Endovascular Treatment of Intracranial Aneurysms
Abstract
1. Introduction
2. Background of Intracranial Aneurysms
2.1. Epidemiology
2.2. Etiology
2.3. Pathophysiology
2.4. Classification
2.5. Anatomy
2.6. Rupture Predicting Scores
3. Endovascular Management of IA
3.1. Detachable Coils
3.2. Balloon-Assisted Coiling (BAC)
3.3. Stent-Assisted Coiling (SAC)
3.4. Laser-Cut Stents
3.4.1. Closed-Cell Stents
3.4.2. Open-Cell Stents
3.4.3. Advances
3.4.4. Braided Stents
3.5. Flow Diversion
3.6. Intrasaccular Flow Disruptors and Woven Endoluminal Bridge (WEB)
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vlak, M.H.M.; Algra, A.; Brandenburg, R.; Rinkel, G.J.E. Prevalence of Unruptured Intracranial Aneurysms, with Emphasis on Sex, Age, Comorbidity, Country, and Time Period: A Systematic Review and Meta-Analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Caranci, F.; Briganti, F.; Cirillo, L.; Leonardi, M.; Muto, M. Epidemiology and Genetics of Intracranial Aneurysms. Eur. J. Radiol. 2013, 82, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, G.J.E.; Djibuti, M.; Algra, A.; van Gijn, J. Prevalence and Risk of Rupture of Intracranial Aneurysms. Stroke 1998, 29, 251–256. [Google Scholar] [CrossRef]
- De Rooij, N.K.; Linn, F.H.H.; Van Der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of Subarachnoid Haemorrhage: A Systematic Review with Emphasis on Region, Age, Gender and Time Trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef]
- Broderick, J.P.; Brott, T.; Miller, R.; Tomsick, T.; Huster, G. The Risk of Subarachnoid and Intracerebral Hemorrhages in Blacks as Compared with Whites. New Engl. J. Med. 1992, 326, 733–736. [Google Scholar] [CrossRef]
- Connolly, E.S.; Poisik, A.; Winfree, C.J.; Kim, L.J.; Huang, J.; McMahon, D.J.; Solomon, R.A. Cigarette Smoking and the Development and Rupture of Cerebral Aneurysms in a Mixed Race Population: Implications for Population Screening and Smoking Cessation. J. Stroke Cerebrovasc. Dis. 1999, 8, 248–253. [Google Scholar] [CrossRef]
- Korja, M.; Lehto, H.; Juvela, S. Lifelong Rupture Risk of Intracranial Aneurysms Depends on Risk Factors: A Prospective Finnish Cohort Study. Stroke 2014, 45, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.K.; van der Spek, R.A.A.; van Rheenen, W.; Morel, S.; Bourcier, R.; Hostettler, I.C.; Alg, V.S.; van Eijk, K.R.; Koido, M.; Akiyama, M.; et al. Genome-Wide Association Study of Intracranial Aneurysms Identifies 17 Risk Loci and Genetic Overlap with Clinical Risk Factors. Nat. Genet. 2020, 52, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Alg, V.S.; Sofat, R.; Houlden, H.; Werring, D.J. Genetic Risk Factors for Intracranial Aneurysms: A Meta-Analysis in More than 116,000 Individuals. Neurology 2013, 80, 2154–2165. [Google Scholar] [CrossRef]
- Schievink, W.I.; Katzmann, J.A.; Piepgras, D.G.; Schaid, D.J. Alpha-1-Antitrypsin Phenotypes among Patients with Intracranial Aneurysms. J. Neurosurg. 1996, 84, 781–784. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Lee, S.T.; Shin, Y.W.; Lee, K.J.; Park, D.K.; Yoo, J.S.; Kim, S.; Kim, M.; Lee, S.K.; et al. Experimental Induction of Cerebral Aneurysms by Developmental Low Copper Diet. J. Neuropathol. Exp. Neurol. 2016, 75. [Google Scholar] [CrossRef]
- Jung, K.-H. New Pathophysiological Considerations on Cerebral Aneurysms. Neurointervention 2018, 13, 73–83. [Google Scholar] [CrossRef]
