A Multi-Scale and Multi-Level Fusion Approach for Deep Learning-Based Liver Lesion Diagnosis in Magnetic Resonance Images with Visual Explanation
Abstract
1. Introduction
2. Related Work
2.1. Traditional CADx Methods of Liver Lesions
2.2. Deep Learning-Based CADx Methods of Liver Lesions
2.3. Deep Neural Network Interpretation Methods
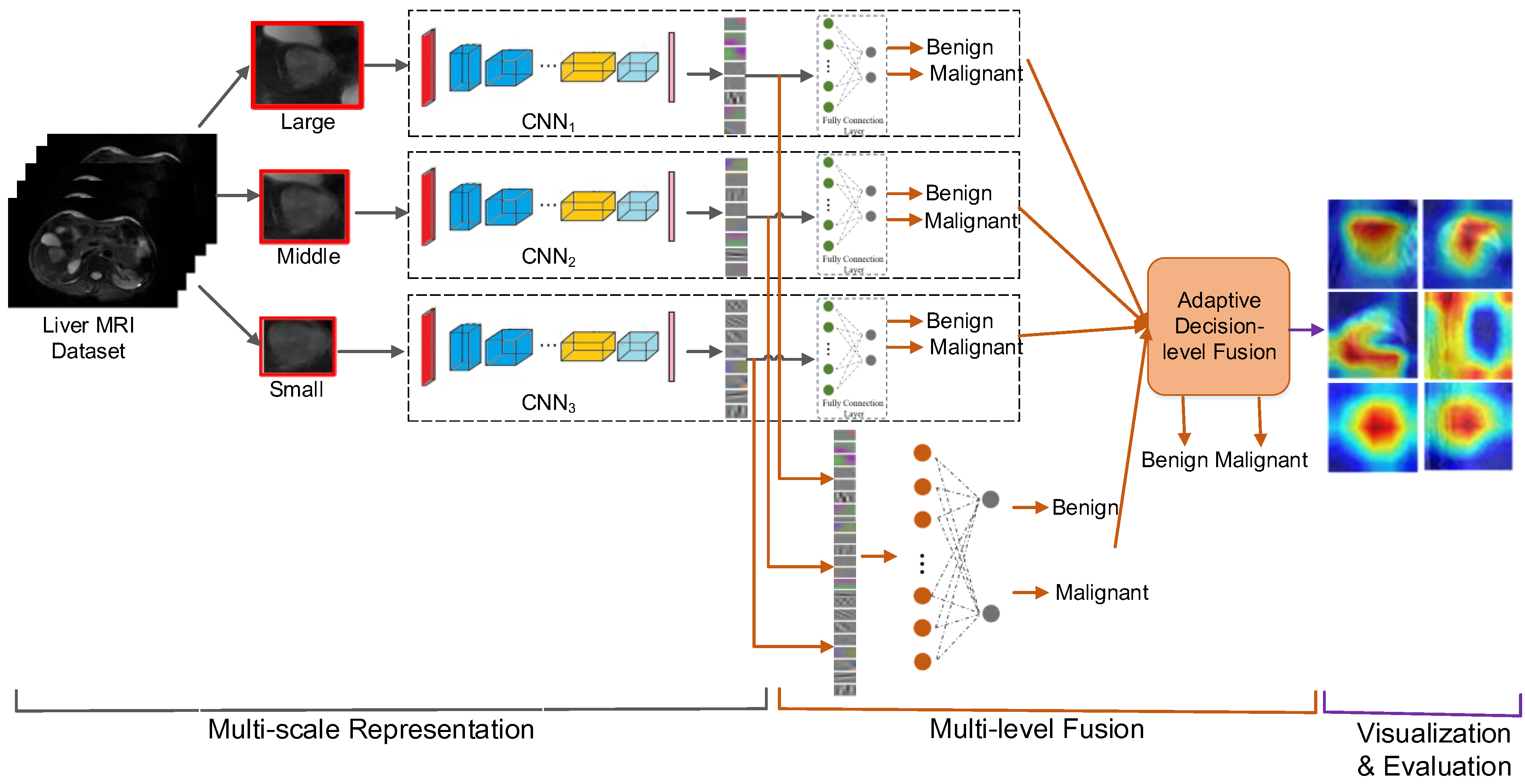
3. The Proposed Methods
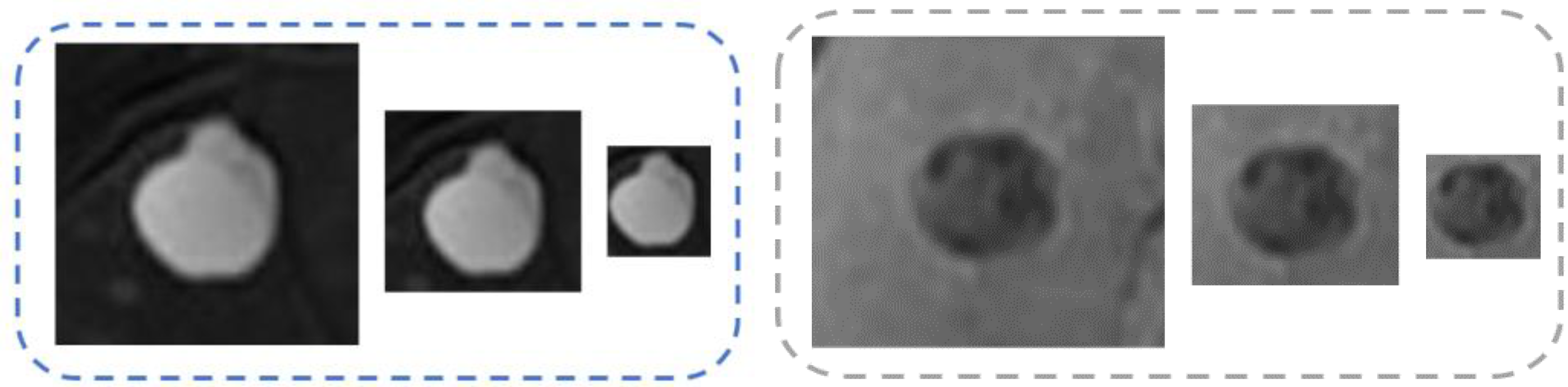
3.1. Multiscale Representation
3.2. Multi-Level Fusion
3.2.1. Feature-Level Fusion
3.2.2. Decision-Level Fusion
| Algorithm 1. The adaptive decision-level fusion method. |
| Inputs: (1) the outputs of four CNN classifiers for one lesion (2) the classification accuracy of each classifier (3) threshold Outputs: the fused final classification decision |
| Steps: 1. Transfer the outputs into probabilities using softmax function; 2. Compute the BPA of each classifier using Equation (3); 3. Compute the conflict coefficient utilizing