Evaluation of Blood Coagulation Parameters and ADMA, NO, IL-6, and IL-18 Serum Levels in Patients with Neovascular AMD before, during, and after the Initial Loading Phase of Intravitreal Aflibercept
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
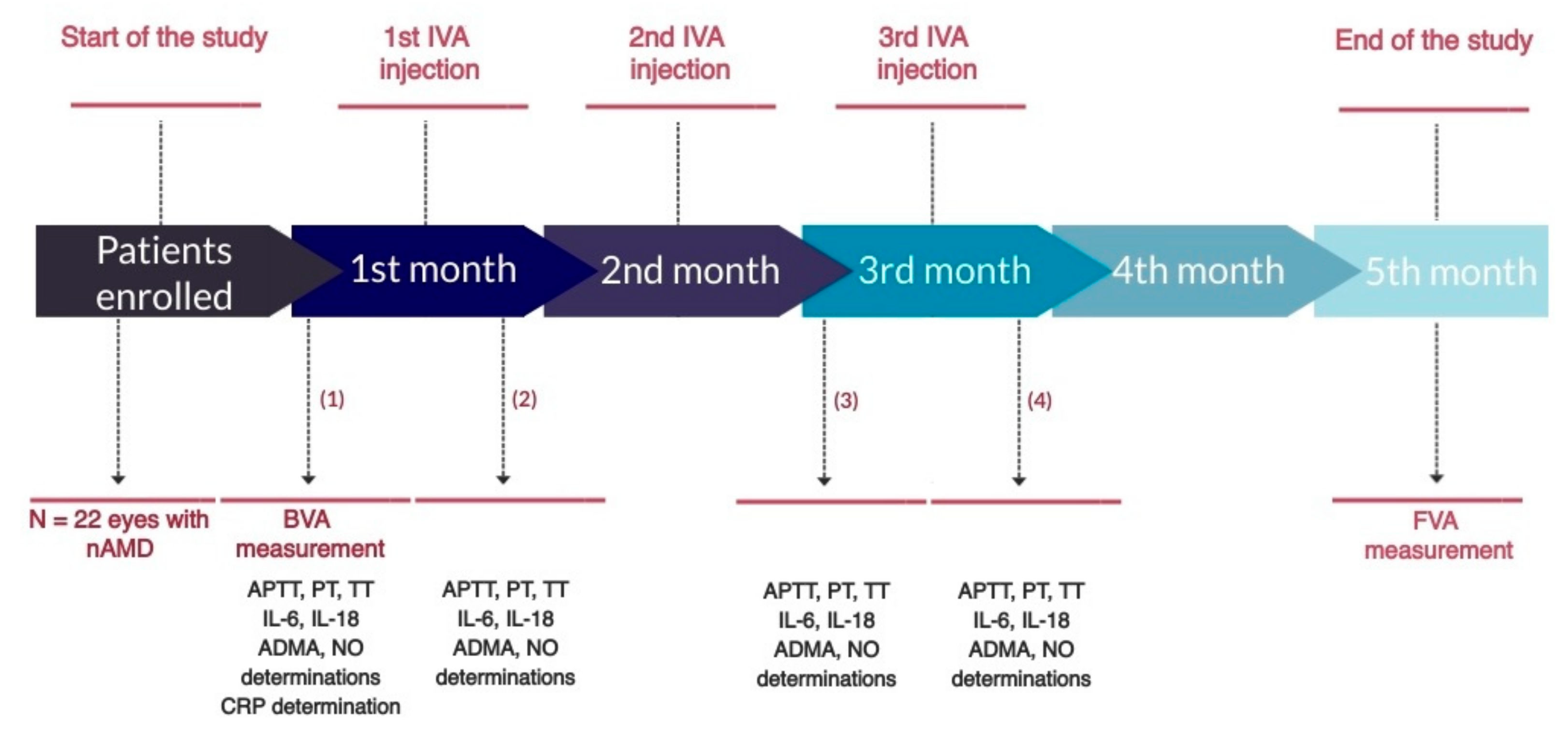
2.2. Study Design
2.3. Sample Collection
2.4. Statistical Analysis
3. Results
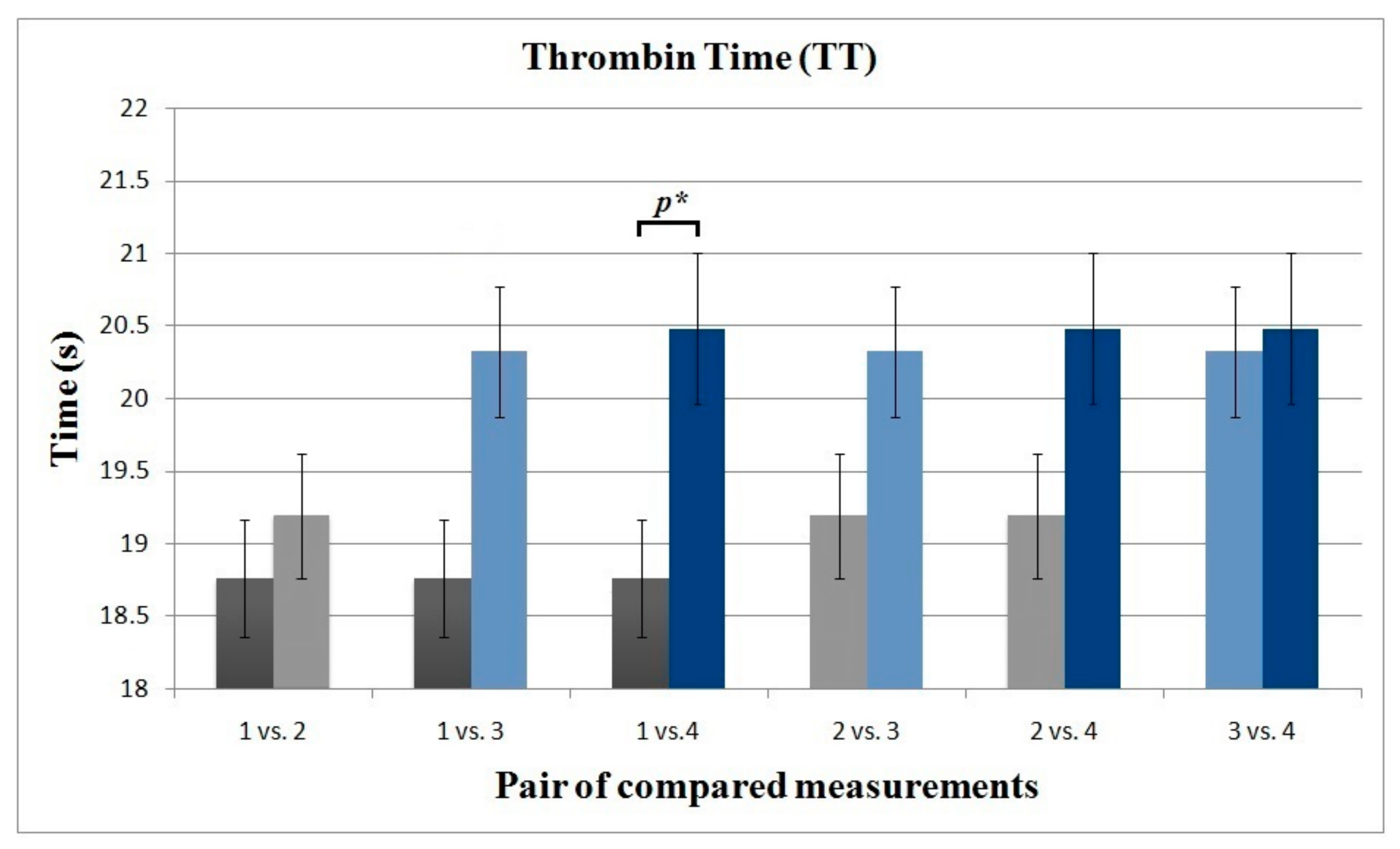
3.1. Blood Coagulation Parameters
3.2. AMDA and NO
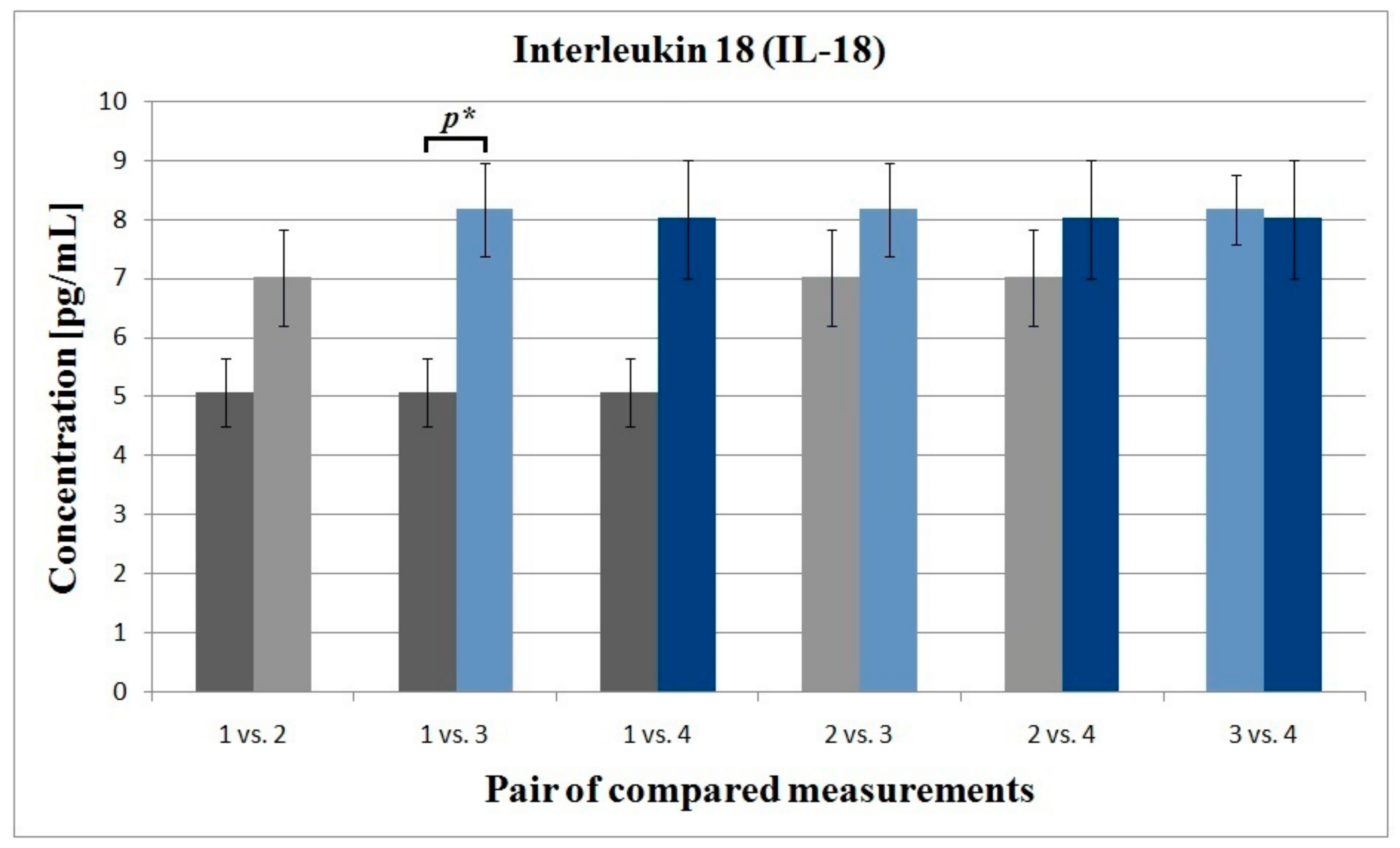
3.3. Interleukin 6 and Interleukin 18
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Chioreso, C.; Schweizer, M.L.; Abramoff, M.D. Effects of aflibercept for neovascular age-related macular degeneration: A systematic review and meta-analysis of observational comparative studies. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5616–5627. [Google Scholar]
- Semeraro, F.; Morescalchi, F.; Duse, S.; Parmeggiani, F.; Gambicorti, E.; Costagliola, C. Aflibercept in wet AMD: Specific role and optimal use. Drug Des. Devel. Ther. 2013, 7, 711–722. [Google Scholar] [CrossRef]
- Cheung, G.C.M.; Lai, T.Y.Y.; Gomi, F.; Ruamviboonsuk, P.; Koh, A.; Lee, W.K. Anti-VEGF therapy for neovascular AMD and polypoidal choroidal vasculopathy. Asia Pac. J. Ophthalmol. 2017, 6, 527–534. [Google Scholar]
- Sato, T.; Takeuchi, M.; Karasawa, Y.; Enoki, T.; Ito, M. Intraocular inflammatory cytokines in patients with neovascular age-related macular degeneration before and after initiation of intravitreal injection of anti-VEGF inhibitor. Sci. Rep. 2018, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Sobolewska, B.; Golenko, J.; Poeschel, S.; Grimmel, C.; Gatsiou, A.; Sopova, K.; Biedermann, T.; Schenke-Layland, K.; Stellos, K.; Ziemssen, F. Influence of aflibercept on platelet activation profile. Exp. Eye Res. 2018, 175, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Ross, J.S.; Sangaralingham, L.R.; Adelman, R.A.; Shah, N.D.; Barkmeier, A.J. Trends of anti-vascular endothelial growth Factor use in ophthalmology among privately insured and medicare advantage patients. Ophthalmology 2017, 124, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847–1858. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, C.; Su, Y.; Li, L.; Zhou, Y. Changes in clotting time, plasma fibrinogen levels, and blood viscosity after administration of ranibizumab for treatment of choroidal neovascularization. Curr. Eye Res. 2015, 40, 1166–1171. [Google Scholar] [CrossRef]