- Fennell, V.S.; Kalani, M.Y.S.; Atwal, G.; Martirosyan, N.L.; Spetzler, R.F. Biology of Saccular Cerebral Aneurysms: A Review of Current Understanding and Future Directions. Front. Surg. 2016, 3, 43. [Google Scholar] [CrossRef]
- Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. Biology of Intracranial Aneurysms: Role of Inflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Pritz, M.B. Cerebral Aneurysm Classification Based on Angioarchitecture. J. Stroke Cerebrovasc. Dis. 2011, 20, 162–167. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.H.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES Score for Prediction of Risk of Rupture of Intracranial Aneurysms: A Pooled Analysis of Six Prospective Cohort Studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Etminan, N.; Brown, R.D.; Beseoglu, K.; Juvela, S.; Raymond, J.; Morita, A.; Torner, J.C.; Derdeyn, C.P.; Raabe, A.; Mocco, J.; et al. The Unruptured Intracranial Aneurysm Treatment Score: A Multidis Ciplinary Consensus. Neurology 2015, 85, 881–889. [Google Scholar] [CrossRef]
- Pagiola, I.; Mihalea, C.; Caroff, J.; Ikka, L.; Chalumeau, V.; Iacobucci, M.; Ozanne, A.; Gallas, S.; Marques, M.; Nalli, D.; et al. The PHASES Score: To Treat or Not to Treat? Retrospective Evaluation of the Risk of Rupture of Intracranial Aneurysms in Patients with Aneurysmal Subarachnoid Hemorrhage. J. Neuroradiol. 2019. [Google Scholar] [CrossRef]
- Hernández-Durán, S.; Mielke, D.; Rohde, V.; Malinova, V. Is the Unruptured Intracranial Aneurysm Treatment Score (UIATS) Sensitive Enough to Detect Aneurysms at Risk of Rupture? Neurosurg. Rev. 2020, 1–7. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Wu, J.; Chen, X.; Li, M.; Li, Z.; Yang, S.; Guo, R.; Gao, B.; Cao, Y.; et al. A Novel Scoring System for Rupture Risk Stratification of Intracranial Aneurysms: A Hemodynamic and Morphological Study. Front. Neurosci. 2018, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Vinuela, F.; Dion, J.; Duckwiler, G. Electrothrombosis of Saccular Aneurysms via Endovascular Approach. Part 2: Preliminary Clinical Experience. J. Neurosurg. 1991, 75, 8–14. [Google Scholar] [CrossRef]
- Pierot, L.; Wakhloo, A.K. Endovascular Treatment of Intracranial Aneurysms: Current Status. Stroke 2013, 44, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Lanzino, G.; Kanaan, Y.; Perrini, P.; Dayoub, H.; Fraser, K. Emerging Concepts in the Treatment of Intracranial Aneurysms: Stents, Coated Coils, and Liquid Embolic Agents. Neurosurgery 2005, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Taschner, C.A.; Chapot, R.; Costalat, V.; Machi, P.; Courthéoux, P.; Barreau, X.; Berge, J.; Pierot, L.; Kadziolka, K.; Jean, B.; et al. Second-Generation Hydrogel Coils for the Endovascular Treatment of Intracranial Aneurysms a Randomized Controlled Trial. Stroke 2018, 49, 667–674. [Google Scholar] [CrossRef]
- Waldau, B.; Fargen, K.M.; Mack, W.J.; Wilson, N.M.; Khaldi, A.; Hoh, B.L.; Mocco, J. Axium MicroFX Coil for the Completing Endovascular Aneurysm Surgery Study (ACCESS): A Prospective Evaluation of the Safety and Durability of Axium MicroFX PGLA Coils. Interv. Neuroradiol. 2012, 18, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Fargen, K.M.; Blackburn, S.; Deshaies, E.M.; Carpenter, J.S.; Jabbour, P.; Mack, W.J.; Rai, A.T.; Siddiqui, A.H.; Turner, R.D.; Mocco, J. Final Results of the Multicenter, Prospective Axium MicroFX for Endovascular Repair of IntraCranial Aneurysm Study (AMERICA). J. Neurointerv. Surg. 2015, 7, 40–43. [Google Scholar] [CrossRef]