Equation (2); 4. If is less than : 5. fuse these four results using standard D-S evidence theory as shown in Equation (2) and get the final classification result of this lesion; 6. Else if is greater than : 7. compute the credibility of each CNN classifier using Equations (4)–(6); 8. select the classifier with the highest credibility, and follow the classification decision of it as the final classification result of this lesion. |
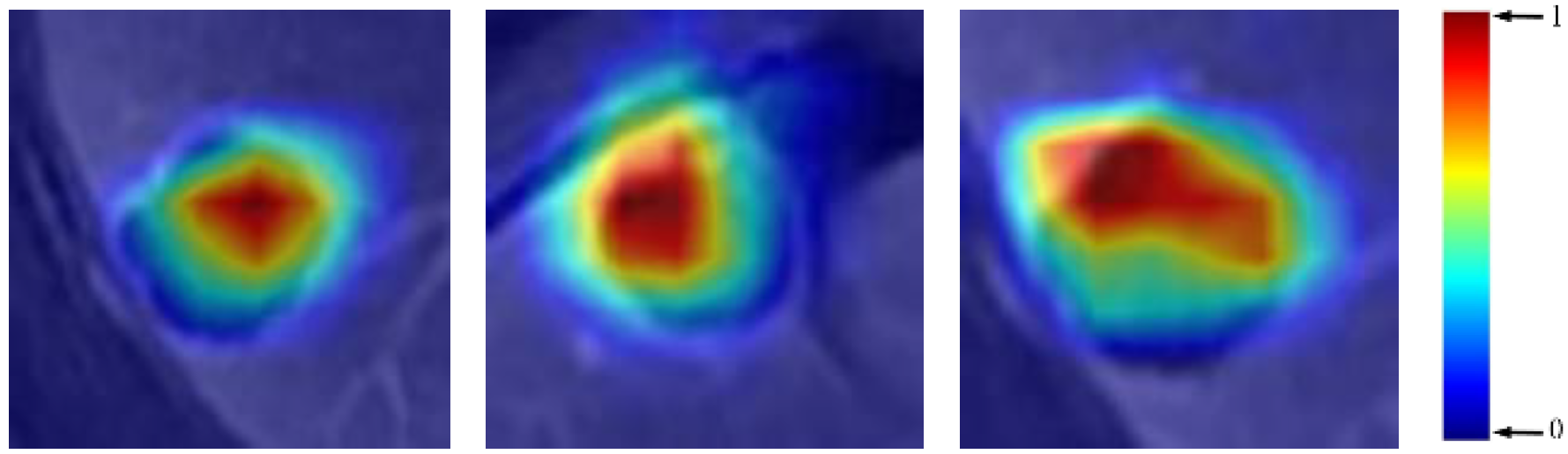
3.3. Visualization and Evaluation
4. Experiments and Results
4.1. Experimental Setup
4.2. Experimental Results
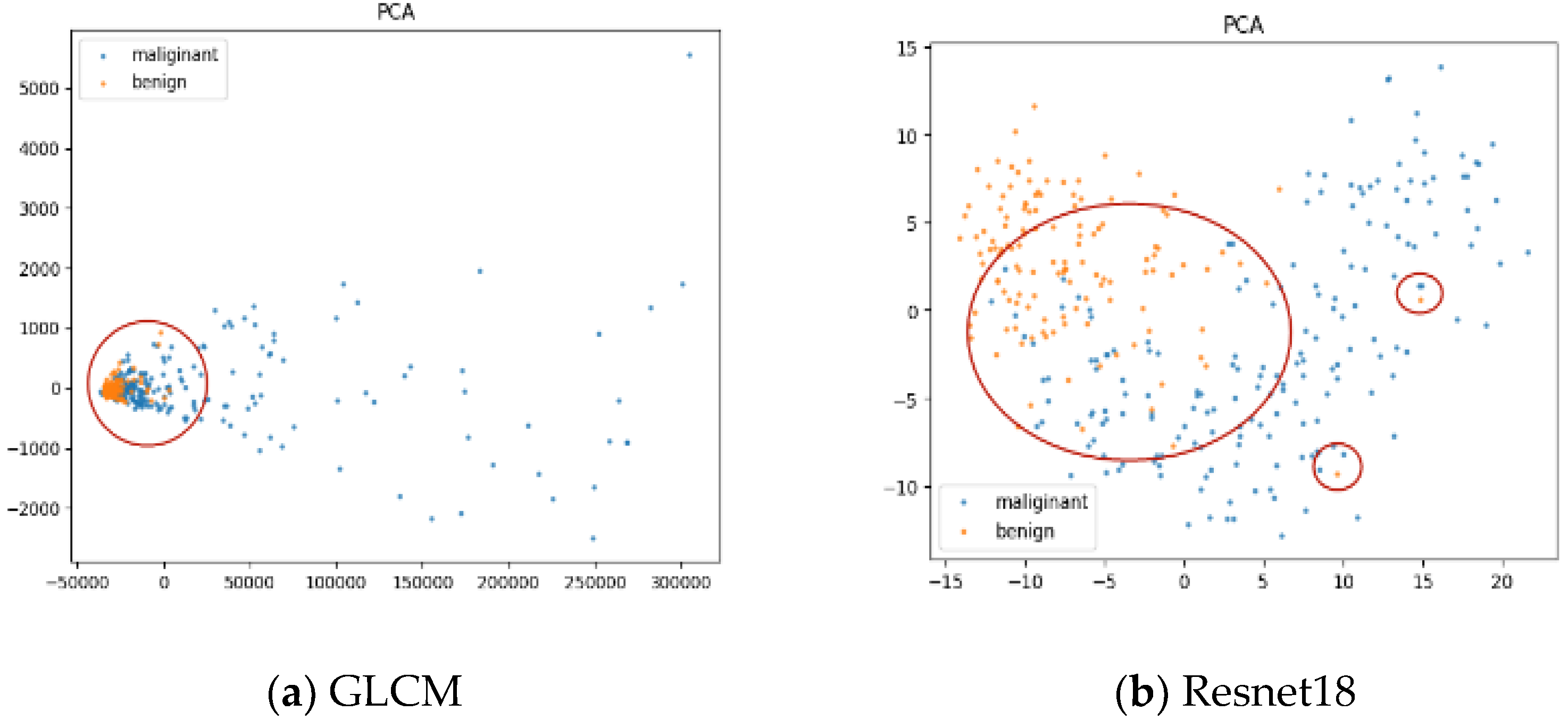
4.2.1. Performance Comparisons
4.2.2. Explanation and Visualization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisma, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer. 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Cokkinides, V.; Brooks, D.; Saslow, D.; Brawley, O.W. Cancer screening in the United States, 2010: A review of current American cancer society guidelines and issues in cancer screening. Cancer J. Clin. 2010, 60, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, K.; Fujisawa, T. Staging and diagnosis of non-small lung cancer: Invasive modalities. Respirology 2007, 12, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Viale, G.; Paganelli, G.; Zurrida, S.; Luini, A.; Galimberti, V.; Veronesi, P.; Intra, M.; Maisonneuve, P.; Zucca, F.; et al. Sentinel lymph node biopsy in breast cancer: Ten-year results of a randomized controlled study. Ann. Surg. 2010, 251, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Clin. Liver Dis. 2018, 68, 723–750. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. EASL J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association-National Cancer Center (KLCANCC) 2018 KLCA-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. KLCA-NCC Website. Available online: http://livercancer.or.kr/study/guidelines.php (accessed on 16 March 2021).
