Mean Platelet Volume as a Potential Marker of Large Vessel Occlusion and Predictor of Outcome in Acute Ischemic Stroke Patients Treated with Reperfusion Therapy
Abstract
1. Background
2. Aim
3. Materials and Methods
Statistical Analysis
4. Results
4.1. Baseline Clinical Characteristics of Studied Cohort
4.2. Characteristics of Studied Subjects Stratified into MPV Quartiles
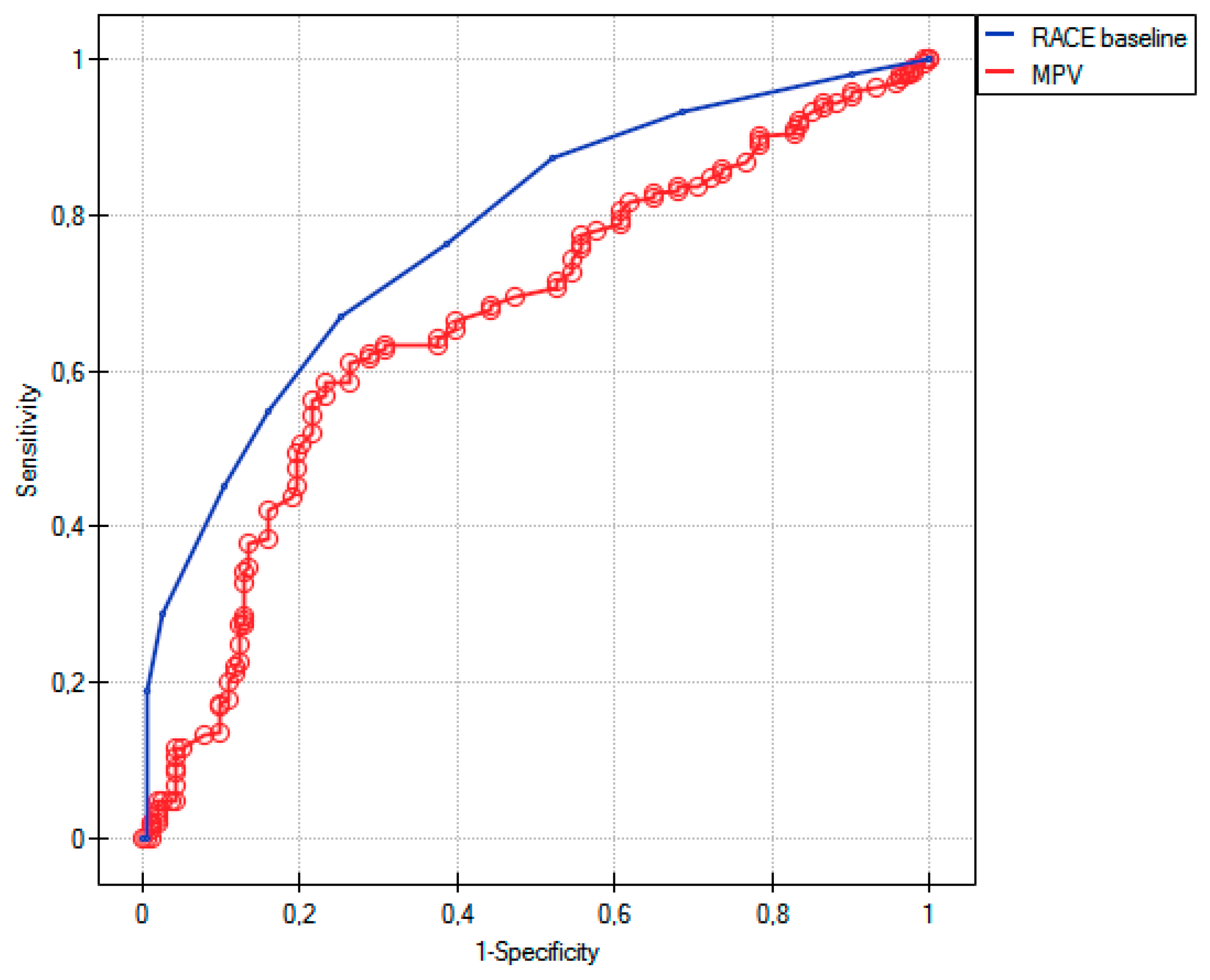
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badhiwala, J.H.; Nassiri, F.; Alhazzani, W.; Selim, M.H.; Farrokhyar, F.; Spears, J.; Kulkarni, A.V.; Singh, S.; Alqahtani, A.; Rochwerg, B.; et al. Endovascular thrombectomy for acute ischemic stroke. JAMA 2015, 314, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Malhotra, K.; Gornbein, J.; Saver, J.L. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: A review. Front. Neurol. 2017, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Navalkele, D.; Vahidy, F.; Kendrick, S.; Traylor, A.; Haydel, M.; Drury, S.; Martin-Schild, S. Vision, aphasia, neglect assessment for large vessel occlusion stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104478. [Google Scholar] [CrossRef]
- Vidale, S.; Agostoni, E. Prehospital stroke scales and large vessel occlusion: A systematic review. Acta Neurol. Scand. 2018, 138, 24–31. [Google Scholar] [CrossRef]
- Colkesen, Y.; Muderrisoglu, H. The role of mean platelet volume in predicting thrombotic events. Clin. Chem. Lab. Med. 2012, 50, 631–634. [Google Scholar] [CrossRef]
- Staszewski, J.; Pogoda, A.; Data, K.; Walczak, K.; Nowocień, M.; Frankowska, E.; Stępień, A. The mean platelet volume on admission predicts unfavorable stroke outcomes in patients treated with IV thrombolysis. Clin. Interv. Aging 2019, 14, 493–503. [Google Scholar] [CrossRef]
- Peng, F.; Zheng, W.; Li, F.; Wang, J.; Liu, Z.; Chen, X.; Xiao, L.; Sun, W.; Liu, X. Elevated mean platelet volume is associated with poor outcome after mechanical thrombectomy. J. NeuroInterv. Surg. 2018, 10, 25–28. [Google Scholar] [CrossRef]
- Sabença, F.; Carvalho, A.; Rocha, M.; Araújo, A.; Rodrigues, M.; Cunha, A.; Ribeiro, M.; Castro, S.; Costa, H.; Barros, P.; et al. Mean platelet volume and mechanical thrombectomy. J. Stroke Cerebrovasc. Dis. 2020, 29, 104971. [Google Scholar] [CrossRef]