- Vajkoczy, P. Editorial on a Paper Entitled “Combined Suture and Clipping for the Reconstruction of a Ruptured Blister-like Aneurysm”. Acta Neurochir. 2016, 158, 1913–1915. [Google Scholar] [CrossRef]
- Jalbert, J.J.; Isaacs, A.J.; Kamel, H.; Sedrakyan, A. Clipping and Coiling of Unruptured Intracranial Aneurysms among Medicare Beneficiaries, 2000 to 2010. Stroke 2015, 46, 2452–2457. [Google Scholar] [CrossRef]
- Moret, J.; Cognard, C.; Weill, A.; Castaings, L.; Rey, A. The “remodelling Technique” in the Treatment of Wide Neck Intracranial Aneurysms: Angiographic Results and Clinical Follow-up in 56 Cases. Interv. Neuroradiol. 1997, 3, 21–35. [Google Scholar] [CrossRef]
- Dmytriw, A.A.; Salem, M.M.; Yang, V.X.D.; Krings, T.; Pereira, V.M.; Moore, J.M.; Thomas, A.J. Endosaccular Flow Disruption: A New Frontier in Endovascular Aneurysm Management. Neurosurgery 2019, 86, 170–181. [Google Scholar] [CrossRef]
- Shapiro, M.; Babb, J.; Becske, T.; Nelson, P.K. Safety and Efficacy of Adjunctive Balloon Remodeling during Endovascular Treatment of Intracranial Aneurysms: A Literature Review. Am. J. Neuroradiol. 2008, 29, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Spelle, L.; Leclerc, X.; Cognard, C.; Bonafé, A.; Moret, J. Endovascular Treatment of Unruptured Intracranial Aneurysms: Comparison of Safety of Remodeling Technique and Standard Treatment with Coils. Radiology 2009, 251, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Sluzewski, M.; Van Rooij, W.J.; Beute, G.N.; Nijssen, P.C. Balloon-Assisted Coil Embolization of Intracranial Aneurysms: Incidence, Complications, and Angiography Results. J. Neurosurg. JNS 2006, 105, 396–399. [Google Scholar] [CrossRef]
- Henkes, H.; Bose, A.; Felber, S.; Miloslavski, E.; Berg-Dammer, E.; Kühne, D. Endovascular Coil Occlusion of Intracranial Aneurysms Assisted by a Novel Self-Expandable Nitinol Microstent (Neuroform). Interv. Neuroradiol. 2002, 8, 107–119. [Google Scholar] [CrossRef]
- Higashida, R.T.; Halbach, V.V.; Dowd, C.F.; Juravsky, L.; Meagher, S. Initial Clinical Experience with a New Self-Expanding Nitinol Stent for the Treatment of Intracranial Cerebral Aneurysms: The Cordis Enterprise Stent. Am. J. Neuroradiol. 2005, 26, 1751–1756. [Google Scholar]
- Wang, F.; Chen, X.; Wang, Y.; Bai, P.; Wang, H.Z.; Sun, T.; Yu, H.L. Stent-Assisted Coiling and Balloon-Assisted Coiling in the Management of Intracranial Aneurysms: A Systematic Review & Meta-Analysis. J. Neurol. Sci. 2016, 364, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Mine, B.; Bonnet, T.; Vazquez-Suarez, J.C.; Iosif, C.; Lubicz, B. Comparison of Stents Used for Endovascular Treatment of Intracranial Aneurysms. Expert Rev. Med. Devices 2018, 15, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Claus, B.; Lee, C.-Y.; Biondi, A.; Benndorf, G. Stent Conformity in Curved Vascular Models with Simulated Aneurysm Necks Using Flat-Panel CT: An In Vitro Study. Am. J. Neuroradiol. 2007, 28, 823–829. [Google Scholar] [PubMed]
