orchard, T.R.; Wordsworth, B.P.; Jewell, D.P. Peripheral arthropathies in inflammatory bowel disease: Their articular distribution and natural history. Gut 1998, 42, 387–391. [Google Scholar] [CrossRef] [PubMed]
- McHugh, N.J.; Balachrishnan, C.; Jones, S.M. Progression of peripheral joint disease in psoriatic arthritis: A 5-yr prospective study. Rheumatology (Oxford) 2003, 42, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Padovano, I.; Costantino, F.; Breban, M.; D’Agostino, M.A. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann. Rheum. Dis. 2016, 75, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Dubash, S.R.; De Marco, G.; Wakefield, R.J.; Tan, A.L.; McGonagle, D.; Marzo-Ortega, H. Ultrasound Imaging in Psoriatic Arthritis: What Have We Learnt in the Last Five Years? Front. Med. 2020, 7, 487. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Finzel, S.; Englbrecht, M.; Engelke, K.; Stach, C.; Schett, G. A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann. Rheum. Dis. 2011, 70, 122–127. [Google Scholar] [CrossRef]
- Eder, L.; Jayakar, J.; Thavaneswaran, A.; Haddad, A.; Chandran, V.; Salonen, D.; Rosen, C.F.; Gladman, D.D. Is the MAdrid Sonographic Enthesitis Index useful for differentiating psoriatic arthritis from psoriasis alone and healthy controls? J. Rheumatol. 2014, 41, 466–472, Epub 2014/02/04. [Google Scholar] [CrossRef]
- Naredo, E.; Moller, I.; De Miguel, E.; Batlle-Gualda, E.; Acebes, C.; Brito, E.; Mayordomo, L.; Moragues, C.; Uson, J.; De Agustin, J.J.; et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: A prospective case-control study. Rheumatology (Oxford) 2011, 50, 1838–1848. [Google Scholar] [CrossRef]
- Rizzo, A.; Ferrante, A.; Guggino, G.; Ciccia, F. Gut inflammation in spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 863–876. [Google Scholar] [CrossRef] [PubMed]
| Age, years | Median (IQR) | 56 (51–65) |
| Female | n (%) | 61 (85) |
| Disease duration, months | Median (IQR) | 14 (9–19) |
| ESR (mm/h) | Median (min–max) | 10 (1–60) |
| ESR ≥ 29 mm/h | n (%) | 11(15) |
| CRP (mg/L) | Median(min–max) | 3(0.3–42) |
| CRP ≥ 5 mg/L | n (%) | 15 (21) |
| Joint pain and swelling | n(%) | 57(79) |
| Tendon pain and swelling | n (%) | 14 (19) |
| Dactylitis | n(%) | 3 (4) |
| ACPA | n (%) | 2 (3) |
| RF | n (%) | 3 (4) |
| ANA | n (%) | |
| NSAIDs | n (%) | 65 (90) |
| csDMARDs | n (%) | 30 (42) |
| CSs | n (%) | 15 (21) |
| bDMARDS | n (%) | 3 (4) |
| 72 HLA-B*35 Positive Patients | |||||
|---|---|---|---|---|---|
| Positive US (n = 33) | Negative US (n = 39) | p-Value † | Difference (95% CI) | ||
| ESR (mm/h) | Median (IQR) (min–max) | 9 (5–15) (1–60) | 10 (5–18) (1–52) | 0.743 † | |
| CRP (mg/L) | Median (IQR) (min–max) | 4 (1.8–7) (0.8–42) | 2.9 (1–3.7) (0.3–15) | 0.039 † | 1.1 (−0.38–2.6) |
| DAS28ESR | Median (IQR) (min–max) | 4.3 (3.1–4.8) (1.8–5.8) | 3.5 (2.5–4.1) (0.4–5.7) | 0.039 † | 0.8 (−0.1–1.72) |
| DAS28CRP | Median (IQR) (min–max) | 4.1 (3.4–4.6) (2.3–5.8) | 3.4 (2.4–3.9) (1.2–4.9) | 0.002 † | 0.7 (−0.03–1.31) |
| HAQ | Median (IQR) (min–max) | 1.25 (1–1.7) (0–2.5) | 1.1 (0.9–1.4) (0.4–2.3) | 0.124 † | |
| 72 HLA-B*35 Positive Patients | |||||
|---|---|---|---|---|---|
| Positive US (n = 33) | Negative US (n = 39) | p-Value * | OR(95%CI) p-Value ** | ||
| ESR (≥29 mmHg) | n (%) | 4 (12) | 5 (13) | 0.955 | 0.76 (0.19–3) 0.691 |
| CRP (≥5 mg/L) | n (%) | 9(27) | 6(15) | 0.344 | 2.1 (0.65–6.6) 0.221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šošo, D.; Aljinović, J.; Lovrić Kojundžić, S.; Marinović, I.; Čečuk Jeličić, E.; Marasović Krstulović, D. Ultrasound-Verified Peripheral Arthritis in Patients with HLA-B*35 Positive Spondyloarthritis. Life 2021, 11, 524. https://doi.org/10.3390/life11060524
Šošo D, Aljinović J, Lovrić Kojundžić S, Marinović I, Čečuk Jeličić E, Marasović Krstulović D. Ultrasound-Verified Peripheral Arthritis in Patients with HLA-B*35 Positive Spondyloarthritis. Life. 2021; 11(6):524. https://doi.org/10.3390/life11060524
Chicago/Turabian StyleŠošo, Daniela, Jure Aljinović, Sanja Lovrić Kojundžić, Ivanka Marinović, Esma Čečuk Jeličić, and Daniela Marasović Krstulović. 2021. "Ultrasound-Verified Peripheral Arthritis in Patients with HLA-B*35 Positive Spondyloarthritis" Life 11, no. 6: 524. https://doi.org/10.3390/life11060524
APA StyleŠošo, D., Aljinović, J., Lovrić Kojundžić, S., Marinović, I., Čečuk Jeličić, E., & Marasović Krstulović, D. (2021). Ultrasound-Verified Peripheral Arthritis in Patients with HLA-B*35 Positive Spondyloarthritis. Life, 11(6), 524. https://doi.org/10.3390/life11060524