- American College of Radiology (ACR) Liver Imaging Reporting and Data System Version 2018. ACR Website. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS (accessed on 15 March 2021).
- Organ Procurement and Transplantation Network (OPTN) policies. OPTN Website. Available online: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (accessed on 21 February 2021).
- Smereka, P.; Doshi, A.M.; Lavelle, L.P.; Shanbhogue, K. New Arterial Phase Enhancing Nodules on MRI of Cirrhotic Liver: Risk of Progression to Hepatocellular Carcinoma and Implications for LI-RADS Classification. AJR Am. J. Roentgenol. 2020, 215, 382–389. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Choudhury, K.R.; Meyer, M.; Furlan, A.; Marin, D. Hepatobiliary phase hypointensity predicts progression to hepatocellular carcinoma for intermediate-high risk observations, but not time to progression. Eur. J. Radiol. 2020, 128, 109018. [Google Scholar] [CrossRef]
- Van der Pol, C.B.; Lim, C.S.; Sirlin, C.B.; McGrath, T.A.; Salameh, J.-P.; Bashir, M.R.; Tang, A.; Singal, A.G.; Costa, A.F.; Fowler, K.; et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 2019, 156, 976–986. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Meyer, M.; Choudhoury, K.R.; Gonzáles, F.; Schwartz, F.R.; Gupta, R.T.; Bashir, M.R.; Furlan, A.; Marin, D. LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10–19 mm with Arterial Phase Hyperenhancement. AJR Am. J. Roentgenol. 2019, 213, 57–65. [Google Scholar] [CrossRef]
- Stoitsis, J.; Valavanis, I.; Mougiakakou, S.G.; Golemati, S.; Nikita, A.; Nikita, K.S. Computer aided diagnosis based on medical image processing and artificial intelligence methods. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2006, 569, 591–595. [Google Scholar] [CrossRef]
- Yasaka, K.; Akai, H.; Abe, O.; Kiryu, S. Deep learning with convolutional neural network for differentiation of liver masses at dynamic contrast-enhanced CT: A preliminary study. Radiology 2017, 3, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Kittler, J.; Hatef, M.; Duin, R.P.W.; Matas, J. On combining classifiers. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 226–239. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Ribeiro, R.; Krishnamurthi, G.; Marinho, R.T.; Sanches, J.; Suri, J.S. Data mining framework for fatty liver disease classification in ultrasound: A hybrid feature extraction paradigm. Med. Phys. 2012, 39, 4255–4264. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Gupta, S. An information fusion based method for liver classification using texture analysis of ultrasound images. Inform. Fusion 2014, 19, 1–96. [Google Scholar] [CrossRef]
