1. Introduction
The mucus gel layer covering the airway surface periciliary layer (PCL) traps inhaled materials and acts as a reservoir for water, buffering hydration of the PCL for needed cell surface lubrication and efficient ciliary beating [
1]. When the hydration of the airway surface liquid (ASL) decreases, the mucus becomes hyperconcentrated, the PCL collapses, and the “thickened” mucus layer adheres to the cell surface, causing plaque/plug formation. Mucus stasis produces the airflow obstruction, infection, and inflammation characteristic of chronic obstructive lung diseases, including cystic fibrosis (CF) and chronic bronchitis (CB) [
2,
3,
4,
5]. Airway surface hydration is maintained by water fluxes mainly driven by active Cl
− and Na
+ transport. Mutations in the CF transmembrane conductance regulator (CFTR) gene that result in abnormal CFTR Cl
− channel expression/activity are also associated with exacerbated Na
+ absorption [
6] and lead to ASL volume depletion in CF. The processes leading to mucus dehydration in non-CF lung diseases are incompletely described, but recent evidence suggests that prolonged cigarette smoke exposure reduces CFTR expression, induces CFTR internalization, and disrupts CFTR channel function, leading to ASL dehydration. Thus, cigarette smoke-induced CFTR dysfunction likely contributes to the onset of CB [
7,
8,
9].
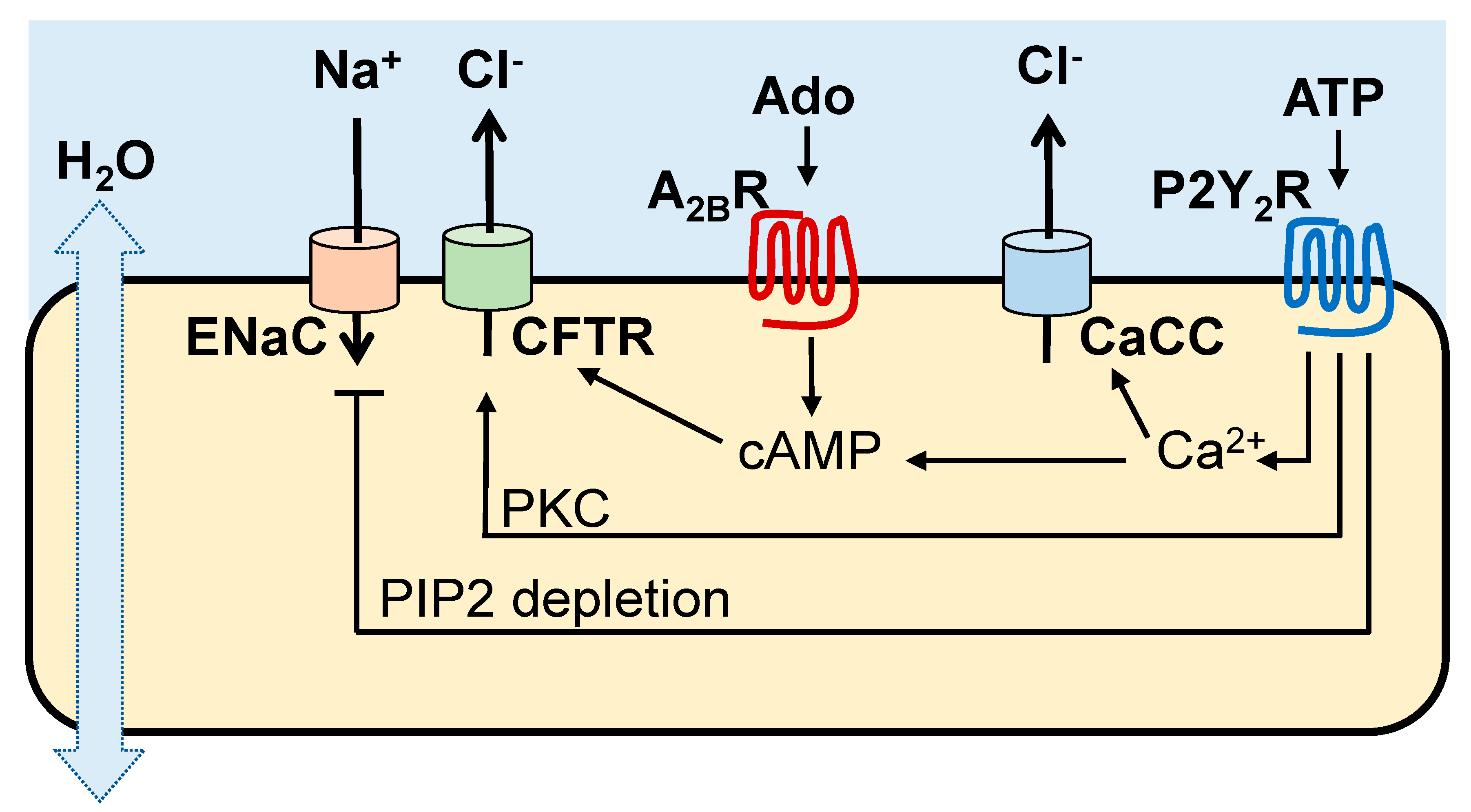
2. Purinergic Receptors Promote MCC Activities
Cl
− and Na
+ transport activities in the airways are regulated by ASL concentrations of ATP and its metabolite adenosine acting on Gq-coupled P2Y
2 receptors (P2Y
2R) and Gs-coupled A
2BR, respectively, expressed in non-mucous cells [
10]. As summarized in
Figure 1, the A
2BR promotes cyclic AMP-regulated CFTR Cl
− secretion, whereas the P2Y
2R promotes Cl
− secretion [via Ca
2+ activated Cl
− channel (CaCC)/TMEM16 [
11,
12,
13], protein kinase C (PKC)-mediated CFTR activation [
14], and Ca
2+-activated adenylyl cyclase I leading to cyclic AMP-mediated CFTR activation [
15]]. In addition, the P2Y
2R contributes to airway surface hydration by inhibiting Na
+ absorption via phospholipase C-catalyzed depletion of phosphatidylinositol 4,5-bisphosphate [
16,
17,
18].