- Kis, B.; Weber, W.; Berlit, P.; Kühne, D. Elective Treatment of Saccular and Broad-Necked Intracranial Aneurysms Using a Closed-Cell Nitinol Stent (Leo). Neurosurgery 2006, 58, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Arat, A.; Sencer, S.; Barburoglu, M.; Men, S. Stent-Assisted Coiling of Wide-Neck Intracranial Aneurysms Using Low-Profile Leo Baby Stents: Initial and Midterm Results. Am. J. Neuroradiol. 2015, 36, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Lubicz, B.; Kadou, A.; Morais, R.; Mine, B. Leo Stent for Endovascular Treatment of Intracranial Aneurysms: Very Long-Term Results in 50 Patients with 52 Aneurysms and Literature Review. Neuroradiology 2017, 59, 271–276. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Jiang, C.; Yang, X.; Wu, Z. Potential Advantages and Limitations of the Leo Stent in Endovascular Treatment of Complex Cerebral Aneurysms. Eur. J. Radiol. 2011, 79, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sedat, J.; Chau, Y.; Gaudart, J.; Sachet, M.; Beuil, S.; Lonjon, M. Stent-Assisted Coiling of Intracranial Aneurysms Using LEO Stents: Long-Term Follow-up in 153 Patients. Neuroradiology 2018, 60, 211–219. [Google Scholar] [CrossRef]
- Pardo, M.I.; Pumar, J.M.; Blanco, M.; Vazquez, F.; Guimaraens, L.; Casasco, A. Medium-Term Results Using the Leo Self-Expanding Stent in the Treatment of Complex Intracranial Aneurysms. Neuroradiol. J. 2008, 21, 704–711. [Google Scholar] [CrossRef]
- Machi, P.; Costalat, V.; Lobotesis, K.; Ruiz, C.; Cheikh, Y.B.; Eker, O.; Gascou, G.; Danière, F.; Riquelme, C.; Bonafé, A. LEO Baby Stent Use Following Balloon-Assisted Coiling: Single- and Dual-Stent Technique-Immediate and Midterm Results of 29 Consecutive Patients. Am. J. Neuroradiol. 2015, 36, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Voigt, P.; Schob, S.; Jantschke, R.; Nestler, U.; Krause, M.; Weise, D.; Lobsien, D.; Hoffmann, K.T.; Quäschling, U. Stent-Assisted Coiling of Ruptured and Incidental Aneurysms of the Intracranial Circulation Using Moderately Flow-Redirecting, Braided Leo Stents-Initial Experience in 39 Patients. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Juszkat, R.; Nowak, S.; Smól, S.; Kociemba, W.; Blok, T.; Zarzecka, A. Leo Stent for Endovascular Treatment of Broad-Necked and Fusiform Intracranial Aneurysms. Interv. Neuroradiol. 2007, 13, 255–269. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, J.; Gao, H.; Xu, F.; Bambakidis, N.C. Endovascular Treatment of Intracranial Aneurysms with the LVIS Device: A Systematic Review. J. Neurointerv. Surg. 2017, 9, 553–557. [Google Scholar] [CrossRef]
- Samaniego, E.A.; Mendez, A.A.; Nguyen, T.N.; Kalousek, V.; Guerrero, W.R.; Dandapat, S.; Dabus, G.; Linfante, I.; Hassan, A.E.; Drofa, A.; et al. LVIS Jr Device for Y-Stent-Assisted Coil Embolization of Wide-Neck Intracranial Aneurysms: A Multicenter Experience. Interv. Neurol. 2018, 7, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, C.K.; Lee, J.W.; Park, K.Y.; Chung, J. Preliminary Experience of Lvis Blue in the Internal Carotid Artery for The Treatment Of Wide-Necked Intracranial Aneurysms. J. Neurointensive Care 2019, 2, 52–57. [Google Scholar] [CrossRef]
- Lv, X.; Jiang, C.; Liang, S. Small Ruptured and Unruptured Complex Cerebral Aneurysms: Single Center Experience of Low-Profile Visualized Intraluminal Support Stent. J. Neurorestoratology 2019, 07, 235–241. [Google Scholar] [CrossRef]
- Tureli, D.; Sabet, S.; Senol, S.; Andac, N.; Donmez, H.; Geyik, S.; Baltacioglu, F.; Cekirge, S. Stent-Assisted Coil Embolization of Challenging Intracranial Aneurysms: Initial and Mid-Term Results with Low-Profile ACCLINO Devices. Acta Neurochir. 2016, 158, 1545–1553. [Google Scholar] [CrossRef]
