Strategies for the Production of Soluble Interferon-Alpha Consensus and Potential Application in Arboviruses and SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
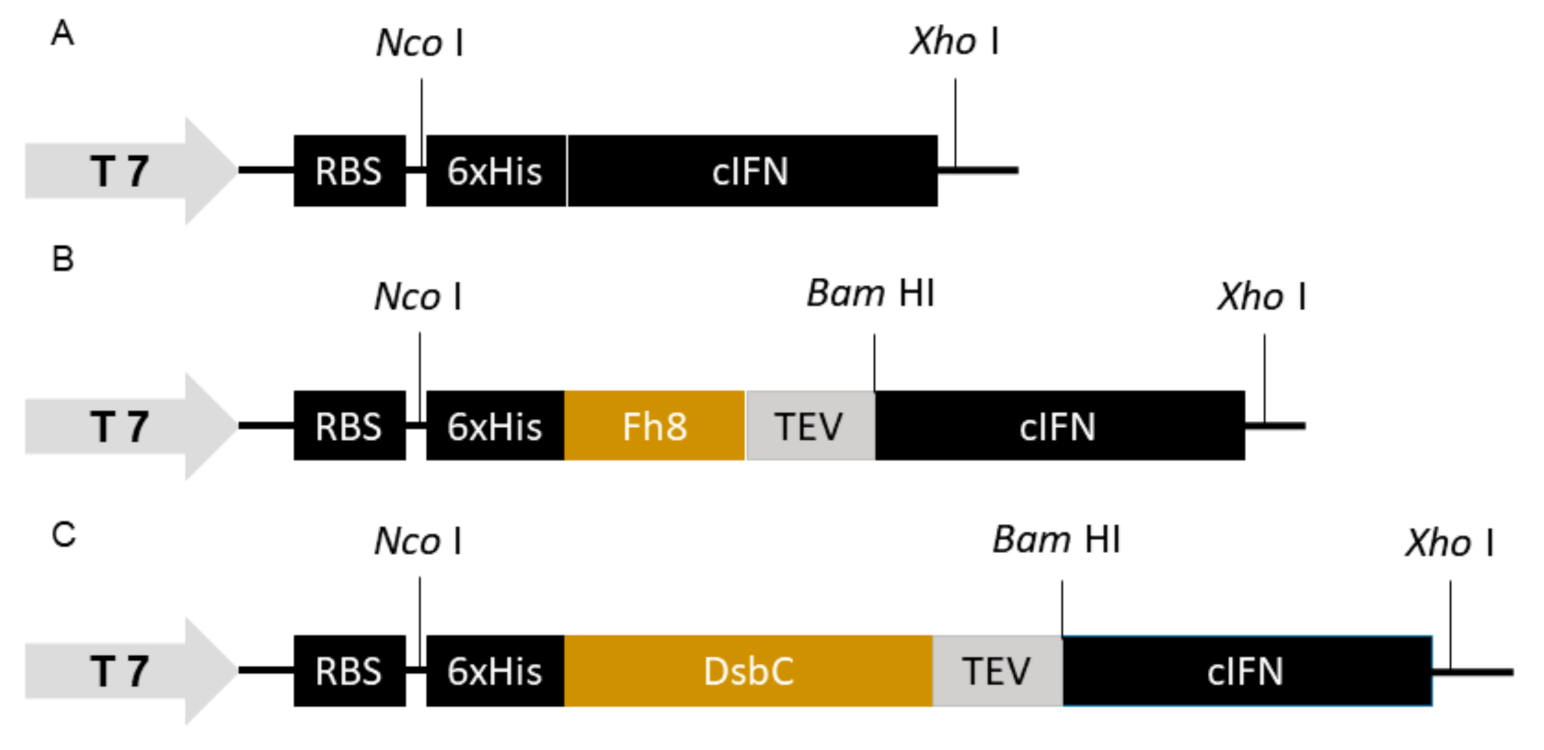
2.1. Design and Cloning of HF-IFN, HD-IFN and H-IFN Genes
2.2. PCR Amplification
2.3. Bacterial Strains
2.4. Culture Media and Conditions