In addition to ion transport regulation, activation of A
2B and P2Y
2 receptors results in increased ciliary beating [
19,
20,
21]. The P2Y
2R also promotes mucin secretion from goblet cells [
19].
3. Nucleotide Homeostasis in the Airways
Nucleotide release and hydrolysis rates must be finely balanced to control ATP and adenosine levels in ASL. Once released, ATP can interact with P2Y
2R and be rapidly converted to adenosine by cell surface ectonucleotidases. Adenosine accumulates in ASL at levels capable of promoting A
2BR/CFTR-dependent water transport in normal cells [
22]. Due to the CFTR defect, the A
2BR/cyclic AMP/CFTR mechanism is impaired in CF and, likely, in cigarette smoke-induced CB. Intriguingly, due to hydrolysis, ATP steady state levels are suboptimal to promote P2Y
2R-mediated fluid secretion and, therefore, the CFTR-independent mechanism of airway hydration downstream of P2Y
2R does not optimally hydrate CFTR-deficient epithelia [
23].
Initial evidence for the purinergic control of ion/water secretion emerged from studies illustrating that: (a) baseline CFTR activity in Calu-3 lung epithelial cells was blocked by A
2BR antagonists [
24]; (b) apical addition of adenosine deaminase to primary cultures of naïve human bronchial epithelial (HBE) cells resulted in a CF-like ASL volume depletion [
22]; and (c) the ASL height measured in CF HBE cells subjected to shear stress-promoted ATP release is markedly reduced following the addition of the ATPase apyrase [
25]. Button and co-workers demonstrated that the hydration status of mucus correlated directly with the luminal rates of ATP release [
26].
Relevant to CF, substantial literature, including our own studies, indicates that airway epithelial ATP release rates are not reduced in CF [
22,
25,
27,
28,
29,
30]. Notably, ATP release increased in HBE cells exposed to inflammatory stresses relevant to CF [
27]. Furthermore, total nucleotide levels, in particular ADP and AMP, were found markedly elevated in CF lung secretions while ATP levels were reduced concomitantly with increased ATPase activity [
23]. ATP concentrations also were reduced in CB lung secretions due to increased ATP hydrolysis activity [
31]. These observations suggest that mucus dehydration and inflammation in CF and CB airways are associated with increased ATP metabolism leading to reduced ASL ATP levels [
23,
31]. Therefore, inhibition of ATP hydrolysis in CF and CB could be an approach to increase steady-state ATP levels on airway surfaces to restore airway hydration via P2Y
2R-promoted activation of Cl
− secretion and inhibition of Na
+ absorption. The recent identification of the polyoxometalate POM-5 as a potent and effective inhibitor of ATPase activities in airway epithelial cells and lung secretions [
23] provided a useful tool to test this hypothesis. As we have recently shown, administration of POM-5 to CF HBE cells results in sustained, increased steady-state ATP levels in airway surfaces and enhanced ASL volume production [
23].
4. Vesicular and Conductive Pathways of Airway Epithelial Nucleotide Release
Given the important role of released nucleotides in the control of airway surface hydration and mucus clearance, the mechanisms of nucleotide release by airway epithelia have been intensively investigated. Airway epithelial cells were initially shown to release ATP acutely, in response to mechanical stimuli, such as plate tilting or touching of the epithelium [
28], and a medium change [
30]. It has since been well-established that epithelial cells release discrete amounts of ATP constitutively [
22,
32] and that enhanced ATP release occurs following pharmacological challenges [
33,
34] or controlled mechanical stimuli, such as hypotonic cell swelling (16,50) or phasic motion that mimics the shear stress that is associated with normal tidal breathing (51). The diversity of conditions in which airway epithelial nucleotide release was observed suggested that different mechanisms (perhaps cell- and stimulus-specific) are involved [
25,
27,
32,
33,
34,
35,
36,
37,
38,
39]. For example, the mechanical strain exerted on the cilia via interaction with the overlying mucus layer promotes ciliated cell ATP release [
26].
Non-lytic mechanisms for ATP release may include regulated exocytosis, which typically requires intracellular Ca
2+, and/or conductive/transporter pathways [
40]. Primary cultures of naïve HBE cells, which are dominated by ciliated cells, exhibited robust release of ATP upon hypotonicity-induced cell swelling, and this release was, largely, not affected by chelation of the intracellular Ca
2+ or by pharmacological inhibition of the secretory pathway [
27,
35,
37], suggesting a conductive mechanism. However, HBE cells exposed to inflammatory challenges that promote goblet cell hyperplasia exhibited enhanced hypotonicity-induced ATP release, which was prevented by intracellular Ca
2+ chelation and by reagents that disrupt vesicle trafficking/exocytosis [
27,
35]. Furthermore, agents that increase intracellular Ca
2+ in HBE cells, such as ionomycin and UTP, caused a minor release of adenine nucleotides and mucins in naïve cultures, but ionomycin- and UTP-promoted ATP release and mucin secretion increased markedly in goblet cell-hyperplasic cultures [
35]. Studies with goblet cell-rich Calu-3 cells demonstrated that mucin secreting cells release nucleotides as co-cargo-molecules from mucin-containing granules [
34,
36].