- Doron, Y.; Mayerwolf, N.; Diamant, I.; Greenspan, H. Texture feature based liver lesion classification. Int. Soc. Opt. Eng. 2014, 2, 9035. [Google Scholar]
- Acharya, U.R.; Faust, O.; Molinari, F.; Sree, S.V.; Junnarkar, S.P.; Sudarshan, V. Ultrasound-based tissue characterization and classification of fatty liver disease: A screening and diagnostic paradigm. Knowl. Based Syst. 2015, 75, 66–77. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Sudhakar, R. Automatic Classification of Liver Diseases from Ultrasound Images Using GLRLM Texture Features. Soft Computing Applications; Springer: Berlin, Germany, 2013; pp. 611–624. [Google Scholar]
- Xian, G.M. An identification method of malignant and benign liver tumors from ultrasonography based on glcm texture features and fuzzy svm. Exp. Syst. Appl. 2010, 37, 6737–6741. [Google Scholar] [CrossRef]
- Vijayarani, S.; Dhayanand, S. Liver disease prediction using svm and naive bayes algorithms. Int. J. Sci. Eng. Technol. Res. 2015, 4, 816–820. [Google Scholar]
- Gulia, A.; Vohra, R.; Rani, P. Liver patient classification using intelligent techniques. Int. J. Comput. Sci. Inform. Technol. 2014, 5, 5110–5115. [Google Scholar]
- Poonguzhali, S.; Ravindran, G. Automatic classification of focal lesions in ultrasound liver images using combined texture features. Inform. Technol. J. 2008, 7, 205–209. [Google Scholar] [CrossRef]
- Fatima, M.; Pasha, M. Survey of machine learning algorithms for disease diagnostic. J. Intell. Learn. Syst. Appl. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Mougiakakou, S.G.; Valavanis, I.; Nikita, K.; Nikita, A.; Kelekis, D. Characterization of CT liver lesions based on texture features and a multiple neural network classification scheme. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Cancun, Mexico, 17–21 September 2003; Volume 2, pp. 1287–1290. [Google Scholar]
- Faust, O.; Acharya, U.R.; Meiburger, K.M.; Molinari, F.; Koh, J.E.; Yeong, C.H.; Kongmebhol, P.; Ng, K.H. Comparative assessment of texture features for the identification of cancer in ultrasound images: A review. Biocybern. Biomed. Eng. 2018, 38, 275–296. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Yang, F.; Yang, C.; Tian, J. Multi-scale convolutional neural networks for lung nodule classification. In International Conference on Information Processing in Medical Imaging; Springer: Berlin, Germany, 2015; pp. 588–599. [Google Scholar]