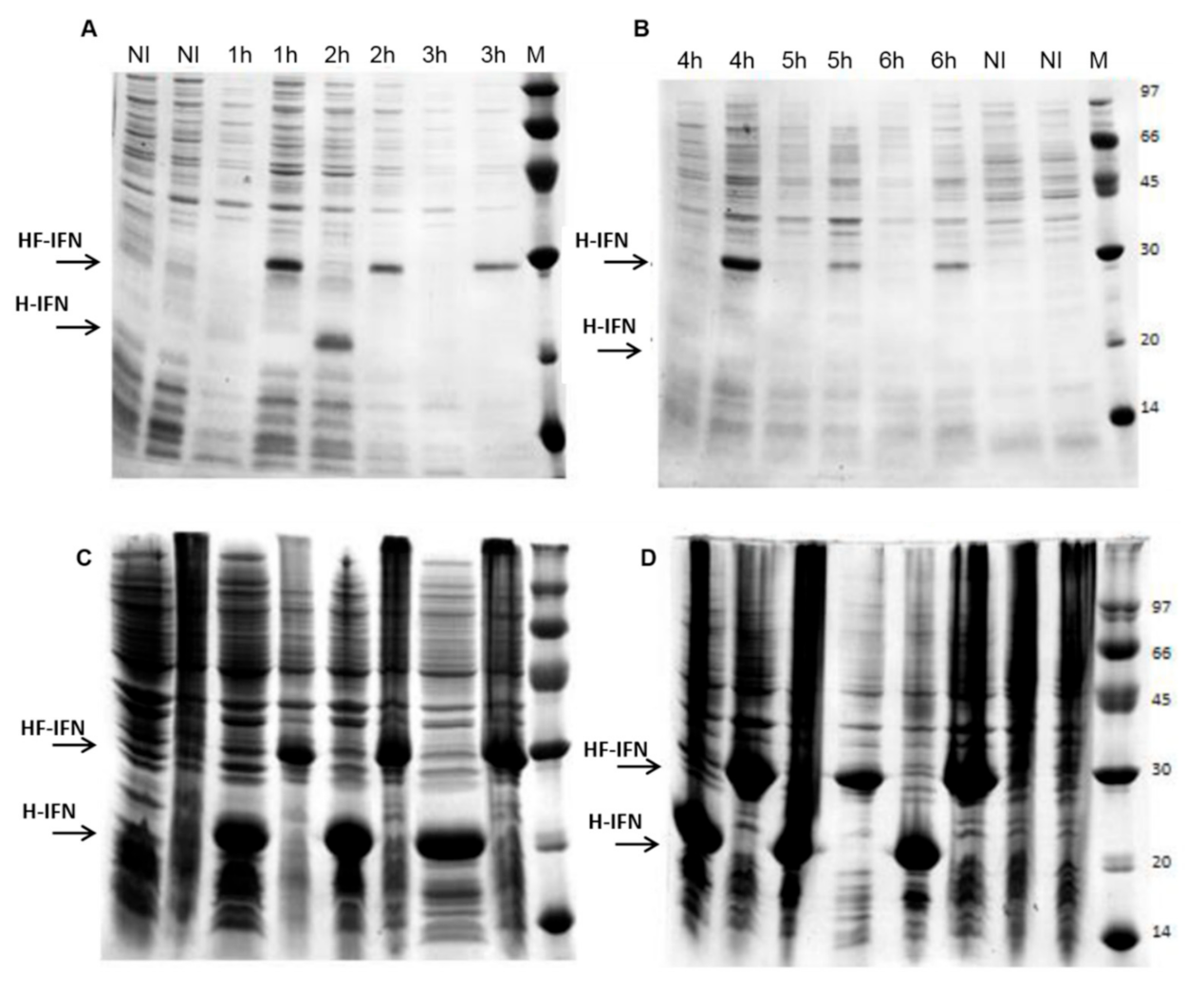
2.5. Evaluation of Protein Synthesis
2.6. Purification of Recombinant H-, HF-IFN and HD-IFN
2.7. Tag Removal
2.8. Purity Determination and Quantification of Target Protein by Densitometry
2.9. High-Performance Size-Exclusion Chromatography (HPSEC)
2.10. Antiviral Activity of cIFN
3. Results