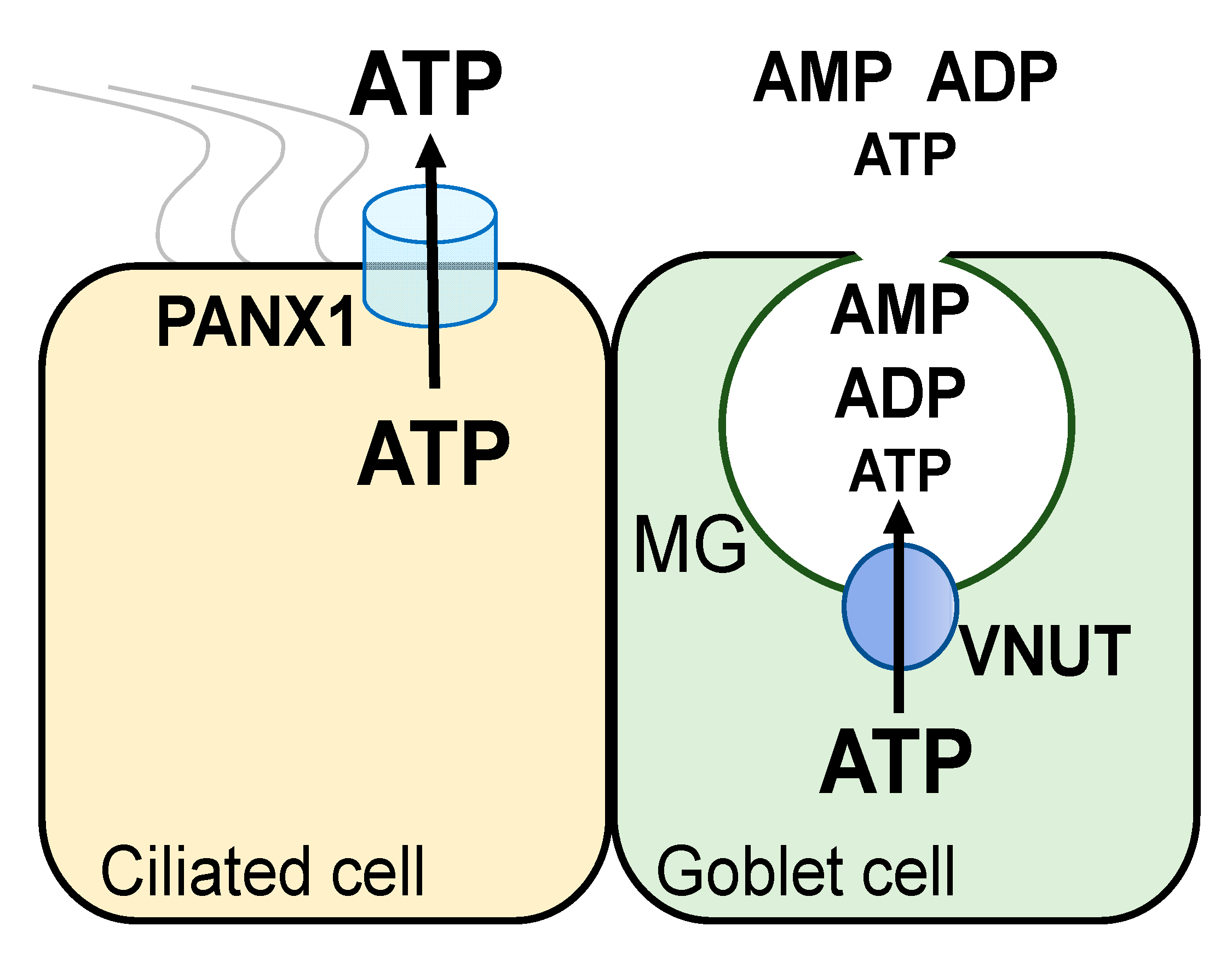
Thus, functional data suggested that conducted and vesicular pathways contribute to ATP release from airway epithelial ciliated and goblet cells, respectively. Major components of these pathways have been identified at the molecular label: (a) pannexin 1 is an ATP release conduit expressed in non-mucous airway epithelial cells [
41,
42]; and (b) the vesicular nucleotide transporter VNUT [the product of the
SLC17A9 gene [
43]] is the ATP transporter mediating ATP storage in (and release from) mucin granules and secretory vesicles [
44] (
Figure 2).
5. VNUT Mediates ATP Release from Mucin Granules and Vesicles
Our initial studies with goblet cell-rich airway epithelia established an association between nucleotide release and mucin secretion [
35,
36]. Calu-3 cells, a lung epithelial cell line comprised by a mixed population of non-mucous and mucin granule-rich (goblet) cells [
36], exhibit both pannexin 1-mediated ATP release in response to cell swelling [
41] and Ca
2+ (ionomycin)-regulated vesicular release of nucleotides that correlates with mucin secretion [
36]. Furthermore, the potent mucin secretagogue thrombin promoted robust nucleotide release in Calu-3 cells after complete inhibition of pannexin 1 [
34]. Strikingly, ADP and AMP were the most abundant species accumulating in thrombin-stimulated Calu-3 cells, following pannexin inhibition. The data suggested that mucin granules store (and release) nucleotides. Analysis of the nucleotide composition in mucin granules isolated from Calu-3 cells supported this hypothesis. Notably, ADP, AMP, and ATP represented 60%, 30%, and 10% of the intragranular nucleotide pool, respectively [
34], supporting the notion that ADP and AMP are the predominant nucleotide species released with mucin granules.