- Oji, S.; Tomohisa, D.; Hara, W.; Tajima, T.; Suzuki, M.; Saito, A.; Yoshida, N.; Nomura, K. Mean platelet volume is associated with early neurological deterioration in patients with branch atheromatous disease: Involvement of platelet activation. J. Stroke Cerebrovasc. Dis. 2018, 27, 1624–1631. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Ay, H.; Furie, K.L.; Singhal, A.; Smith, W.S.; Sorensen, A.G.; Koroshetz, W.J. An evidence-based causative classification system for acute ischemic stroke. Ann. Neurol. 2005, 58, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Pexman, J.H.; Barber, P.A.; Hill, M.D.; Sevick, R.J.; Demchuk, A.M.; Hudon, M.E.; Hu, W.Y.; Buchan, A.M. Use of the Alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. Am. J. Neuroradiol. 2001, 22, 1534–1542. [Google Scholar]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Shi, Z.-S.; Liebeskind, D.S.; Xiang, B.; Ge, S.G.; Feng, L.; Albers, G.W.; Budzik, R.; Devlin, T.; Gupta, R.; Jansen, O.; et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 2014, 45, 1977–1984. [Google Scholar] [CrossRef]
- Smith, E.E.; Kent, D.M.; Bulsara, K.R.; Leung, L.Y.; Lichtman, J.H.; Reeves, M.J.; Towfighi, A.; Whiteley, W.N.; Zahuranec, D.B.; American Heart Association Stroke Council. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: A systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 2018, 49, e111–e122. [Google Scholar] [CrossRef] [PubMed]
- Heldner, M.R.; Zubler, C.; Mattle, H.P.; Schroth, G.; Weck, A.; Mono, M.-L.; Gralla, J.; Jung, S.; El-Koussy, M.; Lüdi, R.; et al. National institutes of health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke 2013, 44, 1153–1157. [Google Scholar] [CrossRef]
- Lima, F.O.; Silva, G.S.; Furie, K.L.; Frankel, M.R.; Lev, M.H.; Camargo, E.C.; Haussen, D.C.; Singhal, A.B.; Koroshetz, W.J.; Smith, W.S.; et al. Field assessment stroke triage for emergency destination. Stroke 2016, 47, 1997–2002. [Google Scholar] [CrossRef]
- De la Ossa, N.P.; Carrera, D.; Gorchs, M.; Querol, M.; Millán, M.; Gomis, M.; Dorado, L.; López-Cancio, E.; Hernández-Pérez, M.; Chicharro, V.; et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion. Stroke 2014, 45, 87–91. [Google Scholar] [CrossRef]