- Edington, M.; Connolly, J.; Chong, N.V. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1217–1224. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through preclinical and clinical studies and theimplications for the management of neovascular age-related macular degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.; Byron, B.K.; Binzel, K.; Bhatia, P.; Wei, L.; Kumar, K.; Knopp, M.V. Systemic biodistribution and intravitreal pharmacokinetic properties of bevacizumab, ranibizumab, and aflibercept in a nonhuman primate model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5636–5645. [Google Scholar] [CrossRef]
- Qian, J.; Jiang, Y.R. Decreased prothrombin time after intravitreal bevacizumab in the early period in patients with proliferative diabetic retinopathy. Acta Ophthal. 2011, 89, 332–335. [Google Scholar] [CrossRef]
- Luaces-Rodríguez, A.; Amo, E.M.D.; Mondelo-García, C.; Gómez-Lado, N.; Gonzalez, F.; Ruibal, Á.; González-Barcia, M.; Zarra-Ferro, I.; Otero-Espinar, F.J.; Fernández-Ferreiro, A.; et al. PET study of ocular and blood pharmacokinetics of intravitreal bevacizumab and aflibercept in rats. Eur. J. Pharm. Biopharm. 2020, 154, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Winter, W.E.; Flax, S.D.; Harris, N.S. Coagulation testing in the core laboratory. Lab. Med. 2017, 48, 295–313. [Google Scholar] [CrossRef]
- Kamal, A.H.; Tefferi, A.; Pruthi, R.K. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin. Proc. 2007, 82, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, W.Z.; Moufak, S.K.; Yusof, Z.; Mohamad, M.S.; Kamarul, I.M. Shortened activated partial thromboplastin time, a hemostatic marker for hypercoagulable state during acute coronary event. Transl. Res. 2010, 155, 315–319. [Google Scholar] [CrossRef]
- Sauls, D.L.; Banini, A.E.; Boyd, L.C.; Hoffman, M. Elevated prothrombin level and shortened clotting times in subjects with type 2 diabetes. J. Thromb. Haemost. 2007, 5, 638–639. [Google Scholar] [CrossRef]
- Korte, W.; Clarke, S.; Lefkowitz, J.B. Short activated partial thromboplastin times are related to increased thrombin generation and an increased risk for thromboembolism. Am. J. Clin. Pathol. 2000, 113, 123–127. [Google Scholar] [CrossRef]