- Wu, K.; Xi, C.; Ding, M. Deep learning based classification of focal liver lesions with contrast-enhanced ultrasound. Int. J. Light Electron. Opt. 2014, 125, 4057–4063. [Google Scholar] [CrossRef]
- Romero, F.P.; Diler, A.; Bisson, G.; Turcotte, S.; Lapointe, R.; Vandenbroucke, F.; Tang, A.; Kadoury, S. End-to-end discriminative deep network for liver lesion classification. arXiv 2019. preprint. [Google Scholar]
- Hassan, T.M.; Elmogy, M.; Sallam, E.S. Diagnosis of Focal Liver Diseases Based on Deep Learning Technique for Ultrasound Images. Arab. J. Sci. Eng. 2017, 42, 3127–3140. [Google Scholar] [CrossRef]
- Lipton, Z.C. The Mythos of Model Interpretability. Commun. ACM 2016, 61, 36–43. [Google Scholar] [CrossRef]
- Ribeiro, M.; Singh, S.; Guestrin, C. Why Should I Trust You?: Explaining the Predictions of Any Classifier. In Proceedings of the ACM SIGKDD Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Xu, Y.; Jia, Z.; Wang, L.; Ai, Y.; Zhang, F.; Lai, M.; Chang, E. Large scale tissue histopathology image classification, segmentation, and visualization via deep convolutional activation features. BMC Bioinform. 2017, 18, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Rieke, J.; Eitel, F.; Weygandt, M.; Haynes, J.; Ritter, K. Visualizing Convolutional Networks for MRI-Based Diagnosis of Alzheimer’s Disease; Springer: Berlin, Germany, 2018. [Google Scholar]
- Zhou, B.; Khosla, A.; Lapedriza, A.; Oliva, A.; Torralba, A. Learning Deep Features for Discriminative Localization. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 26 June–1 July 2016. [Google Scholar]
- Selvaraju, R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-based Localization. In Proceedings of the International Conference on Computer Vision, Vanecia, Italy, 22–29 October 2017. [Google Scholar]
- Tiulpin, A.; Thevenot, J.; Rahtu, E.; Lehenkari, P.; Saarakkala, S. Automatic Knee Osteoarthritis Diagnosis from Plain Radiographs: A Deep Learning-Based Approach. Sci. Rep. 2018, 8, 1727. [Google Scholar] [CrossRef]
- Liu, M.; Shi, J.; Li, Z.; Li, C.; Zhu, J.; Liu, S. Towards Better Analysis of Deep Convolutional Neural Networks. IEEE Trans. Vis. Comput. Graph. 2016, 23, 91–100. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Wasserman, W. Deep Feature Selection: Theory and Application to Identify Enhancers and Promoters. J. Comput. Biol. 2016, 23, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhou, H.; Zhang, X. An Interpretation Architecture for Deep Learning Models with the Application of COVID-19 Diagnosis. Entropy 2021, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Boumaraf, S.; Liu, X.; Wan, Y.; Zheng, Z.; Ferkous, C.; Ma, X.; Li, Z.; Bardou, D. Conventional Machine Learning versus Deep Learning for Magnification Dependent Histopathological Breast Cancer Image Classification: A Comparative Study with Visual Explanation. Diagnostics 2021, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Paszke, A.; Chaurasia, A.; Kim, S.; Culurciello, E. ENet: A Deep Neural Network Architecture for Real-Time Semantic Segmentation. arXiv 2016, arXiv:1606.02147. [Google Scholar]
- Comelli, A.; Dahiya, N.; Stefano, A.; Vernuccio, F.; Portoghese, M.; Cutaia, G.; Bruno, A.; Salvaggio, G.; Yezzi, A. Deep Learning-Based Methods for Prostate Segmentation in Magnetic Resonance Imaging. Appl. Sci. 2021, 11, 782. [Google Scholar] [CrossRef]
- Cuocolo, R.; Comelli, A.; Stefano, A.; Benfante, V.; Dahiya, N.; Stanzione, A.; Castaldo, A.; De Lucia, D.R.; Yezzi, A.; Imbriaco, M. Deep Learning Whole-Gland and Zonal Prostate Segmentation on a Public MRI Dataset. Magn. Reason. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Dong, W.; Socher, R.; Li, L.; Li, K.; Li, F. ImageNet: A large-scale hierarchical image database. In Proceedings of the IEEE Conference on Computer Vision & Pattern Recognition, Miami, FL, USA, 20–25 June 2009. [Google Scholar]
- Shafer, G. A Mathematical Theory of Evidence; Princeton University Press: Princeton, NJ, USA, 1976. [Google Scholar]
- Smets, P. The combination of evidence in the transferable belief model. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 447–458. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X. Combination Method of Conflictive Evidences in D-S Evidence Theory; National Defense Industry Press: Beijing, China, 2010. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), IEEE Computer Society, Las Vegas, NV, USA, 26 June–1 July 2016. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G. ImageNet Classification with Deep Convolutional Neural Networks. Adv. Neural Inform. Process. Syst. 2012, 25, 2. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Dan, Y.; Yao, Y.; Dai, W.; Cai, G.; Yang, G.; Tong, T. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J. Trans. Med. 2020, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Ho-Quang, T.; Le, N. Use Chou’s 5-steps rule with different word embedding types to boost performance of electron transport protein prediction model. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
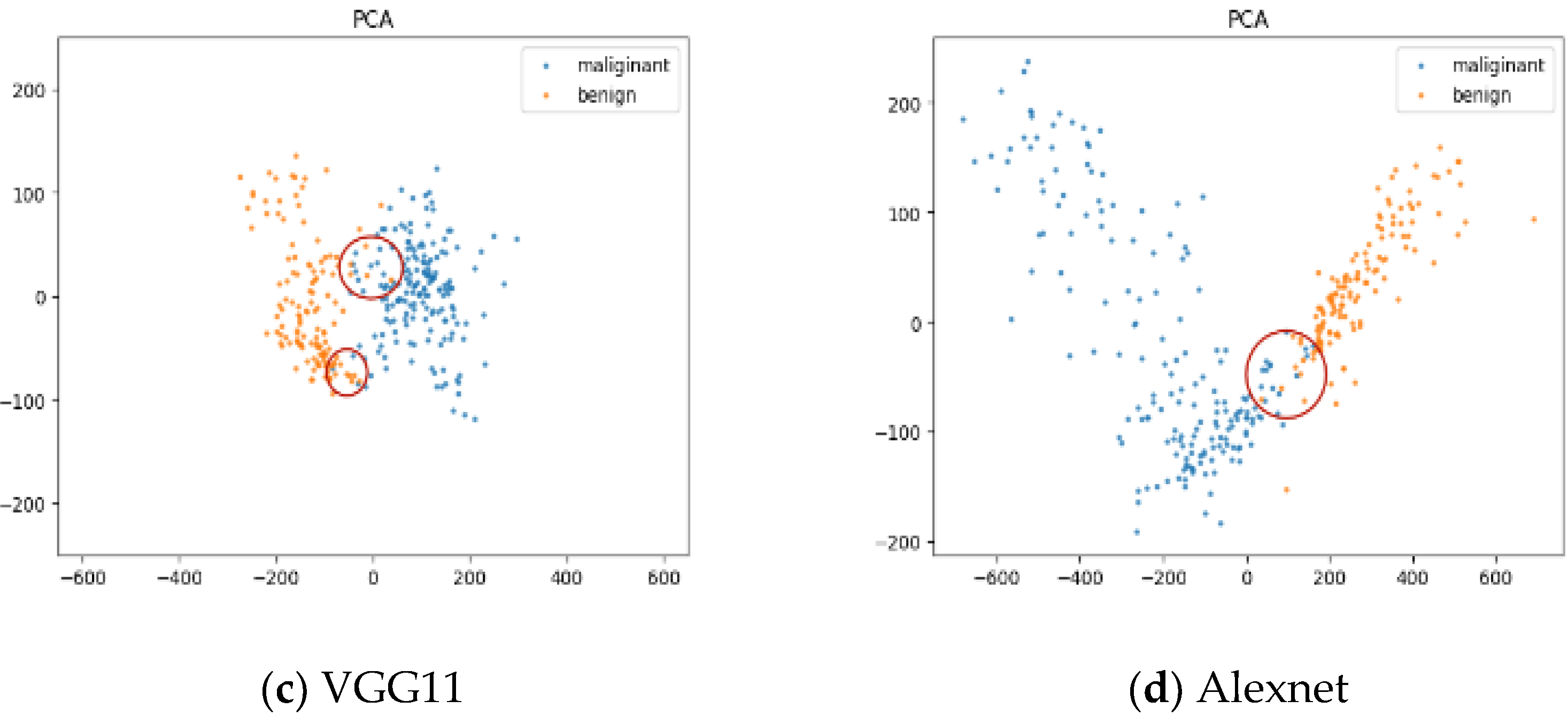