3.1. Consensus IFN and Fusion with Solubility Tags
3.2. The Presence of the Fh8 Tag Increases HF-IFN Solubility
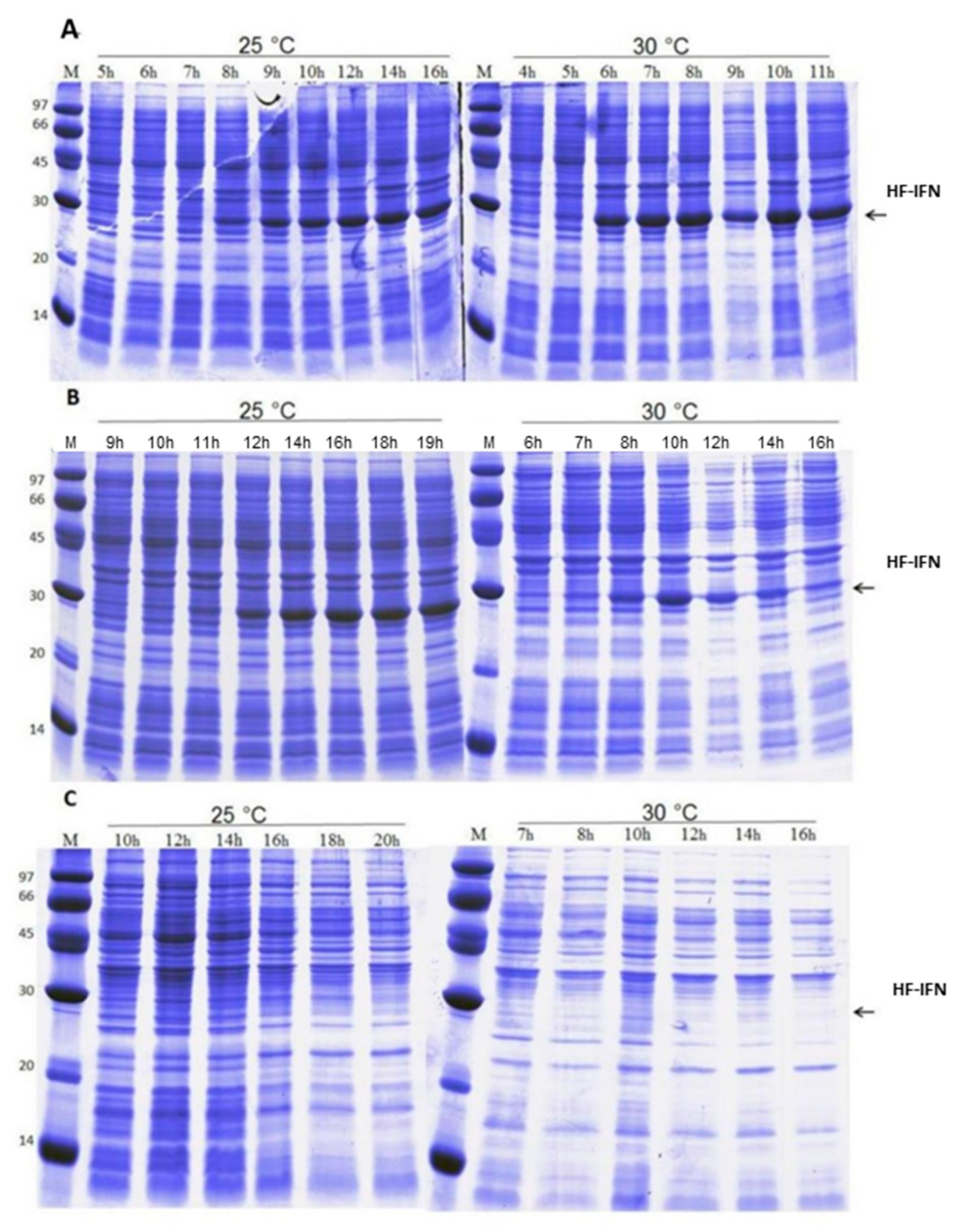
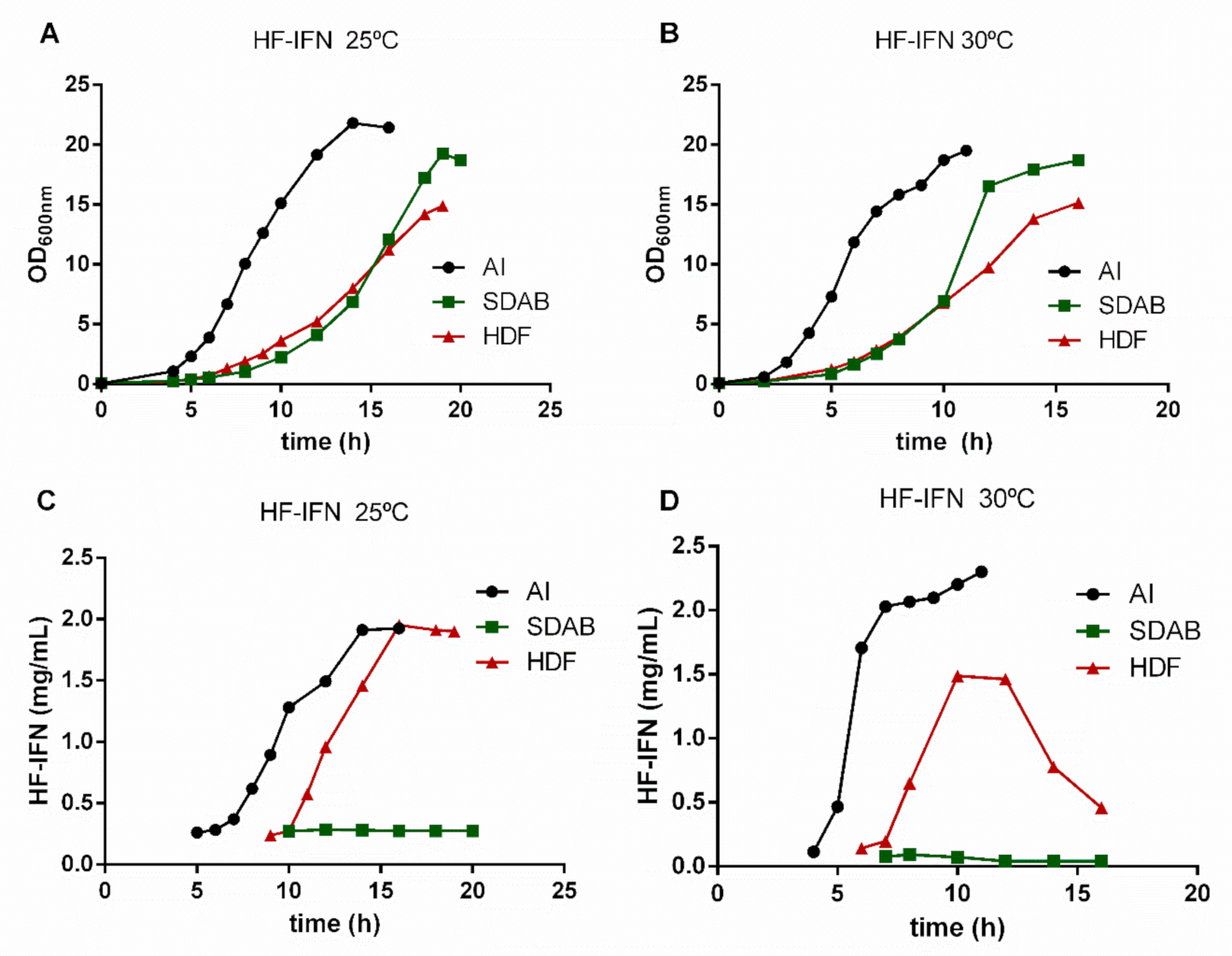
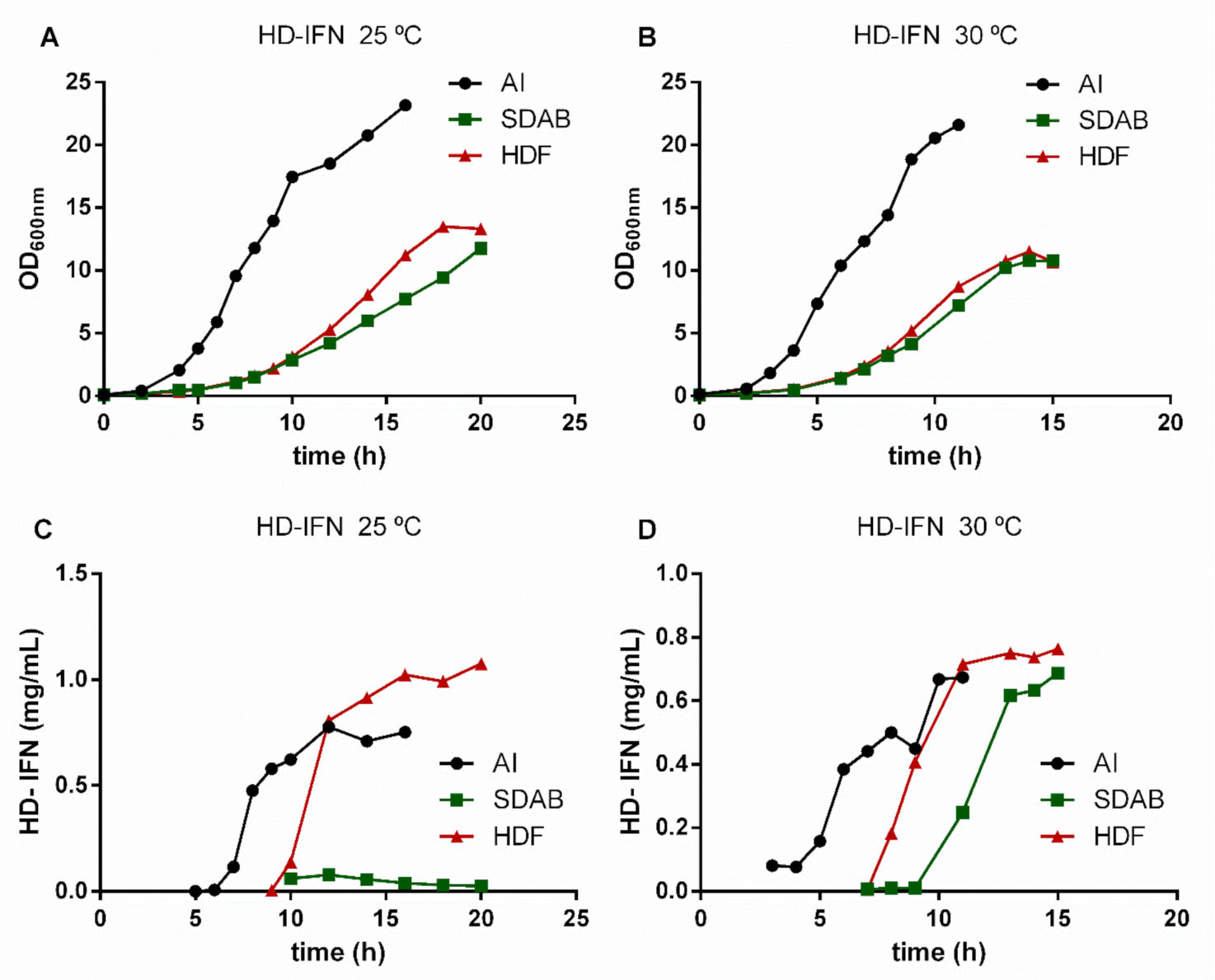
3.3. HF-IFN Production Is Higher When Produced in Autoinduction Medium at 30 °C
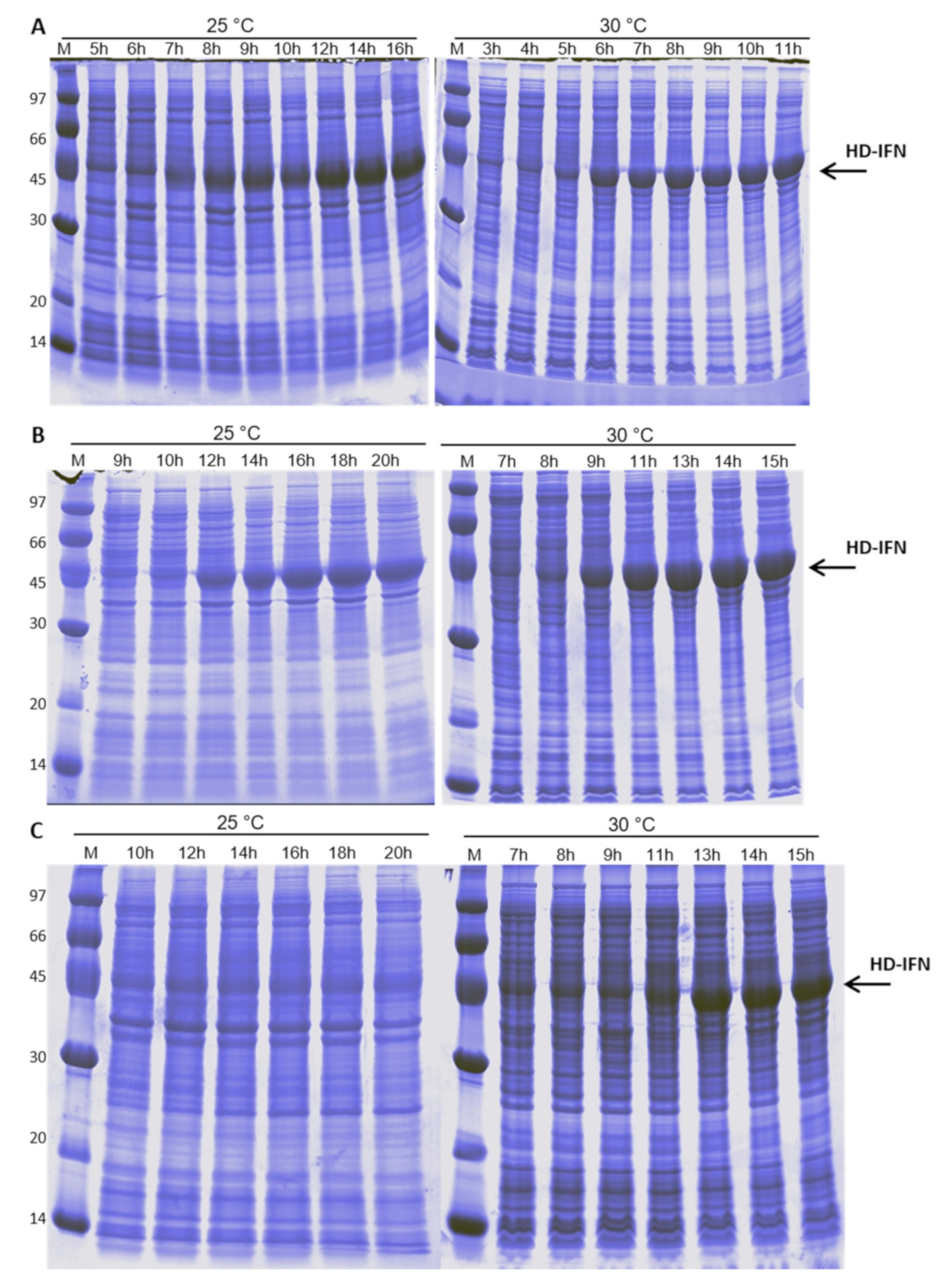