- Brassel, F.; Grieb, D.; Meila, D.; Schlunz-Hendann, M.; Greling, B.; Melber, K. Endovascular Treatment of Complex Intracranial Aneurysms Using Acandis Acclino Stents. J. Neurointerventional Surg. 2017, 9, 854–859. [Google Scholar] [CrossRef]
- Goertz, L.; Smyk, M.A.; Mpotsaris, A.; Borggrefe, J.; Dorn, F.; Liebig, T.; Schlamann, M.; Laukamp, K.; Krischek, B.; Turowski, B.; et al. Long-Term Angiographic Results of the Low-Profile Acandis Acclino Stent for Treatment of Intracranial Aneurysms: A Multicenter Study. Clin. Neuroradiol. 2019, 1–8. [Google Scholar] [CrossRef]
- Kabbasch, C.; Liebig, T.; Faymonville, A.; Dorn, F.; Mpotsaris, A. Initial Clinical Experience with a New Self-Expanding Nitinol Microstent for the Treatment of Wide-Neck Intracranial Cerebral Aneurysms: The Acandis Acclino Stent. J. Vasc. Interv. Neurol. 2015, 8, 1–6. [Google Scholar]
- Dietrich, P.; Gravius, A.; Mühl-Benninghaus, R.; Yilmaz, U.; Kettner, M.; Bomberg, H.; Reith, W.; Simgen, A. Single Center Experience in Stent-Assisted Coiling of Complex Intracranial Aneurysms Using Low-Profile Stents: The ACCLINO® Stent Versus the ACCLINO® Flex Stent. Clin. Neuroradiol. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodayan, Y.; Rhodes, N.; Blackburn, S.; Derdeyn, C.P.; Cross, D.T.; Moran, C.J. Comparison of Enterprise With Neuroform Stent-Assisted Coiling of Intracranial Aneurysms. Am. J. Roentgenol. 2013, 200, 872–878. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J.; Meissner, I.; Brown, R.D.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured Intracranial Aneurysms: Natural History, Clinical Outcome, and Risks of Surgical and Endovascular Treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Sweid, A.; Rahm, S.P.; Das, S.; Baldassari, M.P.; Jabbour, P.; Alexander, T.D.; Velagapudi, L.; Chalouhi, N.; Gooch, M.R.; Herial, N.; et al. Safety and Efficacy of Bilateral Flow Diversion for Treatment of Anterior Circulation Cerebral Aneurysms. World Neurosurg 2019, 130, e1116–e1121. [Google Scholar] [CrossRef] [PubMed]
- Lieber, B.B.; Livescu, V.; Hopkins, L.N.; Wakhloo, A.K. Particle Image Velocimetry Assessment of Stent Design Influence on Intra-Aneurysmal Flow. Ann. Biomed. Eng. 2002, 30, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Xu, X.; Lee, J.S. The Effect of Stent Porosity and Strut Shape on Saccular Aneurysm and Its Numerical Analysis with Lattice Boltzmann Method. Ann. Biomed. Eng. 2010, 38, 2274–2292. [Google Scholar] [CrossRef]
- Liou, T.M.; Liou, S.N.; Chu, K.L. Intra-Aneurysmal Flow with Helix and Mesh Stent Placement across Side-Wall Aneurysm Pore of a Straight Parent Vessel. J. Biomech. Eng. 2004, 126, 36–43. [Google Scholar] [CrossRef]
- Turjman, F.; Acevedo, G.; Moll, T.; Duquesnel, J.; Eloy, R.; Sindou, M. Treatment of Experimental Carotid Aneurysms by Endoprosthesis Implantation: Preliminary Report. Neurol. Res. 1993, 15, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.M.; Kameda-Smith, M.; van Adel, B. A Review of Recent Advances in Endovascular Therapy for Intracranial Aneurysms. Crit. Rev. Biomed. Eng. 2018, 46, 369–397. [Google Scholar] [CrossRef] [PubMed]
