Human Prostate Epithelial Cells Activate the AIM2 Inflammasome upon Cellular Senescence: Role of POP3 Protein in Aging-Related Prostatic Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Primary PrECs, Prostate Cell Line, and Treatments
2.3. Antibodies
2.4. Immunoblotting
2.5. Inflammasome Assay
2.6. RNA Isolation and PCR
2.7. Transfection
2.8. Stable Knockdown of POP3 Expression
2.9. Statistical Methods
3. Results
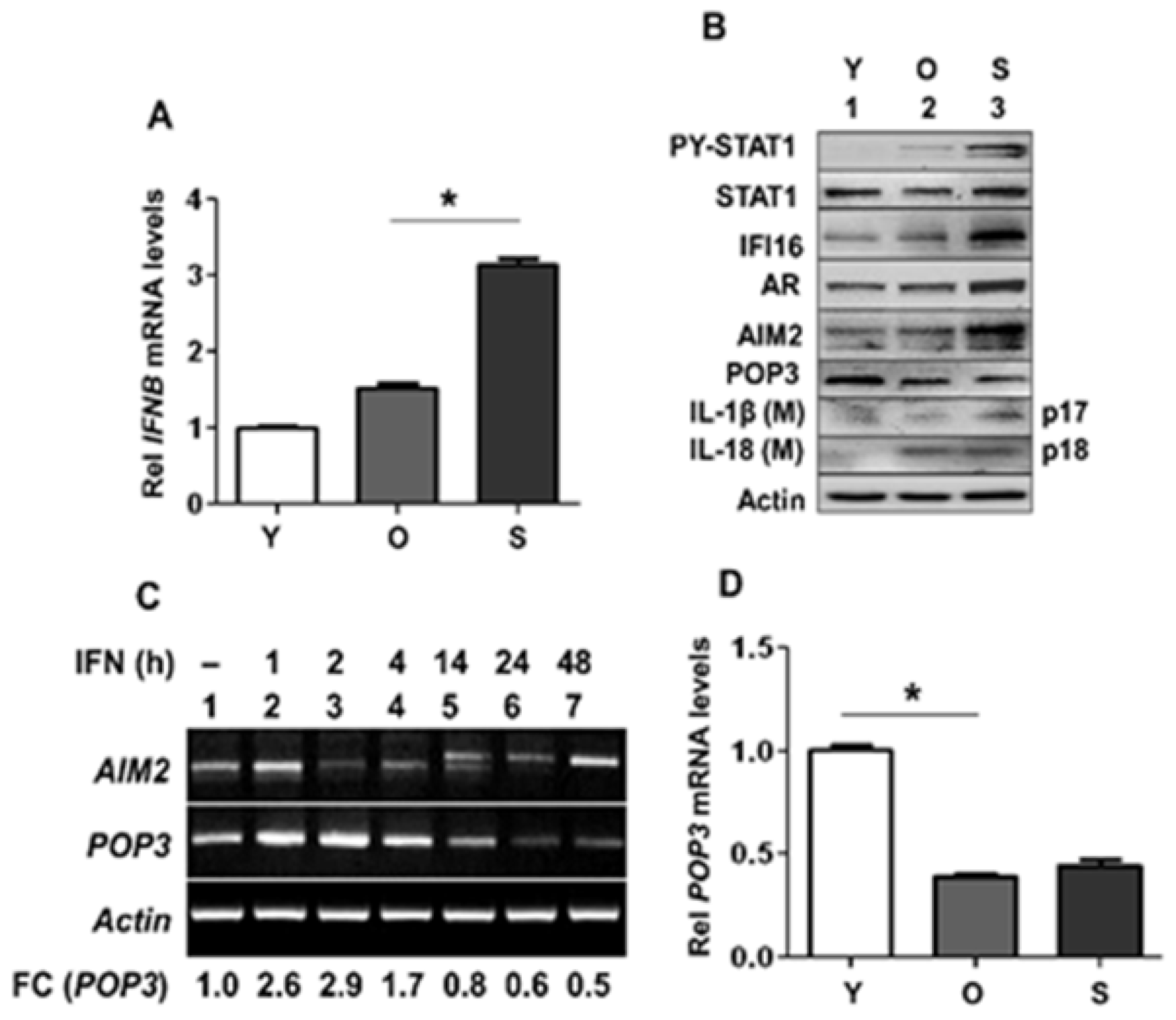
3.1. Activation of Type I Interferon Signaling in Senescent PrECs Differentially Regulated the Expression of POP3 and AIM2 Proteins
3.2. Androgen Receptor Activation in Proliferating PrECs Increased the Expression of POP3
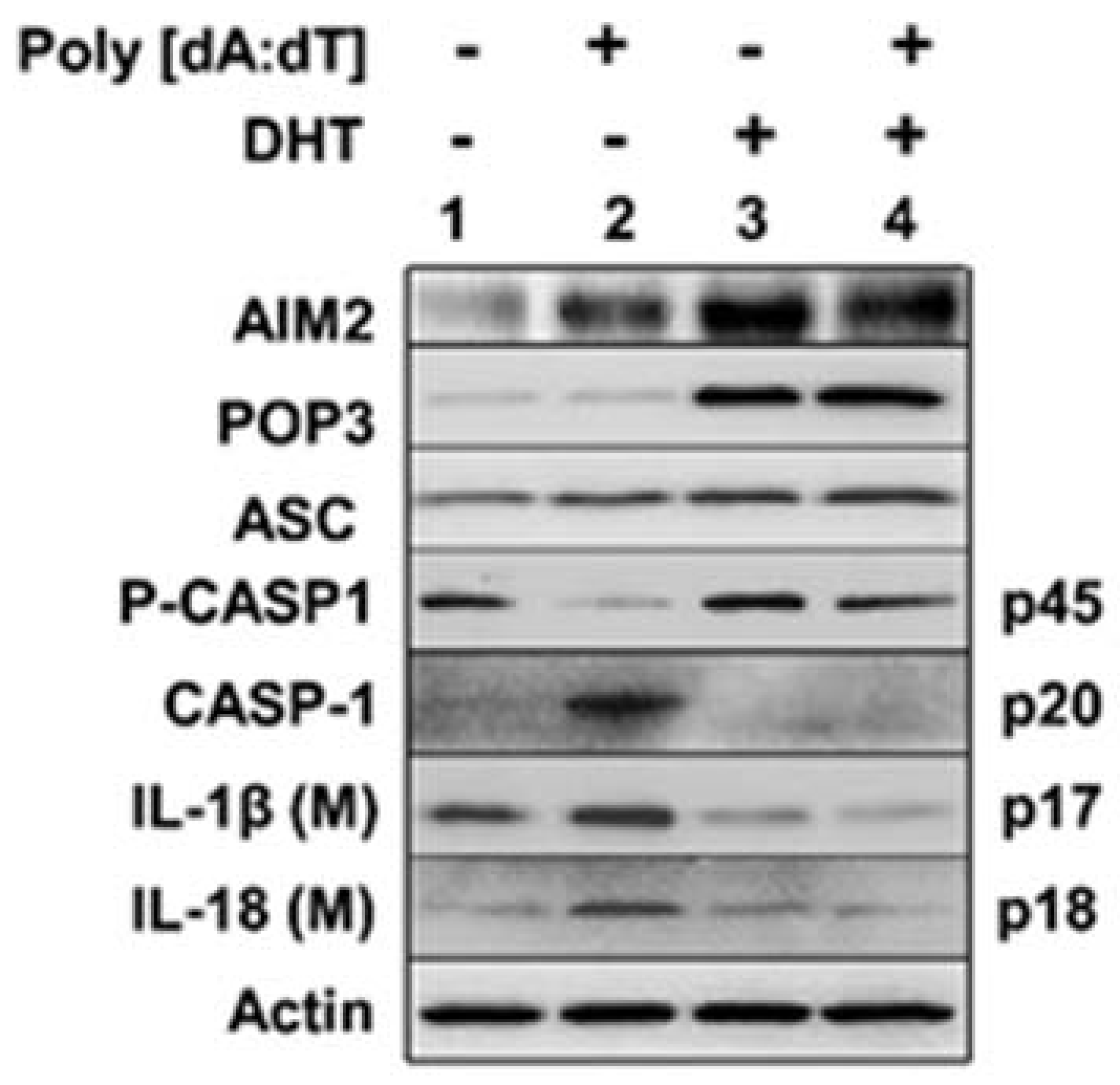
3.3. Androgen Treatment of PrECs Inhibited Cytosolic DNA-Induced Activation of the AIM2 Inflammasome
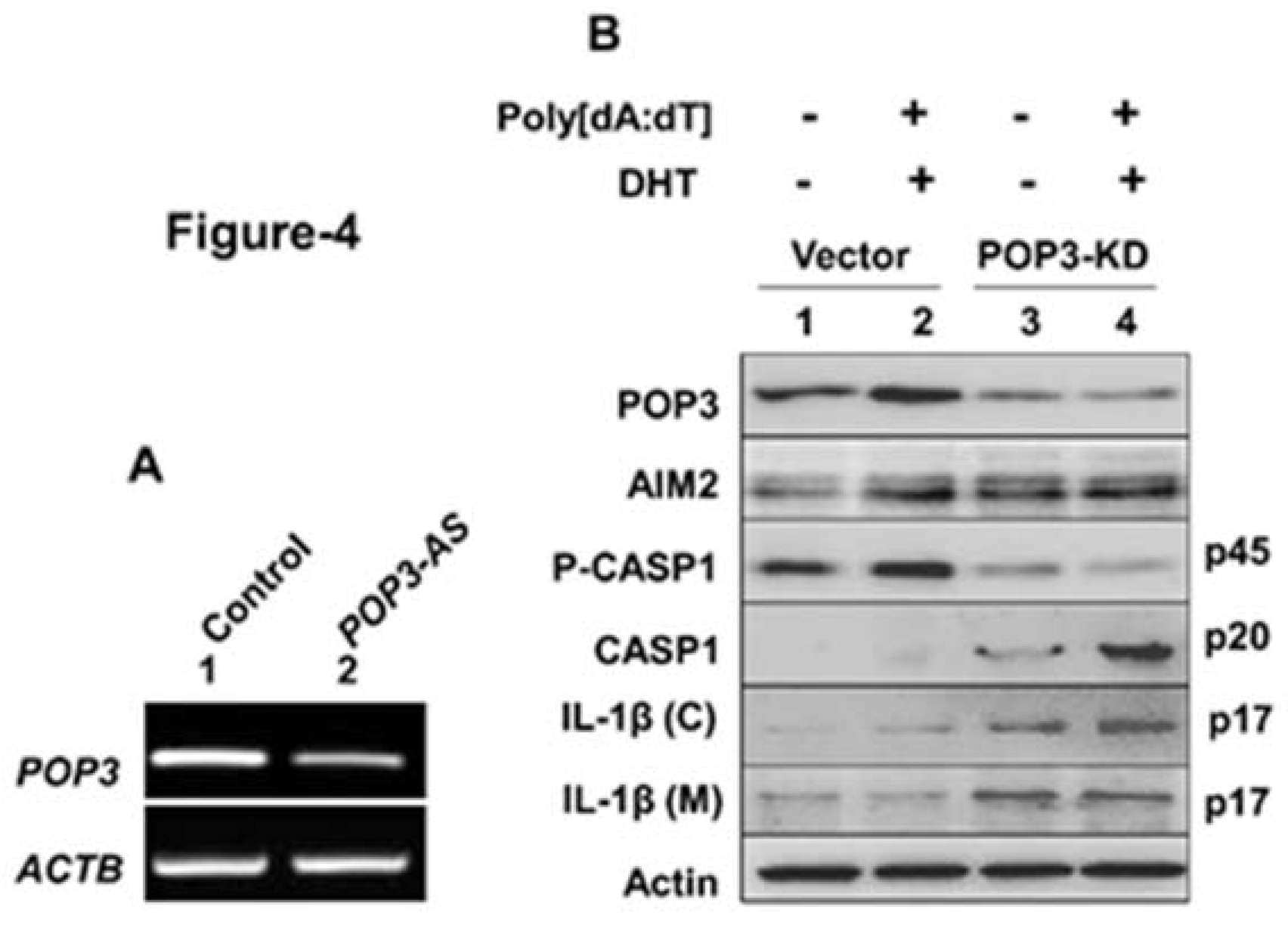
3.4. A Stable Knockdown of POP3 Protein Expression in BPH-1 Cell Line Increased Cytosolic DNA-Induced AIM2 Inflammasome Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Marzo, A.M.; Nakai, Y.; Nelson, W.G. Inflammation, atrophy, and prostate carcinogenesis. Urol. Oncol. 2007, 25, 398–400. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, S.; Platz, E.A. Inflammation and prostate cancer: A focus on infections. Curr. Urol. Rep. 2008, 9, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Silverman, R. Inflammation, infection, and prostate cancer. Curr. Opin. Urol. 2008, 18, 315–319. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Isaacs, W.B.; De Marzo, A.M. Infections and inflammation in prostate cancer. Am. J. Clin. Exp. Urol. 2013, 1, 3–11. [Google Scholar]
- Nakai, Y.; Nonomura, N. Inflammation and prostate carcinogenesis. Int. J. Urol. 2013, 20, 150–160. [Google Scholar] [CrossRef]
- Wallach, D.; Kang, T.B.; Kovalenko, A. Concepts of tissue injury and cell death in inflammation: A historical perspective. Nat. Rev. Immunol. 2014, 14, 51–59. [Google Scholar] [CrossRef]