The identification by Moriyama and co-workers of SLC17A9/VNUT as the nucleotide transporter that transfers cytosolic ATP into secretory granules [
43] provided a tool to investigate the association of mucin secretion and nucleotide release. VNUT mRNA was amplified in Calu-3 cells and strong VNUT immunoreactivity was observed in these cells [
44]. Ca
2+-regulated nucleotide release from Calu-3 cells was blunted after treatment with inhibitors of the secretory pathway and by downregulation of VNUT by shRNA [
36,
44]. Calu-3 cell fractionation yielded a VNUT immunoreactivity-rich fraction that sedimented with mucin granules. The relative distribution of ADP, AMP, and ATP within mucin granules was similar in control and VNUT shRNA-treated cells, but the total nucleotide pool was markedly reduced following VNUT knockdown [
44]. This observation is consistent with the notion that VNUT transports ATP into mucin granules, but ATP is rapidly metabolized within the granular compartment [
34,
44] (
Figure 2). Release of predominantly ADP and AMP from mucin granules minimizes autocrine, P2Y
2R-mediated feedback for mucin secretion. Importantly, released AMP and ADP provide a source for adenosine formation leading to paracrine regulation of the ion/water transport activities needed for the hydration of newly released mucins.
In addition to mucin granules, VNUT immunoreactivity was observed in lysosome-rich and endoplasmic reticulum/Golgi-rich fractions isolated from Calu-3 cells [
44]. Furthermore, confocal microscopy analysis of Calu-3 cells transfected with Myc-tagged VNUT revealed strong Myc immunoreactivity that co-localized with the mucin granule marker MUC5AC as well as vesicular compartments that stained negative for MUC5AC [
44]. Our studies with inflamed airway epithelial cells suggest that a vesicular ATP pool can be released from cells independently from mucins. HBE cells exposed for two days to SMM (sterile supernatant from mucopurulent CF lung secretions) exhibited increased hypotonicity-promoted ATP release that was independent of pannexin 1 activation, was blocked by inhibitors of the secretory pathway, and was associated with increased VNUT expression, but was not accompanied by mucin secretion [
27]. In line with these data, vesicular nucleotide release has been reported with cells lacking biochemically or morphologically defined secretory granules, e.g., lymphocytes [
45], rat hepatoma cells [
46], cholangiocytes [
47], and lung carcinoma A549 cells [
48,
49,
50].
Collectively, these observations suggest that VNUT transports ATP into (a) mucin granules in goblet cells, contributing to nucleotide release in mucin secreting cells, and (b) an unidentified vesicular compartment competent for regulated exocytosis in inflamed airway epithelia.
6. Pannexin 1 Mediated-ATP Release
The report by Dahl and co-workers that pannexin 1 acted as a plasma membrane ATP channel when overexpressed in
Xenopus oocytes [
51] triggered major interest in assessing the involvement of this channel in the release of ATP from mammalian cells, including airway epithelial cells. In 2009, Ransford et al. reported that ATP release from hypotonically swollen HBE cells was markedly inhibited by pannexin channel blockers or by knocking down pannexin 1 via shRNA [
42]. Confocal images revealed pannexin 1 immunoreactivity at the apical membrane of most ciliated cells (the most abundant cell type in these cultures) although expression was not limited to these cells [
42]. Simultaneously, we reported that activation of protease-activated receptors (PAR) in HBE cells resulted in enhanced release of ATP and enhanced uptake of the pannexin 1 permeant dye propidium iodide, and these responses were inhibited by the pannexin 1 blocker carbenoxolone [
33]. Unambiguous assessment of the contribution of pannexin 1 to airway epithelial ATP release was subsequently obtained in our lab, using pannexin 1 knockout (
Panx1 KO) mice. Utilizing a perfusion approach to assess ATP levels in tracheal luminal secretions under controlled flow conditions, we reported that ATP release from wild-type (WT) tracheas increased up to six-fold following a brief exposure to hypotonicity. In contrast,
Panx1 KO animals exhibited impaired hypotonicity-evoked ATP release, and similar data were obtained with tracheal epithelial cell cultures from these mice [
41]. In line with our studies, Workman et al. have recently illustrated that mechanical stimulation (via air puffs) of cultured WT murine nasal epithelial cells resulted in robust ATP release, which was markedly attenuated by the pannexin 1 inhibitor carbenoxolone and was greatly reduced (although not abolished) in cells from
Panx1 KO mice [
52].
It is worth noting that ATP release conductive pathways additional to pannexin 1 have been described. For example, in the above-cited work, Workman and collaborators reported that, similar to
Panx1 KO cells, cells from mice lacking CALHM 1 [Ca
2+ homeostasis modulator 1] exhibited reduced air puff-promoted ATP release. The authors concluded that pannexin 1 and CAHLM1 play complementary roles regulating ATP release in nasal epithelia [
52]. CALHM 1 was initially describe as an ATP channel mediating taste-evoked ATP release from taste buds [
53], but its expression in lower airway epithelia remains to be investigated. It has been recently shown that overexpression of ABCG1 (ABC subfamily G member 1) in HEK-293 cells confers enhanced hypotonicity-induced ATP release through volume-regulated anion channels (VRACs) [
54]. However, the contribution of this pathway to airway epithelial ATP release is not known.