- Lubicz, B.; Collignon, L.; Raphaeli, G.; Pruvo, J.P.; Bruneau, M.; De Witte, O.; Leclerc, X. Flow-Diverter Stent for the Endovascular Treatment of Intracranial Aneurysms: A Prospective Study in 29 Patients with 34 Aneurysms. Stroke 2010, 41, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Lylyk, P.; Miranda, C.; Ceratto, R.; Ferrario, A.; Scrivano, E.; Luna, H.R.; Berez, A.L.; Tran, Q.; Nelson, P.K.; Fiorella, D. Curative Endovascular Reconstruction of Cerebral Aneurysms with the Pipeline Embolization Device: The Buenos Aires Experience. Neurosurgery 2009, 64, 632–642. [Google Scholar] [CrossRef]
- Szikora, I.; Berentei, Z.; Kulcsar, Z.; Marosfoi, M.; Vajda, Z.S.; Lee, W.; Berez, A.; Nelson, P.K. Treatment of Intracranial Aneurysms by Functional Reconstruction of the Parent Artery: The Budapest Experience with the Pipeline Embolization Device. Am. J. Neuroradiol. 2010, 31, 1139–1147. [Google Scholar] [CrossRef]
- Nelson, P.K.; Lylyk, P.; Szikora, I.; Wetzel, S.G.; Wanke, I.; Fiorella, D. The Pipeline Embolization Device for the Intracranial Treatment of Aneurysms Trial. Am. J. Neuroradiol. 2011, 32, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Becske, T.; Kallmes, D.F.; Saatci, I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; Moran, C.J.; Woo, H.H.; Lopes, D.K.; Berez, A.L.; et al. Pipeline for Uncoilable or Failed Aneurysms: Results from a Multicenter Clinical Trial. Radiology 2013, 267, 858–868. [Google Scholar] [CrossRef]
- Atasoy, D.; Kandasamy, N.; Hart, J.; Lynch, J.; Yang, S.H.; Walsh, D.; Tolias, C.; Booth, T.C. Outcome Study of the Pipeline Embolization Device with Shield Technology in Unruptured Aneurysms (PEDSU). Am. Soc. Neuroradiol. 2019, 40, 2094–2101. [Google Scholar] [CrossRef]
- Trivelato, F.P.; Abud, D.G.; Ulhôa, A.C.; Waihrich, E.S.; Abud, T.G.; Castro Afonso, L.H.; Nakiri, G.S.; De Castro, G.D.; Parente, B.D.S.M.; Dos Santos Silva, R.; et al. Derivo Embolization Device for the Treatment of Intracranial Aneurysms: A Multicenter Study of 183 Aneurysms. Stroke 2019, 50, 2351–2358. [Google Scholar] [CrossRef]
- Trivelato, F.P.; Wajnberg, E.; Rezende, M.T.S.; Ulhôa, A.C.; Piske, R.L.; Abud, T.G.; de Castro-Afonso, L.H.; Abath, C.G.C.; Nakiri, G.S.; Araújo, J.F.S.; et al. Safety and Effectiveness of the Pipeline Flex Embolization Device With Shield Technology for the Treatment of Intracranial Aneurysms: Midterm Results From a Multicenter Study. Neurosurgery 2019. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Boogaarts, J.; Van Norden, A.; Wakhloo, A.K. New Generation of Flow Diverter (Surpass) for Unruptured Intracranial Aneurysms: A Prospective Single-Center Study in 37 Patients. Stroke 2013, 44, 1567–1577. [Google Scholar] [CrossRef]
- Wakhloo, A.K.; Lylyk, P.; De Vries, J.; Taschner, C.; Lundquist, J.; Biondi, A.; Hartmann, M.; Szikora, I.; Pierot, L.; Sakai, N.; et al. Surpass Flow Diverter in the Treatment of Intracranial Aneurysms: A Prospective Multicenter Study. Am. J. Neuroradiol. 2015, 36, 98–107. [Google Scholar] [CrossRef]
- Berge, J.; Biondi, A.; Machi, P.; Brunel, H.; Pierot, L.; Gabrillargues, J.; Kadziolka, K.; Barreau, X.; Dousset, V.; Bonafée, A. Flow-Diverter Silk Stent for the Treatment of Intracranial Aneurysms: 1-Year Follow-up in a Multicenter Study. Am. J. Neuroradiol. 2012, 33, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Möhlenbruch, M.A.; Herweh, C.; Jestaedt, L.; Stampfl, S.; Schönenberger, S.; Ringleb, P.A.; Bendszus, M.; Pham, M. The FRED Flow-Diverter Stent for Intracranial Aneurysms: Clinical Study to Assess Safety and Efficacy. Am. J. Neuroradiol. 2015, 36, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Daglioglu, E.; Akmangit, I.; Acik, V.; Alagoz, F.; Sayin, B.; Uckun, O.M.; Belen, A.D.; Arat, A. The Experience of the Derivo® Embolisation Device in Intracranial Aneurysms. Turk. Neurosurg. 2020, 30, 30–37. [Google Scholar] [CrossRef]