- Kwon, O.J.; Zhang, L.; Ittmann, M.M.; Xin, L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl. Acad. Sci. USA 2014, 111, E592–E600. [Google Scholar] [CrossRef]
- Vignozzi, L.; Maggi, M. Prostate cancer: Intriguing data on inflammation and prostate cancer. Nat. Rev. Urol. 2014, 11, 369–370. [Google Scholar] [CrossRef]
- Ho, C.H.; Fan, C.K.; Yu, H.J.; Wu, C.C.; Chen, K.C.; Liu, S.P.; Cheng, P.C. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. PLoS ONE 2017, 12, e0180244. [Google Scholar]
- Fischer, K.; Tschismarov, R.; Pilz, A.; Straubinger, S.; Carotta, S.; McDowell, A.; Decker, T. Cutibacterium acnes infection induces type I interferon synthesis through the cGAS-STING pathway. Front. Immunol. 2020, 11, 571334. [Google Scholar] [CrossRef]
- Stark, G.R.; Kerr, I.M.; Williams, B.R.; Silverman, R.H.; Schreiber, R.D. How cells respond to interferons? Annu. Rev. Biochem. 1998, 67, 227–264. [Google Scholar] [CrossRef]
- Choubey, D.; Duan, X.; Dickerson, E.; Ponomareva, L.; Panchanathan, R.; Shen, H.; Srivastava, R. Interferon-inducible p200-family proteins as novel sensors of cytoplasmic DNA: Role in inflammation and autoimmunity. J. Interferon Cytokine Res. 2010, 30, 371–380. [Google Scholar] [CrossRef]
- Connolly, D.J.; Bowie, A.G. The emerging role of human PYHIN proteins in innate immunity: Implications for health and disease. Biochem. Pharmacol. 2014, 92, 405–414. [Google Scholar] [CrossRef]
- Khare, S.; Ratsimandresy, R.A.; de Almeida, L.; Cuda, C.M.; Rellick, S.L.; Misharin, A.V.; Wallin, M.C.; Gangopadbyay, A.; Forte, E.; Gottwein, E.; et al. The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat. Immunol. 2014, 15, 343–353. [Google Scholar] [CrossRef]