3.4. HD-IFN Production Is Higher When Produced in Chemically Defined Medium at 25 °C
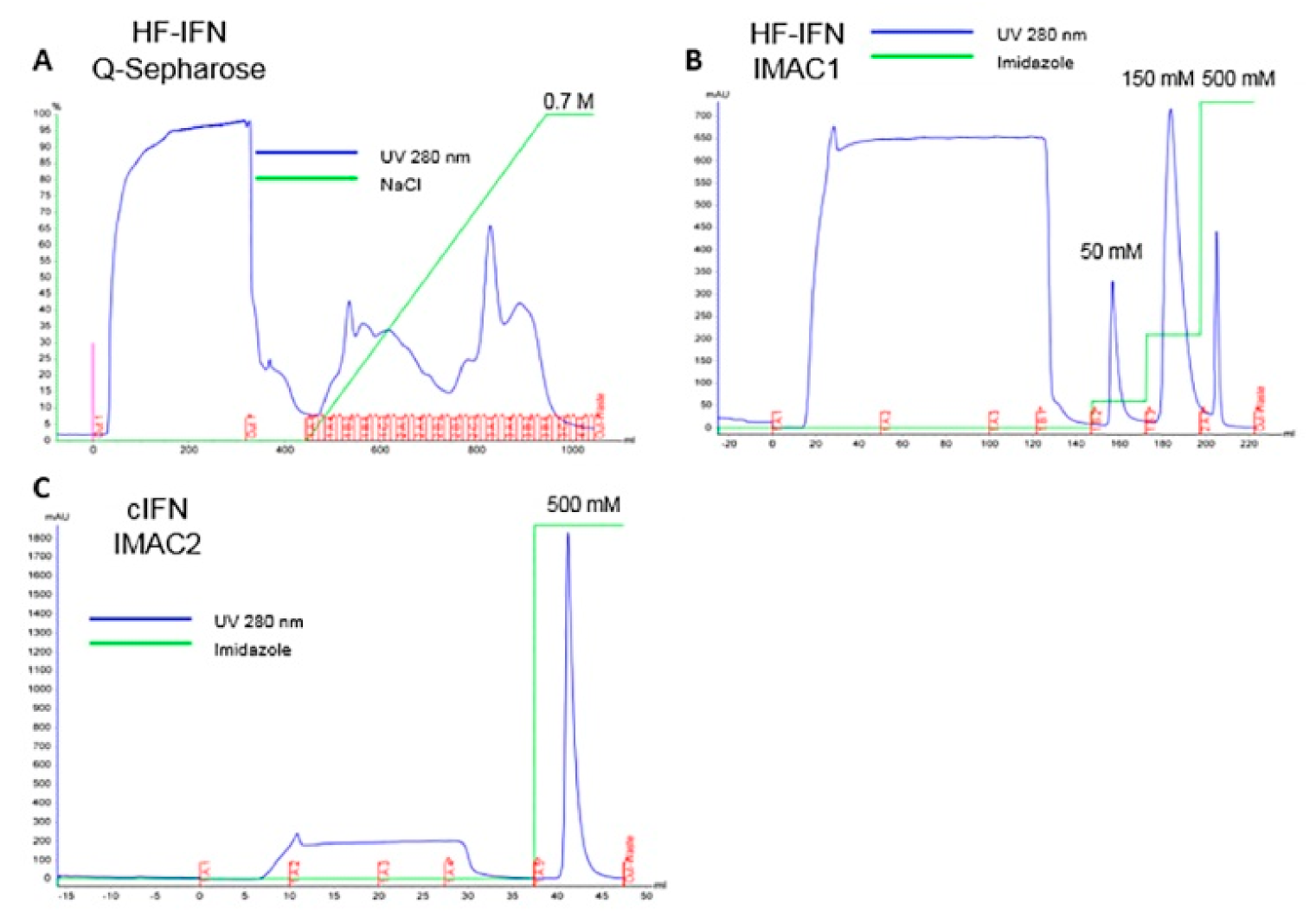
3.5. Purification of Recombinant cIFN with or without Solubility Tag
3.5.1. Purification of H- and HD-IFN
3.5.2. Purification of HF-IFN
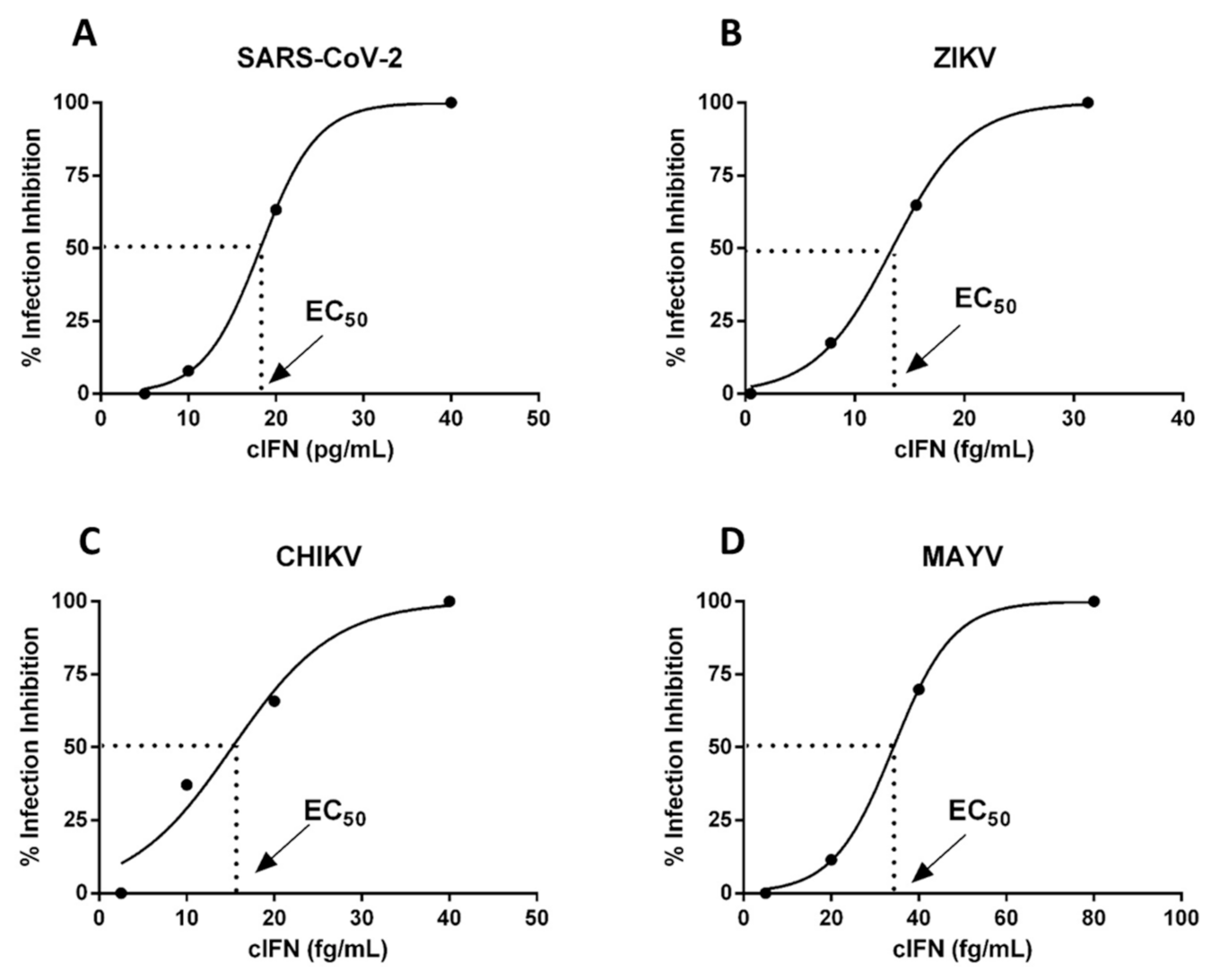
3.6. cIFN Exerts Antiviral Activity in Experimental Model of Arboviruses and SARS-CoV-2 Infection in VERO Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- RNCOS Global Protein Therapeutics Market Outlook 2020. Available online: https://www.researchandmarkets.com/reports/3422491/global-protein-therapeutics-market-outlook-2020 (accessed on 5 March 2021).