- Scheitz, J.F.; Abdul-Rahim, A.H.; MacIsaac, R.L.; Cooray, C.; Sucharew, H.; Kleindorfer, D.; Khatri, P.; Broderick, J.P.; Audebert, H.J.; Ahmed, N.; et al. Clinical selection strategies to identify ischemic stroke patients with large anterior vessel occlusion: Results from SITS-ISTR safe implementation of thrombolysis in stroke international stroke thrombolysis registry. Stroke 2017, 48, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.; Algert, C.; Chapman, N.; Neal, B.; Progress Collaborative Group. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke 2004, 35, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Şengeze, N.; Giray, S. The relationship between mean platelet volume, platelet count, platelet lymphocyte ratio, and recanalization success in first-pass thrombectomy of middle cerebral artery occlusions. Turk. J. Neurol 2020, 26, 133–137. [Google Scholar] [CrossRef]
- Yoon, W.; Kim, S.K.; Park, M.S.; Baek, B.H.; Lee, Y.Y. Predictive factors for good outcome and mortality after stent-retriever thrombectomy in patients with acute anterior circulation stroke. J. Stroke 2017, 19, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Blann, A.; Lip, G. Platelet activation: Assessment and quantification. Eur. Heart J. 2001, 22, 1561–1571. [Google Scholar] [CrossRef]
- Chu, S.G.; Becker, R.C.; Berger, P.B.; Bhatt, D.L.; Eikelboom, J.W.; Konkle, B.; Mohler, E.R.; Reilly, M.P.; Berger, J.S. Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J. Thromb. Haemost. 2010, 8, 148–156. [Google Scholar] [CrossRef]
- Ha, S.-I.; Choi, D.-H.; Ki, Y.-J.; Yang, J.-S.; Park, G.; Chung, J.-W.; Koh, Y.-Y.; Chang, K.-S.; Hong, S.-P. Stroke prediction using mean platelet volume in patients with atrial fibrillation. Platelets 2011, 22, 408–414. [Google Scholar] [CrossRef]
- Maden, O.; Kacmaz, F.; Selcuk, H.; Selcuk, M.T.; Aksu, T.; Tufekcioglu, O.; Senen, E.K.; Balbay, Y.; Ilkay, E. Relationship of admission hematological indexes with myocardial reperfusion abnormalities in acute ST segment elevation myocardial infarction patients treated with primary percutaneous coronary interventions. Can. J. Cardiol. 2009, 25, e164–e168. [Google Scholar] [CrossRef][Green Version]