- Basrawala, Z.; Alimirah, F.; Xin, H.; Mohideen, N.; Campbell, S.C.; Flanigan, R.C.; Choubey, D. Androgen receptor levels are increased by interferons in human prostate stromal and epithelial cells. Oncogene 2006, 25, 2812–2817. [Google Scholar] [CrossRef]
- Coffey, D.S.; Isaacs, J.T. Control of prostate growth. Urology 1981, 17, 17–24. [Google Scholar]
- Zhou, Y.; Bolton, E.C.; Jones, J.O. Androgens and androgen receptor signaling in prostate tumorigenesis. J. Mol. Endocrinol. 2015, 54, R15–R29. [Google Scholar] [CrossRef]
- Alimirah, F.; Chen, J.; Xin, H.; Choubey, D. Androgen receptor auto-regulates its expression by a negative feedback loop through upregulation of IFI16 protein. FEBS Lett. 2006, 580, 1659–1664. [Google Scholar] [CrossRef]
- Xin, H.; Curry, J.; Johnstone, R.W.; Nickoloff, B.J.; Choubey, D. Role of IFI16, a member of the IFN-inducible p200-protein family, in prostate epithelial cellular senescence. Oncogene 2003, 22, 4831–4840. [Google Scholar] [CrossRef]
- Xin, H.; Pereira-Smith, O.M.; Choubey, D. Role of IFI 16 in cellular senescence of human fibroblasts. Oncogene 2004, 23, 6209–6217. [Google Scholar] [CrossRef]
- Duan, X.; Ponomareva, L.; Veeranki, S.; Panchanathan, R.; Dickerson, E.; Choubey, D. Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts. Mol. Cancer Res. 2011, 9, 589–602. [Google Scholar] [CrossRef]
- Choubey, D.; Panchanathan, R. IFI16, an amplifier of DNA-damage response: Role in cellular senescence and aging-associated inflammatory diseases. Ageing Res. Rev. 2016, 28, 27–36. [Google Scholar] [CrossRef]
- Mirochnik, Y.; Veliceasa, D.; Williams, L.; Maxwell, K.; Yemelyanov, A.; Budunova, I.; Volpert, O.V. Androgen receptor drives cellular senescence. PLoS ONE 2012, 7, e31052. [Google Scholar] [CrossRef]
- Campisi, J.; Andersen, J.K.; Kapahi, P.; Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011, 21, 354–359. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Castro, P.; Giri, D.; Lamb, D.; Ittmann, M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate 2003, 55, 30–38. [Google Scholar] [CrossRef]