- Kesik-Brodacka, M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-derived virus-like particles in vaccine development. npj Vaccines 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kesik-Brodacka, M.; Romanik, A.; Mikiewicz-Sygula, D.; Plucienniczak, G.; Plucienniczak, A. A novel system for stable, high-level expression from the T7 promoter. Microb. Cell Fact. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Pacheco, B.; Crombet, L.; Loppnau, P.; Cossar, D. A screening strategy for heterologous protein expression in Escherichia coli with the highest return of investment. Protein Expr. Purif. 2012, 81, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Oppezzo, P. Overcoming the solubility problem in E. coli: Available approaches for recombinant protein production. In Insoluble Proteins: Methods and Protocols; Humana Press: New York, NY, USA, 2014; pp. 27–44. ISBN 9781493922055. [Google Scholar]
- Hammarstrom, M.; Hellgren, N.; van Den Berg, S.; Berglund, H.; Hard, T. Rapid screening for improved solubility of small human proteins produced as fusion proteins in Escherichia coli. Protein Sci. 2002, 11, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Esposito, D.; Chatterjee, D.K. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 2006, 17, 353–358. [Google Scholar] [CrossRef]
- Vincentelli, R.; Cimino, A.; Geerlof, A.; Kubo, A.; Satou, Y.; Cambillau, C. High-throughput protein expression screening and purification in Escherichia coli. Methods 2011, 55, 65–72. [Google Scholar] [CrossRef]
- Nozach, H.; Fruchart-Gaillard, C.; Fenaille, F.; Beau, F.; Ramos, O.H.P.; Douzi, B.; Saez, N.J.; Moutiez, M.; Servent, D.; Gondry, M.; et al. High throughput screening identifies disulfide isomerase DsbC as a very efficient partner for recombinant expression of small disulfide-rich proteins in E. coli. Microb. Cell Factories 2013, 12. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.; Castro, A.; Lopes, A.; Rodrigues, A.; Dias, C.; Conceição, A.; Alonso, J.; Correia da Costa, J.M.; Bastos, M.; Parra, F.; et al. A recombinant antigen recognized by Fasciola hepatica-infected hosts. J. Parasitol. 2004, 90, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.J.; Almeida, A.; Castro, A.; Domingues, L.; Besir, H. The novel Fh8 and H fusion partners for soluble protein expression in Escherichia coli: A comparison with the traditional gene fusion technology. Appl. Microbiol. Biotechnol. 2013, 97, 6779–6791. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.J.; Coelho, E.; Franco, L.; Almeida, A.; Castro, A.; Domingues, L. The Fh8 tag: A fusion partner for simple and cost-effective protein purification in Escherichia coli. Protein Expr. Purif. 2013, 92, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conceição, M.; Costa, S.; Castro, A.; Almeida, A. Fusion Proteins, Its Preparation Process and Its Application on Recombinant Protein Expression Systems. Portugal Patent WO/2010/082097, 22 July 2010. [Google Scholar]
- Zhuo, X.-F.; Zhang, Y.-Y.; Guan, Y.-X.; Yao, S.-J. Co-expression of disulfide oxidoreductases DsbA/DsbC markedly enhanced soluble and functional expression of reteplase in Escherichia coli. J. Biotechnol. 2014, 192 Pt A, 197–203. [Google Scholar] [CrossRef]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Creighton, T.E.; Missiakas, D.; Raina, S. Structural and Functional Characterization of DsbC, a Protein Involved in Disulfide Bond Formation in Escherichia coli. Biochemistry 1995, 34, 5075–5089. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Wang, C.C. The N-terminal sequence (residues 1–65) is essential for dimerization, activities, and peptide binding of Escherichia coli DsbC. J. Biol. Chem. 2000, 275, 22743–22749. [Google Scholar] [CrossRef] [Green Version]
- Turchetto, J.; Sequeira, A.F.; Ramond, L.; Peysson, F.; Brás, J.L.; Saez, N.J.; Duhoo, Y.; Blémont, M.; Guerreiro, C.I.; Quinton, L.; et al. High-throughput expression of animal venom toxins in Escherichia coli to generate a large library of oxidized disulphide-reticulated peptides for drug discovery. Microb. Cell Factories 2017, 16, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, J.C.; Leung, W.S.; Swartz, J.R. Overexpression of Escherichia coli oxidoreductases increases recombinant insulin-like growth factor-I accumulation. Proc. Natl. Acad. Sci. USA 1998, 95, 2773–2777. [Google Scholar] [CrossRef] [Green Version]
- Fish, E.N.; Harrison, S.A.; Hassanein, T. The role of consensus interferon in the current treatment of chronic hepatitis C viral infection. Gastroenterol. Hepatol. 2008, 4, 1–12. [Google Scholar]
- Ozes, O.N.; Reiter, Z.; Klein, S.; Blatt, L.M.; Taylor, M.W. A comparison of interferon-Con1 with natural recombinant interferons-alpha: Antiviral, antiproliferative, and natural killer-inducing activities. J. Interferon Res. 1992, 12, 55–59. [Google Scholar] [CrossRef]
- Blatt, L.M.; Davis, J.M.; Klein, S.B.; Taylor, M.W. The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J. Interferon Cytokine Res. 1996, 16, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Peciak, K.; Tommasi, R.; Choi, J.W.; Brocchini, S.; Laurine, E. Expression of soluble and active interferon consensus in SUMO fusion expression system in E. coli. Protein Expr. Purif. 2014, 99, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Li, J.; Ma, G.; Su, Z. On-column refolding of consensus interferon at high concentration with guanidine-hydrochloride and polyethylene glycol gradients. J. Chromatogr. A 2006, 1115, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; El-Baky, N.A.; Redwan, E.M. Expression, purification, and characterization of recombinant human consensus interferon-alpha in Escherichia coli under λP(L) promoter. Prep. Biochem. Biotechnol. 2012, 42, 426–447. [Google Scholar] [CrossRef]