- Morais, R.; Mine, B.; Bruyère, P.J.; Naeije, G.; Lubicz, B. Endovascular Treatment of Intracranial Aneurysms with the P64 Flow Diverter Stent: Mid-Term Results in 35 Patients with 41 Intracranial Aneurysms. Neuroradiology 2017, 59, 263–269. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhou, Y.; Li, Y.; Li, T.; Leng, B.; Zhang, P.; Liang, G.; Huang, Q.; Yang, P.F.; Shi, H.; et al. Parent Artery Reconstruction for Large or Giant Cerebral Aneurysms Using the Tubridge Flow Diverter: A Multicenter, Randomized, Controlled Clinical Trial (PARAT). Am. J. Neuroradiol. 2018, 39, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Park, K.Y.; Lee, J.W.; Chung, J.; Kim, D.J.; Kim, D.I. A Newly-Developed Flow Diverter (Flowise) for Internal Carotid Artery Aneurysm: Results of a Pilot Clinical Study. Korean J. Radiol. 2019, 20, 505–512. [Google Scholar] [CrossRef]
- Sirakov, S.; Sirakov, A.; Bhogal, P.; Penkov, M.; Minkin, K.; Ninov, K.; Hristov, H.; Karakostov, V.; Raychev, R. The P64 Flow Diverter—Mid-Term and Long-Term Results from a Single Center. Clin. Neuroradiol. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Limbucci, N.; Leone, G.; Renieri, L.; Nappini, S.; Cagnazzo, F.; Laiso, A.; Muto, M.; Mangiafico, S. Expanding Indications for Flow Diverters: Distal Aneurysms, Bifurcation Aneurysms, Small Aneurysms, Previously Coiled Aneurysms and Clipped Aneurysms, and Carotid Cavernous Fistulas. Neurosurgery 2020, 86, S85–S94. [Google Scholar] [CrossRef]
- Briganti, F.; Delehaye, L.; Leone, G.; Sicignano, C.; Buono, G.; Marseglia, M.; Caranci, F.; Tortora, F.; Maiuri, F. Flow Diverter Device for the Treatment of Small Middle Cerebral Artery Aneurysms. J. Neurointerventional Surg. 2016, 8, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Leone, G.; Ugga, L.; Marseglia, M.; Solari, D.; Caranci, F.; Mariniello, G.; Maiuri, F.; Cappabianca, P. Safety and Efficacy of Flow Re-Direction Endoluminal Device (FRED) in the Treatment of Cerebral Aneurysms: A Single Center Experience. Acta Neurochir. 2016, 158, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Spelle, L.; Berge, J.; Januel, A.C.; Herbreteau, D.; Aggour, M.; Piotin, M.; Biondi, A.; Barreau, X.; Mounayer, C.; et al. SAFE Study (Safety and Efficacy Analysis of FRED Embolic Device in Aneurysm Treatment): 1-Year Clinical and Anatomical Results. J. Neurointerventional Surg. 2019, 11, 184–189. [Google Scholar] [CrossRef]
- Strauss, I.; Maimon, S. Silk Flow Diverter in the Treatment of Complex Intracranial Aneurysms: A Single-Center Experience with 60 Patients. Acta Neurochir. 2016, 158, 247–254. [Google Scholar] [CrossRef]
- Pumar, J.M.; Mosqueira, A.; Cuellar, H.; Dieguez, B.; Guimaraens, L.; Masso, J.; Miralbes, S.; Blanco-Ulla, M.; Souto-Bayarri, M.; Vazquez-Herrero, F. Expanding the Use of Flow Diverters beyond Their Initial Indication: Treatment of Small Unruptured Aneurysms. J. Neurointerventional Surg. 2018, 10, 245–248. [Google Scholar] [CrossRef]
- Martínez-Galdámez, M.; Biondi, A.; Kalousek, V.; Pereira, V.M.; Ianucci, G.; Gentric, J.C.; Mosimann, P.J.; Brisbois, D.; Schob, S.; Quäschling, U.; et al. Periprocedural Safety and Technical Outcomes of the New Silk Vista Baby Flow Diverter for the Treatment of Intracranial Aneurysms: Results from a Multicenter Experience. J. Neurointerventional Surg. 2019, 11, 723–727. [Google Scholar] [CrossRef]