- Vital, P.; Castro, P.; Tsang, S.; Ittmann, M. The senescence-associated secretory phenotype promotes benign prostatic hyperplasia. Am. J. Path. 2014, 184, 721–731. [Google Scholar] [CrossRef]
- Kumari, P.; Russo, A.J.; Shivcharan, S.; Rathinam, V.A. AIM2 in health and disease: Inflammasome and beyond. Immunol. Rev. 2020, 297, 83–95. [Google Scholar] [CrossRef]
- Hayward, S.W.; Dahiya, R.; Cunha, G.R.; Bartek, J.; Deshpande, N.; Narayan, P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 14–24. [Google Scholar] [CrossRef]
- Ponomareva, L.; Liu, H.; Duan, X.; Dickerson, E.; Shen, H.; Panchanathan, R.; Choubey, D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol. Cancer Res. 2013, 11, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of pro-IL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Veldscholte, J.; Berrevoets, C.A.; Ris-Stalpers, C.; Kuiper, G.G.; Jenster, G.; Trapman, J.; Brinkmann, A.O.; Mulder, E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J. Steroid Biochem. Mol. Biol. 1992, 41, 665–669. [Google Scholar] [CrossRef]
- Acosta, J.C.; O’Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Da Costa, M.; Brown, C.; Popov, N.; et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef]
- Hoare, M.; Narita, M. Transmitting senescence to the cell neighborhood. Nat. Cell Biol. 2013, 15, 887–889. [Google Scholar] [CrossRef]
- Maggio, M.; Basaria, S.; Ceda, G.P.; Ble, A.; Ling, S.M.; Bandinelli, S.; Valenti, G.; Ferrucci, L. The relationship between testosterone and molecular markers of inflammation in older men. J. Endocrinol. Investig. 2005, 28, 116–119. [Google Scholar]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef]