- Rodriguez, A.K.; Muñoz, A.L.; Segura, N.A.; Rangel, H.R.; Bello, F. Molecular characteristics and replication mechanism of dengue, zika and chikungunya arboviruses, and their treatments with natural extracts from plants: An updated review. EXCLI J. 2019, 18, 988–1006. [Google Scholar] [PubMed]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.M.; Ramírez-Santana, C. Mayaro: An emerging viral threat? Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Colón-González, F.J.; Peres, C.A.; Steiner São Bernardo, C.; Hunter, P.R.; Lake, I.R. After the epidemic: Zika virus projections for Latin America and the Caribbean. PLoS Negl. Trop. Dis. 2017, 11, e0006007. [Google Scholar] [CrossRef] [Green Version]
- Goebel, S.; Snyder, B.; Sellati, T.; Saeed, M.; Ptak, R.; Murray, M.; Bostwick, R.; Rayner, J.; Koide, F.; Kalkeri, R. A sensitive virus yield assay for evaluation of Antivirals against Zika Virus. J. Virol. Methods 2016, 238, 13–20. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Bouayad, A. Innate immune evasion by SARS-CoV-2: Comparison with SARS-CoV. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- De Spiegeleer, P.; Sermon, J.; Lietaert, A.; Aertsen, A.; Michiels, C.W. Source of tryptone in growth medium affects oxidative stress resistance in Escherichia coli. J. Appl. Microbiol. 2004, 97, 124–133. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Campani, G.; dos Santos, M.P.; da Silva, G.G.; Horta, A.C.L.; Badino, A.C.; de Campos Giordano, R.; Gonçalves, V.M.; Zangirolami, T.C. Recombinant protein production by engineered Escherichia coli in a pressurized airlift bioreactor: A techno-economic analysis. Chem. Eng. Process. Process Intensif. 2016, 103, 63–69. [Google Scholar] [CrossRef]
- Li, Z.; Kessler, W.; Van Den Heuvel, J.; Rinas, U. Simple defined autoinduction medium for high-level recombinant protein production using T7-based Escherichia coli expression systems. Appl. Microbiol. Biotechnol. 2011, 91, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Seeger, A.; Schneppe, B.; McCarthy, J.E.G.; Deckwer, W.D.; Rinas, U. Comparison of temperature- and isopropyl-beta-d-thiogalacto-pyranoside-induced synthesis of basic fibroblast growth factor in high-cell-density cultures of recombinant Escherichia coli. Enzym. Microb. Technol. 1995, 17, 947–953. [Google Scholar] [CrossRef]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol. Cell. Biochem. 2007, 307, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.; Asenjo, J.A.; Andrews, B.A. Design and implementation of a high yield production system for recombinant expression of peptides. Microb. Cell Fact. 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeshen, M.N.; Al-Hejin, A.M.; Bora, R.S.; Ahmed, M.M.M.; Ramadan, H.A.I.; Saini, K.S.; Baeshen, N.A.; Redwan, E.M. Production of Biopharmaceuticals in E. coli: Current Scenario and Future Perspectives. J. Microbiol. Biotechnol. 2015, 25, 953–962. [Google Scholar] [CrossRef]
- Mahmoudi Gomari, M.; Saraygord-Afshari, N.; Farsimadan, M.; Rostami, N.; Aghamiri, S.; Farajollahi, M.M. Opportunities and challenges of the tag-assisted protein purification techniques: Applications in the pharmaceutical industry. Biotechnol. Adv. 2020, 45, 107653. [Google Scholar] [CrossRef]
- Protection against Recurrent Genital Herpes by Therapeutic Immunization with Herpes Simplex Virus Type 2 Ribonucleotide Reductas BenMohamed. Lbachir [THE REGENTS OF THE UNIVERSITY OF CALIFORNIA]. Available online: https://uspto.report/patent/app/20200046827 (accessed on 10 March 2021).
- Costa, S.; Almeida, A.; Castro, A.; Domingues, L. Fusion tags for protein solubility, purification, and immunogenicity in Escherichia coli: The novel Fh8 system. Front. Microbiol. 2014, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Arnau, J.; Lauritzen, C.; Petersen, G.E.; Pedersen, J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 2006, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dyson, M.R.; Shadbolt, S.P.; Vincent, K.J.; Perera, R.L.; McCafferty, J. Production of soluble mammalian proteins in Escherichia coli: Identification of protein features that correlate with successful expression. BMC Biotechnol. 2004, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, Z.; Chi, L.; Sun, J.; Shen, Y. Enhanced production of soluble tumor necrosis factor-related apoptosis-inducing ligand in Escherichia coli using a novel self-cleavable tag system Fh8-ΔI-CM. Protein Expr. Purif. 2018, 148, 16–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.-H.; Wang, F.; Fang, M.; Yin, C.-C.; Zhou, Z.-Y.; Lin, Q.; Huang, H.-L. Overexpression of DsbC and DsbG markedly improves soluble and functional expression of single-chain Fv antibodies in Escherichia coli. Protein Expr. Purif. 2002, 26, 218–228. [Google Scholar] [CrossRef]
- Malhotra, A. Tagging for Protein Expression. Methods Enzymol. 2009, 463, 239–258. [Google Scholar] [CrossRef]
- Singh, S.M.; Panda, A.K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrió, M.M.; Cubarsi, R.; Villaverde, A. Fine architecture of bacterial inclusion bodies. FEBS Lett. 2000, 471, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Rabhi-Essafi, I.; Sadok, A.; Khalaf, N.; Fathallah, D.M. A strategy for high-level expression of soluble and functional human interferon alpha as a GST-fusion protein in E. coli. Protein Eng. Des. Sel. 2007, 20, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, A.; González-Montalbán, N.; Arís, A.; Villaverde, A. The conformational quality of insoluble recombinant proteins is enhanced at low growth temperatures. Biotechnol. Bioeng. 2007, 96, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; Baldwin, R.L. Temperature Dependence of the Hydrophobic Interaction in Protein Folding Temperature dependence of the hydrophobic interaction in protein folding (hydrocarbon model). Proc. Natl. Acad. Sci. USA 1986, 83, 8069–8072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, S.D.; King, J. Cold rescue of the thermolabile tailspike intermediate at the junction between productive folding and off-pathway aggregation. Protein Sci. 1998, 7, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Pope, W.H.; Haase-Pettingell, C.; King, J. Protein folding failure sets high-temperature limit on growth of phage P22 in Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2004, 70, 4840–4847. [Google Scholar] [CrossRef] [Green Version]