- Ozdemir, O.; Soylu, M.; Alyan, O.; Geyik, B.; Demir, A.D.; Aras, D.; Cihan, G.; Cagirci, G.; Kacmaz, F.; Balbay, Y.; et al. Association between mean platelet volume and autonomic nervous system functions: Increased mean platelet volume reflects sympathetic overactivity. Exp. Clin. Cardiol. 2004, 9, 243–247. [Google Scholar]
- Dorrance, A.M.; Fink, G. Effects of stroke on the autonomic nervous system. Compr. Physiol. 2015, 5, 1241–1263. [Google Scholar] [CrossRef]
- Bowles, K.M.; Cooke, L.J.; Richards, E.M.; Baglin, T.P. Platelet size has diagnostic predictive value in patients with thrombocytopenia. Int. J. Lab. Hematol. 2005, 27, 370–373. [Google Scholar] [CrossRef] [PubMed]

| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p * | Post Hoc ** | |
|---|---|---|---|---|---|---|
| n | 92 | 88 | 93 | 88 | ||
| Age n (SD) | 68.3 (12.5) | 69 (13.9) | 71.7 (10.8) | 72.2 (12.4) | 0.1 | |
| Sex (F) n (%) | 44 (23.8) | 52 (28.1) | 50 (27) | 39 (21) | 0.2 | |
| AF | 22 (24) | 34 (38.6) | 39 (42) | 39 (44) | 0.01 | |
| Hypertension | 62 (68) | 68 (77) | 75 (81) | 59 (68.6) | 0.1 | |
| Diabetes | 12 (13) | 24 (27) | 26 (28) | 17 (20) | 0.06 | |
| Smoking | 27 (30) | 14 (16) | 25 (27) | 14 (16) | 0.05 | |
| Obesity | 124 (38) | 73 (23) | 54 (17) | 105 (33) | 0.2 | |
| CHD | 24 (26) | 21 (24) | 23 (25) | 19 (22) | 0.9 | |
| Dyslipidemia | 31 (34) | 23 (26) | 32 (34) | 20 (23) | 0.2 | |
| Past stroke | 11 (12) | 14 (16) | 13 (14) | 12 (14) | 0.9 | |
| OTG (MT) min | 257 (77) | 223 (62) | 246 (95) | 246 (73) | 0.6 | |
| OTT (tPA) min | 168 (62) | 172 (62) | 178 (63) | 178 (65) | 0.9 | |
| baseline RACE ≥ 5 | 24 (26) | 39 (44.3) | 56 (60.2) | 52 (59) | <0.01 | |
| NIHSS baseline | 10.9 (5.2) | 13.2 (5.7) | 15 (6.2) | 14 (6.5) | <0.01 | 1 vs. 2,3,4 |
| NIHSS 24 h | 7.4 (5.7) | 10.7 (7.6) | 12.5 (8.7) | 11.8 (8) | <0.01 | 1 vs. 3,4 |
| NIHSS discharge | 3.9 (3.6) | 7 (5.8) | 8 (7.8) | 6.9 (7) | <0.01 | 1 vs. 2,3,4 |
| mRSdischarge | 2.4 (1.8) | 3.5 (1.9) | 3.6 (1.8) | 3.5 (1.9) | <0.01 | 1 vs. 2,3,4 |
| mRS ≤ 2 discharge | 57 (62) | 29 (33) | 27 (29) | 33 (37.5) | <0.01 | |
| In-hospital mortality | 8 (8.7) | 14 (15.9) | 16 (17.2) | 13 (14.7) | 0.35 | |
| Hyperdense MCA sign | 16 (18.3) | 25 (30.5) | 23 (28.8) | 28 (38.9) | 0.04 | 1 vs. 2,3,4 |
| sICH hemorrhage | 10 (11) | 13 (15) | 21 (23) | 16 (18) | 0.17 | |