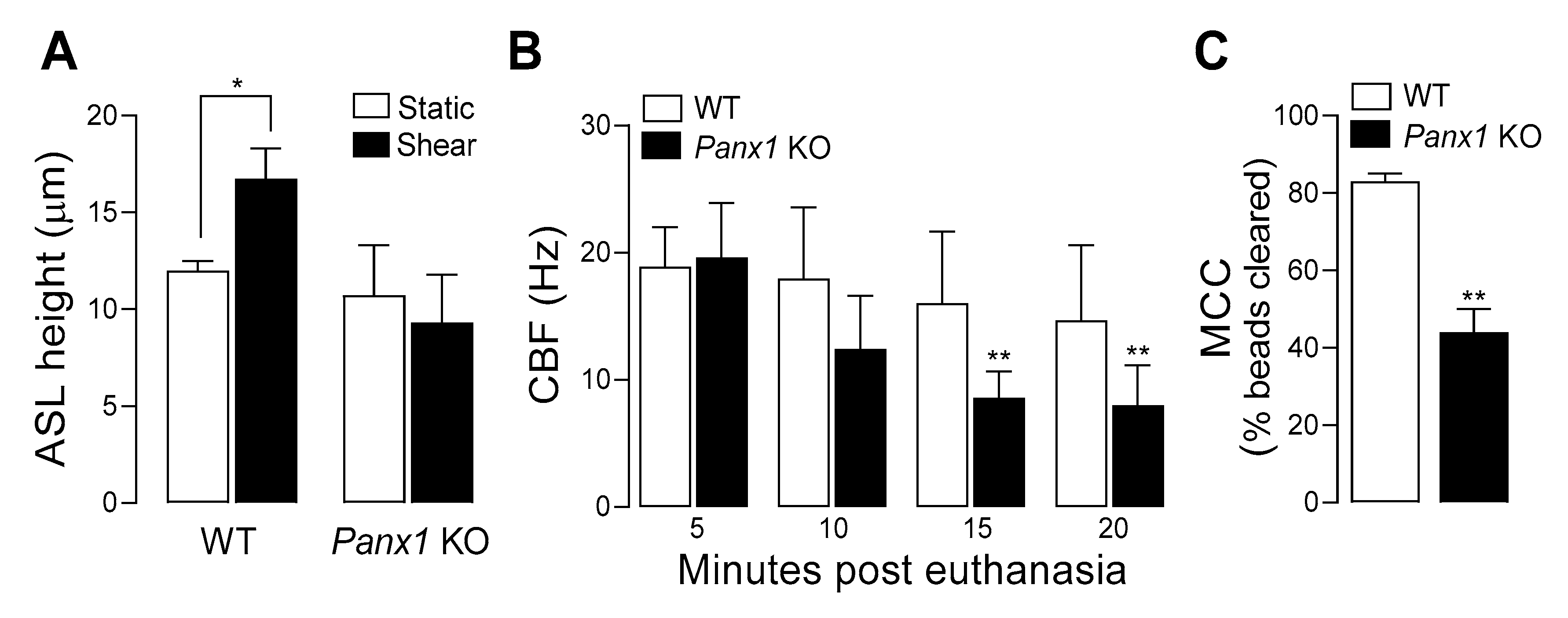
7. Pannexin 1 KO Mice Exhibit ASL Dehydration and Deficient Mucociliary Clearance
Capitalizing on the availability of
Panx1 KO mice, we investigated the contribution of pannexin 1 to mucociliary clearance (MCC) activities. First, we evaluated the role of pannexin 1 in the regulation of airway surface hydration. ASL height was assessed in primary cultures of murine tracheal epithelial (MTE) cells, as an index of ASL volume production [
26]. Under resting conditions, ASL height, as surrogate of the hydration status of the airway luminal surface [
55] was similar in WT and
Panx1 KO cell cultures. However, when MTE cells were subjected to oscillatory shear stress [which mimics the phasic mechanical motion of the lung in vivo and results in increased ATP release [
25,
38,
39]] ASL height increased in WT cells, but not in cells from
Panx1 KO mice (
Figure 3A). These observations suggest that ASL height in static murine airway epithelia is not controlled by pannexin 1, but pannexin 1 promotes airway surface hydration under conditions relevant to the mechanical stresses exerted in normal lung physiology [
25]. Ciliary beat frequency (CBF) and mucociliary transport rates were subsequently measured in murine tracheas in situ. CBF remained unchanged over time in WT mice but was markedly and significantly lower in
Panx1 KO mice after 15- and 20-min post euthanasia (
Figure 3B). Lastly, utilizing a fluorescence micro-bead clearance assay previously described [
56], MCC rates were found to be ~50% reduced in
Panx1 KO mice, compared to WT mouse (
Figure 3C).
Collectively, these observations suggest that pannexin 1-mediated ATP release promotes airway surface hydration and ciliary beating, thus contributing to MCC activities in normal epithelia.