- Scharnagl, C.; Reif, M.; Friedrich, J. Stability of proteins: Temperature, pressure and the role of the solvent. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1749, 187–213. [Google Scholar] [CrossRef]
- Schellman, J.A. Temperature, stability, and the hydrophobic interaction. Biophys. J. 1997, 73, 2960–2964. [Google Scholar] [CrossRef] [Green Version]
- Strandberg, L.; Enfors, S.O. Factors influencing inclusion body formation in the production of a fused protein in Escherichia coli. Appl. Environ. Microbiol. 1991, 57, 1669–1674. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guidelines on Transmissible Spongiform Encephalopathies in Relation to Biological and Pharmaceutical Products; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Takahashi, M.; Aoyagi, H. Practices of shake-flask culture and advances in monitoring CO2 and O2. Appl. Microbiol. Biotechnol. 2018, 102, 4279–4289. [Google Scholar] [CrossRef]
- EL-Baky, N.A.; Linjawi, M.H.; Redwan, E.M. Auto-induction expression of human consensus interferon-alpha in Escherichia coli. BMC Biotechnol. 2015, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brown, T. Clonagem Gênica e Análise de DNA-Uma Introdução, 4th ed.; Artmed: Porto Alegre, Brazil, 2003; ISBN 85-363-0095-7. [Google Scholar]
- Cardoso, V.M.; Campani, G.; Santos, M.P.; Silva, G.G.; Pires, M.C.; Gonçalves, V.M.; Giordano, R.D.C.; Sargo, C.R.; Horta, A.C.; Zangirolami, T.C. Cost analysis based on bioreactor cultivation conditions: Production of a soluble recombinant protein using Escherichia coli BL21(DE3). Biotechnol. Rep. 2020, 26, e00441. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yan, Z.; Xue, X.; Hao, Q.; Wan, Y.; Qin, X.; Zhang, C.; You, Y.; Han, W.; Zhang, Y. High-yield expression, purification and characterization of tumor-targeted IFN-α2a. Cytotherapy 2007, 9, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Bhattacharaya, P.; Pandey, G.; Mukherjee, K.J. Overexpression and purification of recombinant human interferon alpha2b in Escherichia coli. Protein Expr. Purif. 2005, 41, 313–322. [Google Scholar] [CrossRef]
- Babu, K.R.; Swaminathan, S.; Marten, S.; Khanna, N.; Rinas, U. Production of interferon-α in high cell density cultures of recombinant Escherichia coli and its single step purification from refolded inclusion body proteins. Appl. Microbiol. Biotechnol. 2000, 53, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Bashir, H.; Zafar, A.U.; Khan, M.A.; Tahir, S.; Khan, F.; Khan, M.I.; Akram, M.; Husnain, T. Optimization of conditions for high-level expression and purification of human recombinant consensus interferon (rh-cIFN) and its characterization. Biotechnol. Appl. Biochem. 2015, 62, 699–708. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Redwan, E.M. Therapeutic Alpha-Interferons Protein: Structure, Production, and Biosimilar. Prep. Biochem. Biotechnol. 2015, 45, 109–127. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Minakami, H.; Koshizuka, S.; Sato, I. Administration of interferon-alpha during pregnancy: Effects on fetus. J. Perinat. Med. 2000, 28, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Egberts, F.; Lischner, S.; Russo, P.; Kampen, W.U.; Hauschild, A. Diagnostic and therapeutic procedures for management of melanoma during pregnancy: Risks for the fetus? J. Dtsch. Dermatol. Ges. 2006, 4, 717–720. [Google Scholar] [CrossRef]



| Protein | TAG | Gene Size | Protein Size |
|---|---|---|---|
| H-IFN | 6xHis | 522 bp | 20.2 kDa |
| HF-IFN | 6xHis-Fh8 | 753 bp | 28.8 kDa |
| HD-IFN | 6xHis-DsbC | 1194 bp | 44.6 kDa |
| Fraction | Total Protein (mg) | Relative Purity (%) | HF-IFN (mg) | Step Recovery (%) | Total Recovery (%) | Purification Factor |
| Soluble Load | 456 | 15.4 | 70.5 | 100 | 100 | - |
| Q-Sepharose FF Elution | 112 | 17.4 | 19.5 | 27.8 | 27.8 | 1.12 |
| IMAC Elution | 5 | 81.6 | 4.08 | 21 | 5.8 | 4.69 |
| Fraction | Total Protein (mg) | Relative Purity (%) | HD-IFN (mg) | Step Recovery (%) | Total Recovery (%) | Purification Factor |
| Soluble Load | 2649 | 52.7 | 1397.3 | 100 | 100 | - |
| IMAC Elution | 68 | 32.7 | 22.2 | 1.6 | 1.6 | 0.62 |
| Fraction | Total Protein (mg) | Relative Purity (%) | H-IFN (mg) | Step Recovery (%) | Total Recovery (%) | |
| Pellet | 835 | 58 | 484.8 | |||
| Soluble Load | 832 | 0 | 4.74# | 100 | 100 | |
| IMAC Elution | 52 | 9 | 4.74 | 100 | 1 | |
| Construct | Untagged cIFN Recovery after Hydrolysis (%) |
|---|---|
| HF-IFN -> cIFN | 58 |
| HD-IFN -> cIFN | 14 |
| cIFN + Virus | EC50 |
|---|---|
| Zika (ZIKV) | 14 ± 8 fg/mL |
| Mayaro (MAYV) | 35 ± 5 fg/mL |
| Chikungunya (CHIV) | 12 ± 2 fg/mL |
| SARS-CoV-2 | 11 ± 7 fg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabarz, F.; Lopes, A.P.Y.; Barbosa, F.F.; Barazzone, G.C.; Santos, J.C.; Botosso, V.F.; Jorge, S.A.C.; Nascimento, A.L.T.O.; Astray, R.M.; Gonçalves, V.M. Strategies for the Production of Soluble Interferon-Alpha Consensus and Potential Application in Arboviruses and SARS-CoV-2. Life 2021, 11, 460. https://doi.org/10.3390/life11060460
Grabarz F, Lopes APY, Barbosa FF, Barazzone GC, Santos JC, Botosso VF, Jorge SAC, Nascimento ALTO, Astray RM, Gonçalves VM. Strategies for the Production of Soluble Interferon-Alpha Consensus and Potential Application in Arboviruses and SARS-CoV-2. Life. 2021; 11(6):460. https://doi.org/10.3390/life11060460
Chicago/Turabian StyleGrabarz, Felipe, Alexandre Paulo Yague Lopes, Flávia Ferreira Barbosa, Giovana Cappio Barazzone, Jademilson Celestino Santos, Viviane Fongaro Botosso, Soraia Attie Calil Jorge, Ana Lucia Tabet Oller Nascimento, Renato Mancini Astray, and Viviane Maimoni Gonçalves. 2021. "Strategies for the Production of Soluble Interferon-Alpha Consensus and Potential Application in Arboviruses and SARS-CoV-2" Life 11, no. 6: 460. https://doi.org/10.3390/life11060460