- Chen, C.S.; Chang, P.J.; Lin, W.Y.; Huang, Y.C.; Ho, D.R. Evidences of the inflammasome pathway in chronic prostatitis and chronic pelvic pain syndrome in an animal model. Prostate 2013, 73, 391–397. [Google Scholar] [CrossRef]
- Kashyap, M.; Pore, S.; Wang, Z.; Gingrich, J.; Yoshimura, N.; Tyagi, P. Inflammasomes are important mediators of prostatic inflammation associated with BPH. J. Inflamm. 2015, 12, 37. [Google Scholar] [CrossRef]
- Karan, D.; Tawfik, O.; Dubey, S. Expression analysis of inflammasome sensors and implication of NLRP12 inflammasome in prostate cancer. Sci. Rep. 2017, 7, 4378. [Google Scholar] [CrossRef]
- Karan, D.; Dubey, S. From inflammation to prostate cancer: The role of inflammasomes. Adv. Urol. 2016, 2016, 3140372. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchanathan, R.; Ramalingam, V.; Liu, H.; Choubey, D. Human Prostate Epithelial Cells Activate the AIM2 Inflammasome upon Cellular Senescence: Role of POP3 Protein in Aging-Related Prostatic Inflammation. Life 2021, 11, 366. https://doi.org/10.3390/life11040366
Panchanathan R, Ramalingam V, Liu H, Choubey D. Human Prostate Epithelial Cells Activate the AIM2 Inflammasome upon Cellular Senescence: Role of POP3 Protein in Aging-Related Prostatic Inflammation. Life. 2021; 11(4):366. https://doi.org/10.3390/life11040366
Chicago/Turabian StylePanchanathan, Ravichandran, Vaikundamoorthy Ramalingam, Hongzhu Liu, and Divaker Choubey. 2021. "Human Prostate Epithelial Cells Activate the AIM2 Inflammasome upon Cellular Senescence: Role of POP3 Protein in Aging-Related Prostatic Inflammation" Life 11, no. 4: 366. https://doi.org/10.3390/life11040366
APA StylePanchanathan, R., Ramalingam, V., Liu, H., & Choubey, D. (2021). Human Prostate Epithelial Cells Activate the AIM2 Inflammasome upon Cellular Senescence: Role of POP3 Protein in Aging-Related Prostatic Inflammation. Life, 11(4), 366. https://doi.org/10.3390/life11040366