- Altinkaynak, H.; Kars, M.E.; Kurkcuoglu, P.Z.; Ugurlu, N. Blood coagulation parameters after intravitreal injection of aflibercept in patients with neovascular age-related macular degeneration. Int. Ophthalmol. 2018, 38, 2397–2402. [Google Scholar] [CrossRef]
- Georgakopoulos, C.G.; Makri, O.E.; Pallikari, A.; Kagkelaris, K.; Plotas, P.; Grammenou, V.; Emmanuil, A. Effect of intravitreal injection of aflibercept on blood coagulation parameters in patients with age-related macular degeneration. Ther. Adv. Ophthalmol. 2020, 12, 2515841420903929. [Google Scholar] [CrossRef]
- Yamashita, M.; Matsumoto, M.; Hayakawa, M.; Sakai, K.; Fujimura, Y.; Ogata, N. Intravitreal injection of aflibercept, an anti-VEGF antagonist, down-regulates plasma von Willebrand factor in patients with age-related macular degeneration. Sci. Rep. 2018, 8, 1491. [Google Scholar] [CrossRef]
- Peyvandi, F.; Garagiola, I.; Baronciani, L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011, 9, 3–8. [Google Scholar]
- Machlus, K.R.; Cardenas, J.C.; Church, F.C.; Wolberg, A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 2011, 117, 4953–4963. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wolf, P.A.; Castelli, W.P.; D’Agostino, R.B. Fibrinogen and risk of cardiovascular disease: The Framingham Study. JAMA 1987, 258, 1183–1186. [Google Scholar] [CrossRef]
- Floyd, C.N.; Ferro, A. The platelet fibrinogen receptor: From megakaryocyte to the mortuary. JRSM Cardiovasc. Dis. 2012, 1, 1–13. [Google Scholar]
- Nomura, Y.; Kaneko, M.; Miyata, K.; Yatomi, Y.; Yanagi, Y. Bevacizumab and aflibercept activate platelets via FcγRIIa. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8075–8082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhutto, I.A.; Baba, T.; Merges, C.; McLeod, D.S.; Lutty, G.A. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp. Eye Res. 2010, 90, 155–167. [Google Scholar] [CrossRef]
- Farah, C.; Mithchel, L.Y.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292. [Google Scholar] [CrossRef]
- Freedman, J.E.; Loscalzo, J. Nitric oxide and its relationship to thrombotic disorders. J. Thromb. Haemost. 2003, 1, 1183–1188. [Google Scholar] [CrossRef]
- Fiedler, L.R.; Bachetti, T.; Leiper, J.; Zachary, I.; Chen, L.; Renné, T.; Wojciak-Stothard, B. The ADMA/DDAH pathway regulates VEGF-mediated angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2117–2124. [Google Scholar] [CrossRef]