| TICI 2b,3 (MT) | 11 (85) | 13(76) | 27 (69) | 44 (80) | 0.6 | |
| ASPECTS score | 9.2 (0.5) | 9.1 (0.8) | 8.1 (1.2) | 8 (1.4) | 0.8 | |
| LVO | 36 (39.1) | 38 (43.2) | 54 (58) | 66 (75) | <0.01 | |
| Cardioembolic stroke | 24 (27) | 36 (40) | 41 (46) | 39 (43) | <0.05 | |
| Atherothrombotic stroke | 33 (37) | 40 (44) | 42 (47) | 48 (53) | <0.05 |
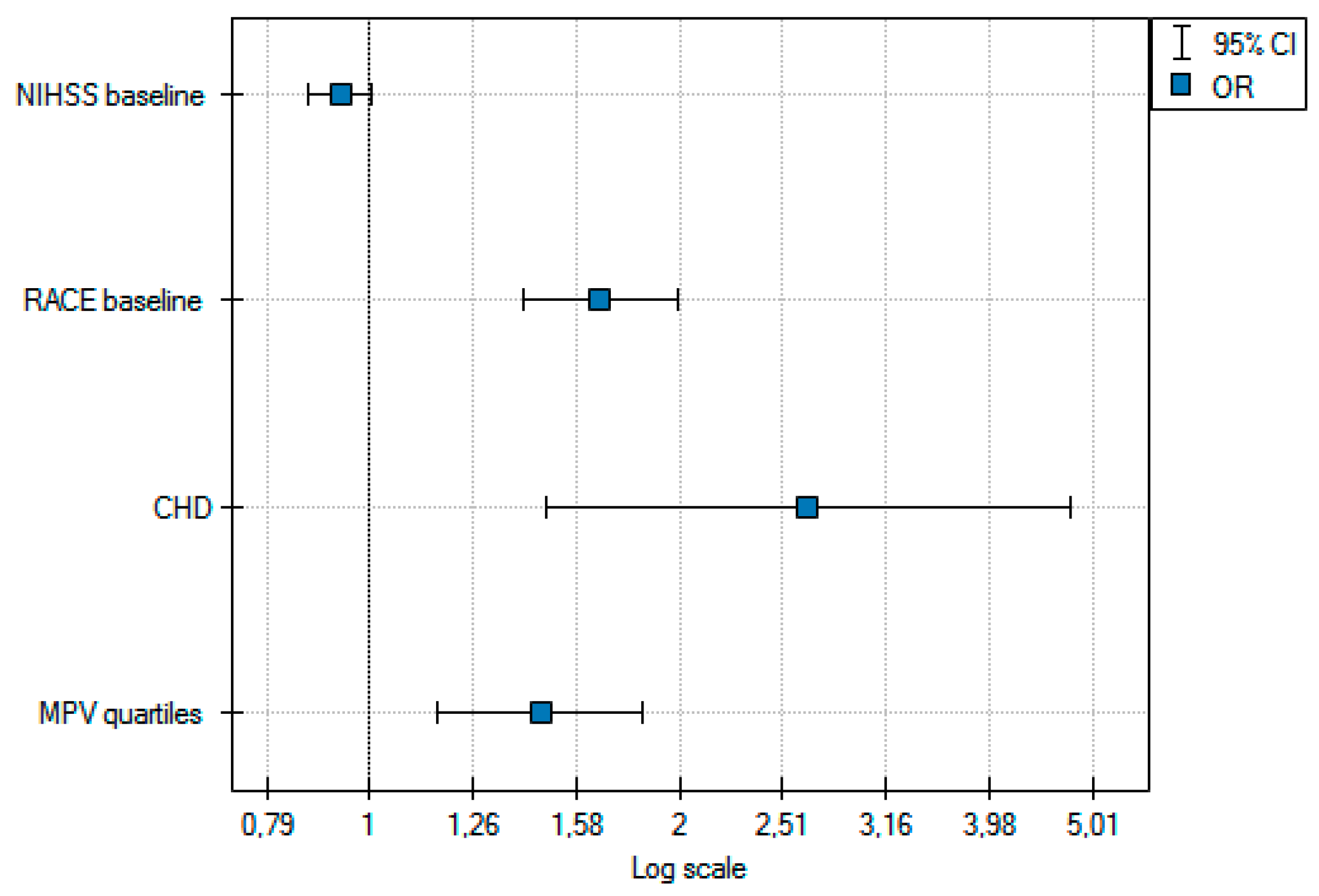
| Odds Ratio | −95%CI | +95%CI | p Value | |
|---|---|---|---|---|
| NIHSS baseline | 1.11 | 1.07 | 1.16 | <0.01 |
| RACE baseline | 1.51 | 1.37 | 1.67 | <0.01 |
| Age | 1.0 | 0.98 | 1.02 | 0.5 |
| Age ≥ 65 | 1.11 | 0.89 | 1.39 | 0.3 |
| MPV | 1.43 | 1.22 | 1.67 | <0.01 |
| MPV quartile | 1.66 | 1.36 | 2.03 | <0.01 |
| Hypertension | 1.0 | 0.62 | 1.61 | 0.9 |
| AF | 1.33 | 0.86 | 2.05 | 0.2 |
| Diabetes | 1.57 | 0.94 | 2.62 | 0.08 |
| Smoking | 1.4 | 0.84 | 2.33 | 0.2 |
| CHD | 2.21 | 1.33 | 3.69 | <0.01 |
| Dyslipidemia | 1.19 | 0.75 | 1.88 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębiec, A.; Pogoda-Wesołowska, A.; Piasecki, P.; Stępień, A.; Staszewski, J. Mean Platelet Volume as a Potential Marker of Large Vessel Occlusion and Predictor of Outcome in Acute Ischemic Stroke Patients Treated with Reperfusion Therapy. Life 2021, 11, 469. https://doi.org/10.3390/life11060469
Dębiec A, Pogoda-Wesołowska A, Piasecki P, Stępień A, Staszewski J. Mean Platelet Volume as a Potential Marker of Large Vessel Occlusion and Predictor of Outcome in Acute Ischemic Stroke Patients Treated with Reperfusion Therapy. Life. 2021; 11(6):469. https://doi.org/10.3390/life11060469
Chicago/Turabian StyleDębiec, Aleksander, Aleksandra Pogoda-Wesołowska, Piotr Piasecki, Adam Stępień, and Jacek Staszewski. 2021. "Mean Platelet Volume as a Potential Marker of Large Vessel Occlusion and Predictor of Outcome in Acute Ischemic Stroke Patients Treated with Reperfusion Therapy" Life 11, no. 6: 469. https://doi.org/10.3390/life11060469
APA StyleDębiec, A., Pogoda-Wesołowska, A., Piasecki, P., Stępień, A., & Staszewski, J. (2021). Mean Platelet Volume as a Potential Marker of Large Vessel Occlusion and Predictor of Outcome in Acute Ischemic Stroke Patients Treated with Reperfusion Therapy. Life, 11(6), 469. https://doi.org/10.3390/life11060469