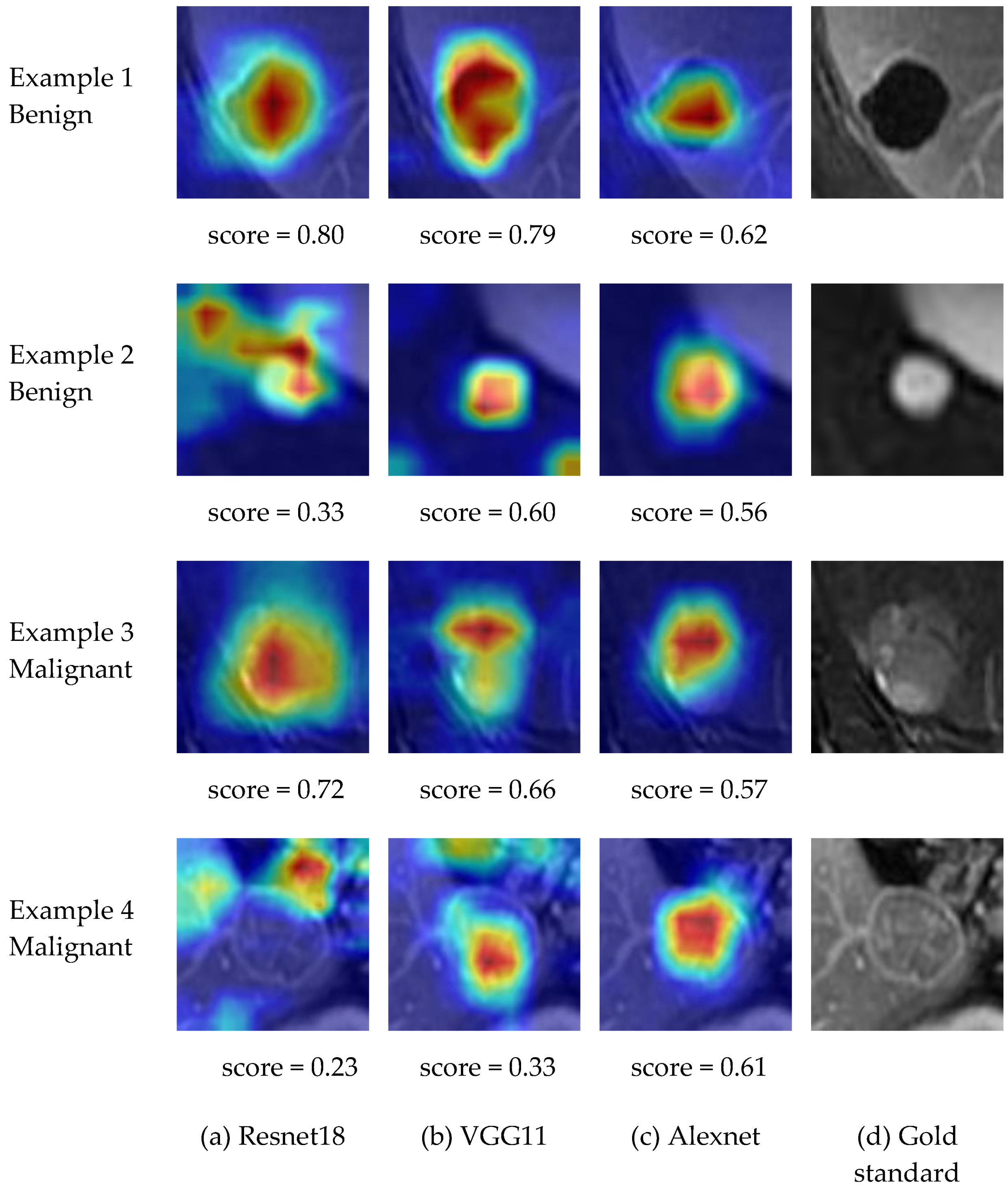
| Method | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Traditional | GLCM + AdaBoost | 85.31 | 87.97 | 81.23 | 87.31 | 75.45 |
| GLCM + SVM | 84.30 | 93.20 | 71.62 | 82.53 | 75.64 | |
| GLCM + RF | 87.09 | 90.69 | 81.63 | 88.02 | 80.83 | |
| Resnet18 | Large Scale | 92.20 | 94.34 | 88.63 | 92.45 | 91.64 |
| Middle Scale | 90.58 | 92.77 | 87.38 | 91.52 | 89.17 | |
| Small Scale | 92.15 | 92.94 | 91.00 | 93.83 | 89.79 | |
| Feature-Level Fusion | 92.56 | 94.47 | 89.75 | 93.17 | 91.65 | |
| Multi-Level Fusion | 95.70 | 97.02 | 93.75 | 95.80 | 95.54 | |
| VGG11 | Large Scale | 94.02 | 95.74 | 91.50 | 94.32 | 93.67 |
| Middle Scale | 95.65 | 96.94 | 93.75 | 95.80 | 95.43 | |
| Small Scale | 94.28 | 94.89 | 93.38 | 95.48 | 92.59 | |
| Feature-Level Fusion | 97.97 | 98.30 | 97.50 | 98.30 | 97.53 | |
| Multi-Level Fusion | 98.99 | 98.72 | 99.38 | 99.57 | 98.15 | |
| Alexnet | Large Scale | 96.60 | 97.45 | 95.38 | 96.91 | 96.15 |
| Middle Scale | 95.04 | 95.74 | 94.00 | 95.91 | 93.77 | |
| Small Scale | 98.03 | 98.21 | 97.75 | 98.51 | 97.39 | |
| Feature-Level Fusion | 98.86 | 98.93 | 98.75 | 99.14 | 98.46 | |
| Multi-Level Fusion | 99.49 | 99.15 | 100 | 100 | 98.77 | |
| Resnet18 | VGG11 | Alexnet | |
|---|---|---|---|
| Average Score | 0.45 | 0. 51 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Zheng, Z.; Liu, R.; Zhu, Z.; Zhou, H.; Zhang, X.; Boumaraf, S. A Multi-Scale and Multi-Level Fusion Approach for Deep Learning-Based Liver Lesion Diagnosis in Magnetic Resonance Images with Visual Explanation. Life 2021, 11, 582. https://doi.org/10.3390/life11060582
Wan Y, Zheng Z, Liu R, Zhu Z, Zhou H, Zhang X, Boumaraf S. A Multi-Scale and Multi-Level Fusion Approach for Deep Learning-Based Liver Lesion Diagnosis in Magnetic Resonance Images with Visual Explanation. Life. 2021; 11(6):582. https://doi.org/10.3390/life11060582
Chicago/Turabian StyleWan, Yuchai, Zhongshu Zheng, Ran Liu, Zheng Zhu, Hongen Zhou, Xun Zhang, and Said Boumaraf. 2021. "A Multi-Scale and Multi-Level Fusion Approach for Deep Learning-Based Liver Lesion Diagnosis in Magnetic Resonance Images with Visual Explanation" Life 11, no. 6: 582. https://doi.org/10.3390/life11060582
APA StyleWan, Y., Zheng, Z., Liu, R., Zhu, Z., Zhou, H., Zhang, X., & Boumaraf, S. (2021). A Multi-Scale and Multi-Level Fusion Approach for Deep Learning-Based Liver Lesion Diagnosis in Magnetic Resonance Images with Visual Explanation. Life, 11(6), 582. https://doi.org/10.3390/life11060582