- Keles, S.; Ates, O.; Kartal, B.; Alp, H.H.; Ekinci, M.; Ceylan, E.; Ondas, O.; Arpali, E.; Dogan, S.; Yildirim, K.; et al. Evaluation of cardiovascular biomarkers in patients with age-related wet macular degeneration. Clin. Ophthalmol. 2014, 8, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.E.; Gilchrist, S.E.C.M.; Woodside, J.V.; Jiang, J.; Ni, Z.; Wang, J.; Sun, X. Homocysteine, B–vitamin status, ADMA and risk of age-related macular disease–A case-control study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2197. [Google Scholar]
- Pinna, A.; Zinellu, A.; Tendas, D.; Blasetti, F.; Carru, C.; Castiglia, P. Plasma homocysteine and asymmetrical dimethyl-L-arginine (ADMA) and whole blood DNA methylation in early and neovascular age-related macular degeneration: A pilot study. Med. Sci. Monit. 2014, 41, 88–96. [Google Scholar] [CrossRef]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.W.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Winnik, S.; Lohmann, C.; Siciliani, G.; Von Lukowicz, T.; Kuschnerus, K.; Kraenkel, N.; Brokopp, C.E.; Enseleit, F.; Michels, S.; Ruschitzka, F.; et al. Systemic VEGF inhibition accelerates experimental atherosclerosis and disrupts endothelial homeostasis—Implications for cardiovascular safety. Int. J. Cardiol. 2013, 168, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Sümbül, A.T.; Dişel, U.; Sezgin, N.; Sezer, A.; Köse, F.; Beşen, A.A.; Sümbül, Z.; Abalı, H.; Özyılkan, Ö. Can serial monitoring of serum vascular endothelial growth factor (VEGF), nitric oxide (NO), and angiotensin II (ANGII) levels have predictive role during bevacizumab treatment? Med. Sci. Monit. 2014, 20, 428–433. [Google Scholar]
- Ghasemi, A.; Zahediasl, S.; Azizi, F. Reference values for serum nitric oxide metabolites in anadult population. Clin. Biochem. 2010, 43, 89–94. [Google Scholar] [CrossRef]
- Hov, G.G.; Sagen, E.; Bigonah, A.; Asberg, A. Health-associated reference values for arginine, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) measured with high-performance liquid chromatography. Scand. J. Clin. Lab. Investig. 2007, 67, 868–876. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Chan, C.C.; Sen, H.N.; Ferris, F.L.; Nussenblatt, R.B. Inflammatory mechanisms of age-related macular degeneration. Int. Ophthalmol. Clin. 2015, 55, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Doyle, S.; Humphries, P. IL-18: A new player in immunotherapy for age-related macular degeneration? Expert Rev. Clin. Immunol. 2014, 10, 1273–1275. [Google Scholar] [CrossRef][Green Version]