8. Summary and Conclusions
ATP and its metabolite adenosine present within the ASL, via activation of airway epithelial purinergic receptors, regulate multiple components of the MCC. Major pathways of nucleotide release and metabolism have been identified and their contribution to MCC activities is beginning to be understood. ATP is released onto the luminal surface from: (a) ciliated cells via the apical membrane channel pannexin 1; and (b) goblet cell mucin granules and secretory vesicles via VNUT-mediated transport. Released ATP interacts with the P2Y2R but is also rapidly converted to adenosine, which, in turn, promotes activation of the A2BR. Both the P2Y2R and the A2BR regulate ASL volume production and ciliary beating. Thus, decreased ATP release is predicted to result in defective MCC. Indeed, in normal, ciliated cell-dominated airway epithelia, a reduction in ATP released via ablation of pannexin 1 reduced, at least in part, ASL volume regulation, ciliary beating, and MCC rates. However, mucus hyperproduction and inflammation are associated with increased VNUT-mediated nucleotide release, suggesting that secretion of nucleotides from mucin granules and vesicles drives the hydration of newly secreted mucins. The extent to which pannexin 1- and VNUT-mediated nucleotide release contributes to mucous hydration in vivo in healthy and diseased airways is intensively investigated.
Due to the CFTR defect, the A2BR/cyclic AMP/CFTR-dependent mechanism of fluid secretion is impaired in CF and likely in cigarette smoke-induced CB. Intriguingly, due to hydrolysis, ATP steady-state levels are suboptimal to promote P2Y2R-mediated water secretion and, therefore, the CFTR-independent mechanism of airway hydration downstream of P2Y2R does not optimally compensate for the CFTR defect. Therefore, reducing the rates of hydrolysis of released ATP is predicted to facilitate airway surface hydration in CFTR-deficient airways.
9. Methods
Wild-type and
Panx1 KO mice were on C57BL/6 background, as previously described [
41]. Mice 4 months of age and both genders were used. Mice were housed in individually ventilated micro-isolator cages, in a specific pathogen-free facility maintained at the University of North Carolina at Chapel Hill, on a 12-h day/night cycle. They were fed a regular chow diet and given water ad libitum.
MTE cells from WT and
Panx1 KO mice. Cells were harvested from tracheas from 6–8 WT and Panx1 KO mice and grown to confluence in ASL for 10 days, as described (39). ASL height was measure by confocal microscopy, as in [
26]. For these studies, oscillatory shear stress was applied at 0.5 dynes/cm at 14 cycles per minute, as previously described (25).
CBF was measured in situ in the closed trachea, immediately after exsanguination of the mouse anesthetized with 3% isoflurane. For the tracheal preparation, the skin was opened, the muscle and connective tissue overlying the trachea were retracted and the exposed trachea was covered with a piece of plastic wrap dipped in water equilibrated mineral oil. This preparation was immediately placed under a dissecting scope (10× magnification) outfitted with a digital camera (Basler, Germany) interfaced with Basler software. The preparation was lighted with a DC red light allowing light reflected from the beating cilia to be easily seen. The temperature of the preparation was closely monitored using a temperature microprobe (T Type insect probe, Physitemp Inst., Clifton, NJ, USA) placed alongside the trachea. The output from the temperature probe was displayed digitally on a Physitemp TCAT-2ac Controller and a small ceramic heater (Wuhostam, Shenzhen, China) positioned close to the preparation was used to maintain the temperature of the preparation at 37 (±0.1) °C. Preparations were placed on an air table (TMC, Peabody, MA, USA) to minimize vibrations that interfered with the CBF measurements. It took approximately 3 min from the time of euthanasia until the preparation was under the scope and data acquisition commenced. CBF was measured for a 2 s period at the indicated times after euthanasia. The data were collected at 100 frames/second and analyzed using SAVA software. CBF is reported as the mean of values from the three time points for each mouse.
MCC activities in the trachea were assessed using the fluorescence micro-bead clearance assay previously described [
56].
Author Contributions
Conceptualization, E.R.L.; methodology, C.v.H., B.R.G. and B.B.; investigation, and formal analysis, E.R.L., B.R.G. and B.B.; writing—original draft preparation, E.R.L.; writing—review and editing, C.v.H., B.B., B.R.G. and E.R.L.; funding acquisition, E.R.L. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by NIH Grants R56 HL136909, R01 HL125280, and P30 DK065988, and Cystic Fibrosis Foundation Grants LAZARO19GO and BUTTON19GO.
Institutional Review Board Statement
Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill (IACUC ID: 17-050.0; 03/02/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors are grateful to Wanda O’Neal and Lisa Morton (Molecular Biology Core) for assisting with mouse genotype studies, to Scott Randell and the University of North Carolina Tissue Core for assisting with MTE cell cultures techniques, and to Eric Roe for editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Button, B.; Cai, L.H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef]
- Boucher, R.C. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu. Rev. Med. 2007, 58, 157–170. [Google Scholar] [CrossRef]
- Boucher, R.C. Relationship of airway epithelial ion transport to chronic bronchitis. Proc. Am. Thorac. Soc. 2004, 1, 66–70. [Google Scholar] [CrossRef]
- Evans, C.M.; Koo, J.S. Airway mucus: The good, the bad, the sticky. Pharmacol. Ther. 2009, 121, 332–348. [Google Scholar] [CrossRef]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N. Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Gentzsch, M.; Dang, H.; Dang, Y.; Garcia-Caballero, A.; Suchindran, H.; Boucher, R.C.; Stutts, M.J. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J. Biol. Chem. 2010, 285, 32227–32232. [Google Scholar] [CrossRef] [PubMed]
- Clunes, L.A.; Davies, C.M.; Coakley, R.D.; Aleksandrov, A.A.; Henderson, A.G.; Zeman, K.L.; Worthington, E.N.; Gentzsch, M.; Kreda, S.M.; Cholon, D.; et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012, 26, 533–545. [Google Scholar] [CrossRef]
- Tyrrell, J.; Qian, X.; Freire, J.; Tarran, R. Roflumilast combined with adenosine increases mucosal hydration in human airway epithelial cultures after cigarette smoke exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1068–L1077. [Google Scholar] [CrossRef][Green Version]
- Cantin, A.M. Cystic Fibrosis Transmembrane Conductance Regulator. Implications in Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. Ann. ATS 2016, 13, S150–S155. [Google Scholar]
- Lazarowski, E.R.; Boucher, R.C. Purinergic receptors in airway epithelia. Curr. Opin. Pharmacol. 2009, 9, 262–267. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef]
- Jia, Y.; Mathews, C.J.; Hanrahan, J.W. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J. Biol. Chem. 1997, 272, 4978–4984. [Google Scholar] [CrossRef]
- Namkung, W.; Finkbeiner, W.E.; Verkman, A.S. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol. Biol. Cell 2010, 21, 2639–2648. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Bachhuber, T.; Regeer, R.; Markovich, D.; Sun, J.; Schreiber, R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005, 19, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.P.; Eaton, D.C. Acute regulation of epithelial sodium channel by anionic phospholipids. J. Am. Soc. Nephrol. 2005, 16, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.P.; Chou, C.F.; Wei, S.P.; Eaton, D.C. Regulation of the epithelial sodium channel by phosphatidylinositides: Experiments, implications, and speculations. Pflügers Arch. Eur. J. Physiol. 2007, 455, 169–180. [Google Scholar] [CrossRef]
- Davis, C.W.; Lazarowski, E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir. Physiol. Neurobiol. 2008, 163, 208–213. [Google Scholar] [CrossRef]
- Winters, S.L.; Davis, C.W.; Boucher, R.C. Mechanosensitivity of mouse tracheal ciliary beat frequency: Roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L614–L624. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.M.; Smullen, J.L.; Davis, C.W. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am. J. Physiol. Cell Physiol. 2001, 280, C1485–C1497. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R.; Tarran, R.; Grubb, B.R.; van Heusden, C.A.; Okada, S.; Boucher, R.C. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 2004, 279, 36855–36864. [Google Scholar] [CrossRef] [PubMed]
- van Heusden, C.; Button, B.; Anderson, W.H.; Ceppe, A.; Morton, L.C.; O’Neal, W.K.; Dang, H.; Alexis, N.E.; Donaldson, S.; Stephan, H.; et al. Inhibition of ATP hydrolysis restores airway surface liquid production in cystic fibrosis airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L356–L365. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.B.; Lazarowski, E.R.; Tarran, R.; Milgram, S.L.; Boucher, R.C.; Stutts, M.J. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc. Natl. Acad. Sci. USA 2001, 98, 14120–14125. [Google Scholar] [CrossRef] [PubMed]
- Tarran, R.; Button, B.; Picher, M.; Paradiso, A.M.; Ribeiro, C.M.; Lazarowski, E.R.; Zhang, L.; Collins, P.L.; Pickles, R.J.; Fredburg, J.J.; et al. Normal and cystic fbrosis airway surface liquid homeostasis: The effects of phasic shear stress and viral infections. J. Biol. Chem. 2005, 280, 35751–35759. [Google Scholar] [CrossRef] [PubMed]
- Button, B.; Okada, S.F.; Frederick, C.B.; Thelin, W.R.; Boucher, R.C. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci. Signal. 2013, 6, ra46. [Google Scholar] [CrossRef]
- Okada, S.F.; Ribeiro, C.M.; Sesma, J.I.; Seminario-Vidal, L.; Abdullah, L.H.; van Heusden, C.; Lazarowski, E.R.; Boucher, R.C. Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways. Am. J. Respir. Cell Mol. Biol. 2013, 49, 814–820. [Google Scholar] [CrossRef]
- Grygorczyk, R.; Hanrahan, J.W. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am. J. Physiol. 1997, 272, C1058–C1066. [Google Scholar] [CrossRef]
- Hazama, A.; Shimizu, T.; Ando-Akatsuka, Y.; Hayashi, S.; Tanaka, S.; Maeno, E.; Okada, Y. Swelling-induced, CFTR-independent ATP release from a human epithelial cell line. J. Gen. Physiol. 1999, 114, 525–533. [Google Scholar] [CrossRef]