- Shen, J.; Choy, D.F.; Yoshida, T.; Iwase, T.; Hafiz, G.; Xie, B.; Hackett, S.F.; Arron, J.R.; Campochiaro, P.A. Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: Reciprocal suppression with VEGF. J. Cell Physiol. 2014, 229, 974–983. [Google Scholar] [CrossRef]
- Echevarria, F.D.; Formichella, C.R.; Sappington, R.M. Interleukin-6 deficiency attenuates retinal ganglion cell axonopathy and glaucoma-related vision loss. Front. Neurosci. 2017, 11, 318. [Google Scholar] [CrossRef]
- Pham, B.H.; Hien, D.L.; Matsumiya, W.; Ngoc, T.T.T.; Doan, H.L.; Akhavanrezayat, A.; Yaşar, C.; Nguyen, H.V.; Halim, M.S.; Nguyen, Q.D. Anti-interleukin-6 receptor therapy with tocilizumab for refractory pseudophakic cystoid macular edema. Am. J. Ophthalmol. Case Rep. 2020, 20, 100881. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.Y.; Boehlke, C.S.; Zheng, Q.D.; Zhang, L.; Han, Y.; Zacks, D.N. Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. IOVS 2008, 49, 3193–3200. [Google Scholar]
- Wainstein, M.V.; Mossmann, M.; Araujo, G.N.; Gonçalves, S.C.; Gravina, G.L.; Sangalli, M.; Veadrigo, F.; Matte, R.; Reich, R.; Costa, F.G.; et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol. Metab. Syndr. 2017, 9, 67. [Google Scholar] [CrossRef]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.G.; Bedirian, R.; Singh, G.; Pinheiro, G.D. Interleukin 6 inhibition and coronary artery disease in a high-risk population: A prospective community-based clinical study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, Z.; Li, X.; Chen, Y.; Zhang, Y.; Ding, D.; Deng, X.; Xia, M.; Qiu, J.; Ling, W. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediat. Inflamm. 2013, 2013, 726178. [Google Scholar] [CrossRef]
- Jefferis, B.J.; Papacosta, O.; Owen, C.G.; Wannamethee, S.G.; Humphries, S.E.; Woodward, M.; Lennon, L.T.; Thomson, A.; Welsh, P.; Rumley, A.; et al. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis 2011, 217, 227–233. [Google Scholar] [CrossRef]
- Blankenberg, S.; Luc, G.; Ducimetière, P.; Arveiler, D.; Ferrières, J.; Amouyel, P.; Evans, A.; Cambien, F.; Tiret, L. Interleukin-18 and the risk of coronary heart disease in European men: The prospective epidemiological study of myocardial infarction (PRIME). Circulation 2003, 108, 2453–2459. [Google Scholar] [CrossRef]
- Formanowicz, D.; Wanic-Kossowska, M.; Pawliczak, E.; Radom, M.; Formanowicz, P. Usefulness of serum interleukin-18 in predicting cardiovascular mortality in patients with chronic kidney disease—Systems and clinical approach. Sci. Rep. 2015, 5, 18332. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wei, R.; Miao, X.; Sun, S.; Liang, G.; Chu, C.; Zhao, L.; Zhu, X.; Guo, Q.; et al. IL (interleukin)-6 contributes to deep vein thrombosis and is negatively regulated by miR-338–5p. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 323–334. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin 6 and haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar]
- Senchenkova, E.Y.; Komoto, S.; Russell, J.; Almeida-Paula, L.D.; Yan, L.S.; Zhang, S.; Grangder, D.N. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am. J. Pathol. 2013, 183, 173–181. [Google Scholar] [CrossRef]
- Li, G.; Zhou, R.; Zhao, X.; Liu, R.; Ye, C. Correlation between the expression of IL-18 and deep venous thrombosis. Int. J. Mol. Med. 2018, 42, 883–896. [Google Scholar]
- Trøseid, M.; Seljeflot, I.; Hjerkinn, E.M.; Arnesen, H. Interleukin-18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: Synergistic effect of inflammation and hyperglycemia. Diabetes Care 2009, 32, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013, 434010. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Takahashi, H.; Tan, X.; Inoue, Y.; Nomura, Y.; Arai, Y.; Fujino, Y.; Kawashima, H.; Yanagi, Y. Changes in multiple cytokine concentrations in the aqueous humour of neovascular age-related macular degeneration after 2 months of ranibizumab therapy. Br. J. Ophthalmol. 2018, 102, 448–454. [Google Scholar] [CrossRef]
- Kotake, O.; Noma, H.; Yasuda, K.; Motohashi, R.; Goto, H.; Shimura, M. Comparing cytokine kinetics between ranibizumab and aflibercept in central retinal vein occlusion with macular edema. Ophthalmic Res. 2019, 61, 210–217. [Google Scholar] [CrossRef]
- Smidowicz, A.; Regula, J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 Concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef]