- Watt, W.C.; Lazarowski, E.R.; Boucher, R.C. Cystic fibrosis transmembrane regulator-independent release of ATP—Its implications for the regulation of P2Y(2) receptors in airway epithelia. J. Biol. Chem. 1998, 273, 14053–14058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, W.H.; Coakley, R.D.; Button, B.; Henderson, A.G.; Zeman, K.L.; Alexis, N.E.; Peden, D.B.; Lazarowski, E.R.; Davis, C.W.; Bailey, S.; et al. The Relationship of Mucus Concentration (Hydration) to Mucus Osmotic Pressure and Transport in Chronic Bronchitis. Am. J. Respir. Crit. Care Med. 2015, 192, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R.; Boucher, R.C.; Harden, T.K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000, 275, 31061–31068. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Vidal, L.; Kreda, S.; Jones, L.; O’Neal, W.; Trejo, J.; Boucher, R.C.; Lazarowski, E.R. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J. Biol. Chem. 2009, 284, 20638–20648. [Google Scholar] [CrossRef] [PubMed]
- Kreda, S.M.; Seminario-Vidal, L.; van Heusden, C.A.; O’Neal, W.; Jones, L.; Boucher, R.C.; Lazarowski, E.R. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J. Physiol. 2010, 588, 2255–2267. [Google Scholar] [CrossRef]
- Okada, S.F.; Zhang, L.; Kreda, S.M.; Abdullah, L.H.; Davis, C.W.; Pickles, R.J.; Lazarowski, E.R.; Boucher, R.C. Coupled Nucleotide and Mucin Hypersecretion from Goblet Cell Metaplastic Human Airway Epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 253–260. [Google Scholar] [CrossRef]
- Kreda, S.M.; Okada, S.F.; van Heusden, C.A.; O’Neal, W.; Gabriel, S.; Abdullah, L.; Davis, C.W.; Boucher, R.C.; Lazarowski, E.R. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. 2007, 584, 245–259. [Google Scholar] [CrossRef]
- Okada, S.F.; Nicholas, R.A.; Kreda, S.M.; Lazarowski, E.R.; Boucher, R.C. Physiological regulation of ATP release at the apical surface of human airway epithelia. J. Biol. Chem. 2006, 281, 22992–23002. [Google Scholar] [CrossRef]
- Tarran, R.; Button, B.; Boucher, R.C. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu. Rev. Physiol. 2006, 68, 543–561. [Google Scholar] [CrossRef]
- Button, B.; Picher, M.; Boucher, R.C. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J. Physiol. 2007, 580, 577–592. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef]
- Seminario-Vidal, L.; Okada, S.F.; Sesma, J.I.; Kreda, S.M.; van Heusden, C.A.; Zhu, Y.; Jones, L.C.; O’Neal, W.K.; Penuela, S.; Laird, D.W.; et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 2011, 286, 26277–26286. [Google Scholar] [CrossRef]
- Ransford, G.A.; Fregien, N.; Qiu, F.; Dahl, G.; Conner, G.E.; Salathe, M. Pannexin 1 Contributes to ATP Release in Airway Epithelia. Am. J. Respir. Cell Mol. Biol. 2009, 41, 525–534. [Google Scholar] [CrossRef]
- Sawada, K.; Echigo, N.; Juge, N.; Miyaji, T.; Otsuka, M.; Omote, H.; Yamamoto, A.; Moriyama, Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 5683–5686. [Google Scholar] [CrossRef]
- Sesma, J.I.; Kreda, S.M.; Okada, S.F.; van Heusden, C.; Moussa, L.; Jones, L.C.; O’Neal, W.K.; Togawa, N.; Hiasa, M.; Moriyama, Y.; et al. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am. J. Physiol. Cell Physiol. 2013, 304, C976–C984. [Google Scholar] [CrossRef][Green Version]
- Tokunaga, A.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J. Biol. Chem. 2010, 285, 17406–17416. [Google Scholar] [CrossRef]
- Feranchak, A.P.; Lewis, M.A.; Kresge, C.; Sathe, M.; Bugde, A.; Luby-Phelps, K.; Antich, P.P.; Fitz, J.G. Initiation of Purinergic Signaling by Exocytosis of ATP-containing Vesicles in Liver Epithelium. J. Biol. Chem. 2010, 285, 8138–8147. [Google Scholar] [CrossRef]
- Sathe, M.N.; Woo, K.; Kresge, C.; Bugde, A.; Luby-Phelps, K.; Lewis, M.A.; Feranchak, A.P. Regulation of Purinergic Signaling in Biliary Epithelial Cells by Exocytosis of SLC17A9-dependent ATP-enriched Vesicles. J. Biol. Chem. 2011, 286, 25363–25376. [Google Scholar] [CrossRef]
- Tatur, S.; Kreda, S.; Lazarowski, E.; Grygorczyk, R. Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 2008, 4, 139–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akopova, I.; Tatur, S.; Grygorczyk, M.; Luchowski, R.; Gryczynski, I.; Gryczynski, Z.; Borejdo, J.; Grygorczyk, R. Imaging exocytosis of ATP-containing vesicles with TIRF 1 microscopy in lung epithelial A549 cells. Purinergic Signal. 2012, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Tsukimoto, M.; Harada, H.; Sawada, K.; Moriyama, Y.; Kojima, S. Autocrine regulation of TGF-beta1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J. Cell Sci. 2012, 125, 5051–5060. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Carey, R.M.; Chen, B.; Saunders, C.J.; Marambaud, P.; Mitchell, C.H.; Tordoff, M.G.; Lee, R.J.; Cohen, N.A. CALHM1-Mediated ATP Release and Ciliary Beat Frequency Modulation in Nasal Epithelial Cells. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Taruno, A.; Vingtdeux, V.; Ohmoto, M.; Ma, Z.; Dvoryanchikov, G.; Li, A.; Adrien, L.; Zhao, H.; Leung, S.; Abernethy, M.; et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013, 495, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.J.; Salm, E.J.; Tomita, S. ABC transporters control ATP release through cholesterol-dependent volume-regulated anion channel activity. J. Biol. Chem. 2020, 295, 5192–5203. [Google Scholar] [CrossRef]
- Matsui, H.; Grubb, B.R.; Tarran, R.; Randell, S.H.; Gatzy, J.T.; Davis, C.W.; Boucher, R.C. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998, 95, 1005–1015. [Google Scholar] [CrossRef]
- Ostrowski, L.E.; Yin, W.; Rogers, T.D.; Busalacchi, K.B.; Chua, M.; O’Neal, W.K.; Grubb, B.R. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am. J. Respir. Cell Mol. Biol. 2010, 43, 55–63. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).