- Trøseid, M.; Arnesen, H.; Hijerkinn, E.M.; Seljeflot, I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism 2009, 58, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Tsikas, D.; Lehmann, A.C.; Bönke, J.; Haas, V.; Strauß, A.; Jordan, J. Influence of dietary fat ingestion on asymmetrical dimethylarginine in lean and obese human subjects. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 720–726. [Google Scholar]
- Tayeh, O.; Fahmi, A.; Islam, M.; Saied, M. Asymmetric dimethylarginine as a prognostic marker for cardiovascular complications in hypertensive patients. Egypt Heart J. 2011, 63, 117–124. [Google Scholar] [CrossRef][Green Version]
- Pascale, V.; Pascale, W.; Lavanga, V.; Sansone, V.; Ferrario, P.; Colonna, V.D.G. L-arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in plasma and synovial fluid of patients with knee osteoarthritis. Med. Sci. Monit. 2013, 19, 1057–1062. [Google Scholar] [CrossRef]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Winkler, U.H. Effects of androgens on haemostasis. Maturitas 1996, 24, 147–155. [Google Scholar] [CrossRef]
| Number of Patients (Eyes), N (%) | |
|---|---|
| All | 22 |
| Female | 13 (59.01) |
| Male | 9 (40.9) |
| Age in years (mean value) | |
| Female | 78.69 |
| Male | 76.89 |
| Range | 66–99 |
| Visual Acuity (mean LogMAR) | |
| BVA a | 0.2944 |
| FVA b | 0.2399 |
| p-value c | 0.001 |
| Compared Measurements | Parameter | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | APTT | TT | IL-6 | IL-18 | ADMA | NO | |||||||||||||||
| MV (s) | SE | p-Val. | MV (s) | SE | p-Val. | MV (s) | SE | p-Val. | MV (pg/mL) | SE | p-Val. | MV (pg/mL) | SE | p-Val. | MV (µmol/L) | SE | p-Val. | MV (µmol/L) | SE | p-Val. | |
| 1 2 | 14.87 14.79 | 0.22 0.23 | 0.981 | 26.31 27.83 | 0.35 0.60 | 0.311 | 18.76 19.19 | 0.40 0.43 | 0.891 | 11.74 11.13 | 1.54 1.17 | 0.278 | 5.06 7.02 | 0.58 0.81 | 0.317 | 0.87 0.79 | 0.10 0.12 | 0.946 | 9.38 6.86 | 1.23 0.83 | 0.387 |
| 1 3 | 14.87 15.20 | 0.22 0.20 | 0.864 | 26.31 27.75 | 0.35 0.65 | 0.360 | 18.76 20.32 | 0.40 0.45 | 0.076 | 11.74 11.54 | 1.54 1.82 | 0.702 | 5.06 8.16 | 0.58 1.00 | 0.037 | 0.87 0.76 | 0.10 0.06 | 0.874 | 9.38 10.01 | 1.23 1.12 | 0.979 |
| 1 4 | 14.87 15.40 | 0.22 0.45 | 0.584 | 26.31 28.58 | 0.35 0.78 | 0.053 | 18.76 20.48 | 0.40 0.52 | 0.041 | 11.74 12.21 | 1.54 2.58 | 0.385 | 5.06 8.01 | 0.58 0.78 | 0.052 | 0.87 0.91 | 0.10 0.13 | 0.992 | 9.38 9.73 | 1.23 1.24 | 0.996 |
| 2 3 | 14.79 15.20 | 0.23 0.20 | 0.758 | 27.83 27.75 | 0.60 0.65 | 0.891 | 19.19 20.32 | 0.43 0.45 | 0.291 | 11.13 11.54 | 1.17 1.82 | 0.330 | 7.02 8.16 | 0.81 1.00 | 0.719 | 0.79 0.76 | 0.12 0.06 | 0.997 | 6.86 10.01 | 0.83 1.12 | 0.199 |
| 2 4 | 14.79 15.40 | 0.23 0.45 | 0.455 | 27.83 28.58 | 0.60 0.78 | 0.802 | 19.19 20.48 | 0.43 0.52 | 0.186 | 11.13 12.21 | 1.17 2.58 | 0.074 | 7.02 8.01 | 0.81 0.78 | 0.792 | 0.79 0.91 | 0.12 0.13 | 0.877 | 6.86 9.73 | 0.83 1.24 | 0.272 |
| 3 4 | 15.20 15.40 | 0.20 0.45 | 0.961 | 27.75 28.58 | 0.65 0.78 | 0.750 | 20.32 20.48 | 0.45 0.52 | 0.902 | 11.54 12.21 | 1.82 2.58 | 0.459 | 8.16 8.01 | 0.81 0.78 | 0.899 | 0.76 0.91 | 0.06 0.13 | 0.774 | 10.01 9.73 | 1.12 1.24 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Seredyka-Burduk, M.; Liberski, S.; Marczak, D.; Pol, M.; Malinowski, B.; Pawlak-Osińska, K.; Kaluzny, B.J. Evaluation of Blood Coagulation Parameters and ADMA, NO, IL-6, and IL-18 Serum Levels in Patients with Neovascular AMD before, during, and after the Initial Loading Phase of Intravitreal Aflibercept. Life 2021, 11, 441. https://doi.org/10.3390/life11050441
Wiciński M, Seredyka-Burduk M, Liberski S, Marczak D, Pol M, Malinowski B, Pawlak-Osińska K, Kaluzny BJ. Evaluation of Blood Coagulation Parameters and ADMA, NO, IL-6, and IL-18 Serum Levels in Patients with Neovascular AMD before, during, and after the Initial Loading Phase of Intravitreal Aflibercept. Life. 2021; 11(5):441. https://doi.org/10.3390/life11050441
Chicago/Turabian StyleWiciński, Michał, Małgorzata Seredyka-Burduk, Sławomir Liberski, Daria Marczak, Magdalena Pol, Bartosz Malinowski, Katarzyna Pawlak-Osińska, and Bartlomiej J. Kaluzny. 2021. "Evaluation of Blood Coagulation Parameters and ADMA, NO, IL-6, and IL-18 Serum Levels in Patients with Neovascular AMD before, during, and after the Initial Loading Phase of Intravitreal Aflibercept" Life 11, no. 5: 441. https://doi.org/10.3390/life11050441
APA StyleWiciński, M., Seredyka-Burduk, M., Liberski, S., Marczak, D., Pol, M., Malinowski, B., Pawlak-Osińska, K., & Kaluzny, B. J. (2021). Evaluation of Blood Coagulation Parameters and ADMA, NO, IL-6, and IL-18 Serum Levels in Patients with Neovascular AMD before, during, and after the Initial Loading Phase of Intravitreal Aflibercept. Life, 11(5), 441. https://doi.org/10.3390/life11050441