- Chalouhi, N.; Zanaty, M.; Whiting, A.; Tjoumakaris, S.; Hasan, D.; Ajiboye, N.; Hann, S.; Rosenwasser, R.H.; Jabbour, P. Treatment of Ruptured Intracranial Aneurysms with the Pipeline Embolization Device. Neurosurgery 2015, 76, 165–172. [Google Scholar] [CrossRef]
- McAuliffe, W.; Wenderoth, J.D. Immediate and Midterm Results Following Treatment of Recently Ruptured Intracranial Aneurysms with the Pipeline Embolization Device. Am. J. Neuroradiol. 2012, 33, 487–493. [Google Scholar] [CrossRef]
- Piotin, M.; Blanc, R.; Spelle, L.; Mounayer, C.; Piantino, R.; Schmidt, P.J.; Moret, J. Stent-Assisted Coiling of Intracranial Aneurysms: Clinical and Angiographic Results in 216 Consecutive Aneurysms. Stroke 2010, 41, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Park, H.; Bang, J.S.; Jin, S.C.; Kim, B.C.; Oh, C.W.; Kang, H.S.; Han, M.H.; Kwon, O.K. Comparison of 2-Year Angiographic Outcomes of Stent- and Nonstent-Assisted Coil Embolization in Unruptured Aneurysms with an Unfavorable Configuration for Coiling. Am. J. Neuroradiol. 2011, 32, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Jahshan, S.; Abla, A.A.; Natarajan, S.K.; Drummond, P.S.; Kan, P.; Karmon, Y.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Results of Stent-Assisted vs Non-Stent-Assisted Endovascular Therapies in 489 Cerebral Aneurysms: Single-Center Experience. Clin. Neurosurg. 2013, 72, 232–239. [Google Scholar] [CrossRef]
- van Rooij, S.; Sprengers, M.; Peluso, J.; Daams, J.; Verbaan, D.; van Rooij, W.; Majoie, C. A Systematic Review and Meta-Analysis of Woven EndoBridge Single Layer for Treatment of Intracranial Aneurysms. Interv. Neuroradiol. 2020, 159101992090442. [Google Scholar] [CrossRef] [PubMed]
- Haffaf, I.; Clarençon, F.; Shotar, E.; Rolla-Bigliani, C.; Vande Perre, S.; Mathon, B.; Drir, M.; Sourour, N.A. Medina Embolization Device for the Treatment of Intracranial Aneurysms: 18 Months’ Angiographic Results. J. Neurointerventional Surg. 2019, 11, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Bhogal, P.; Moreno, R.M.; Bäzner, H.; Ganslandt, O.; Henkes, H. The Medina Embolic Device: Early Clinical Experience from a Single Center. J. Neurointerventional Surg. 2017, 9, 77–87. [Google Scholar] [CrossRef]
- Sourour, N.-A.; Vande Perre, S.; Maria, F.D.; Papagiannaki, C.; Gabrieli, J.; Pistocchi, S.; Bartolini, B.; Degos, V.; Carpentier, A.; Chiras, J.; et al. Medina® Embolization Device for the Treatment of Intracranial Aneurysms: Safety and Angiographic Effectiveness at 6 Months. Neurosurgery 2017, 82, 155–162. [Google Scholar] [CrossRef]
- Bhogal, P.; Brouwer, P.A.; Yeo, L.; Svensson, M.; Söderman, M. The Medina Embolic Device: Karolinska Experience. Interv. Neuroradiol. 2018, 24, 4–13. [Google Scholar] [CrossRef]
- Piotin, M.; Biondi, A.; Sourour, N.; Mounayer, C.; Jaworski, M.; Mangiafico, S.; Andersson, T.; Soderman, M.; Goffette, P.; Anxionnat, R.; et al. The LUNA Aneurysm Embolization System for Intracranial Aneurysm Treatment: Short-Term, Mid-Term and Long-Term Clinical and Angiographic Results. J. Neurointerventional Surg. 2018, 10, E34. [Google Scholar] [CrossRef]
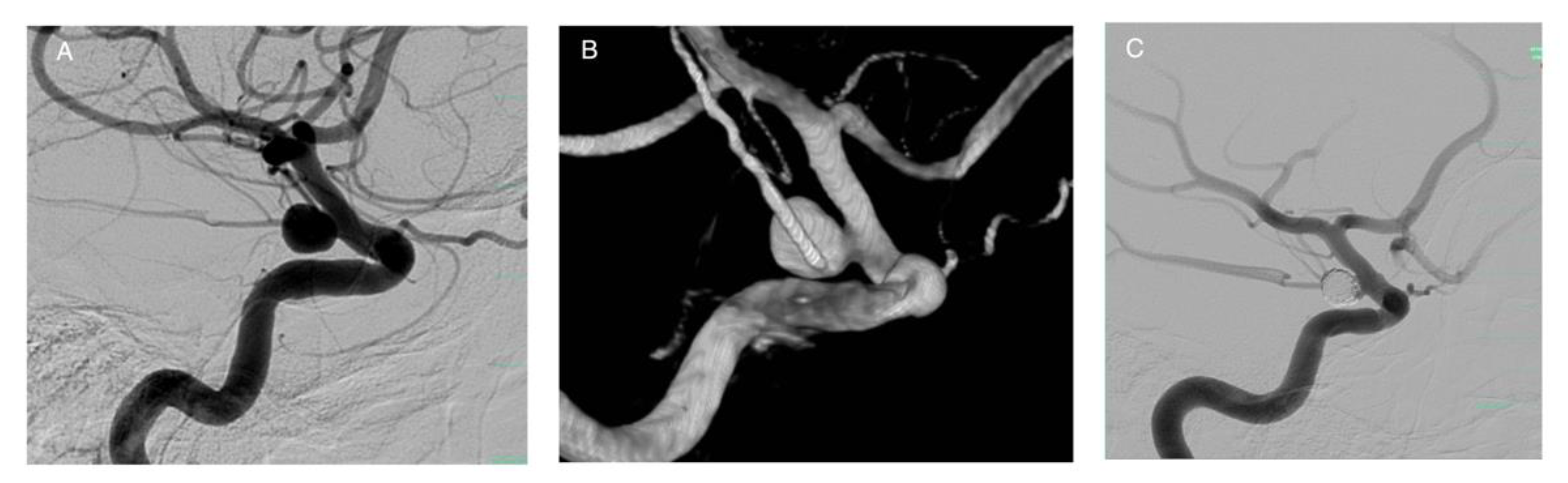
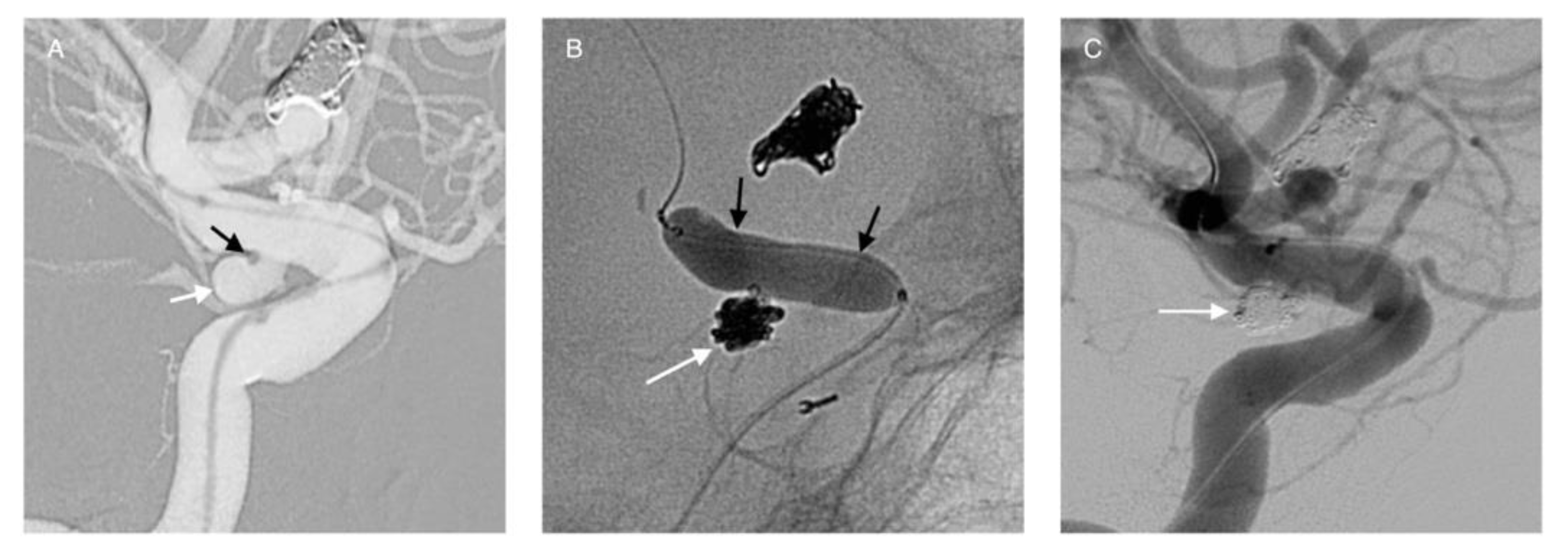
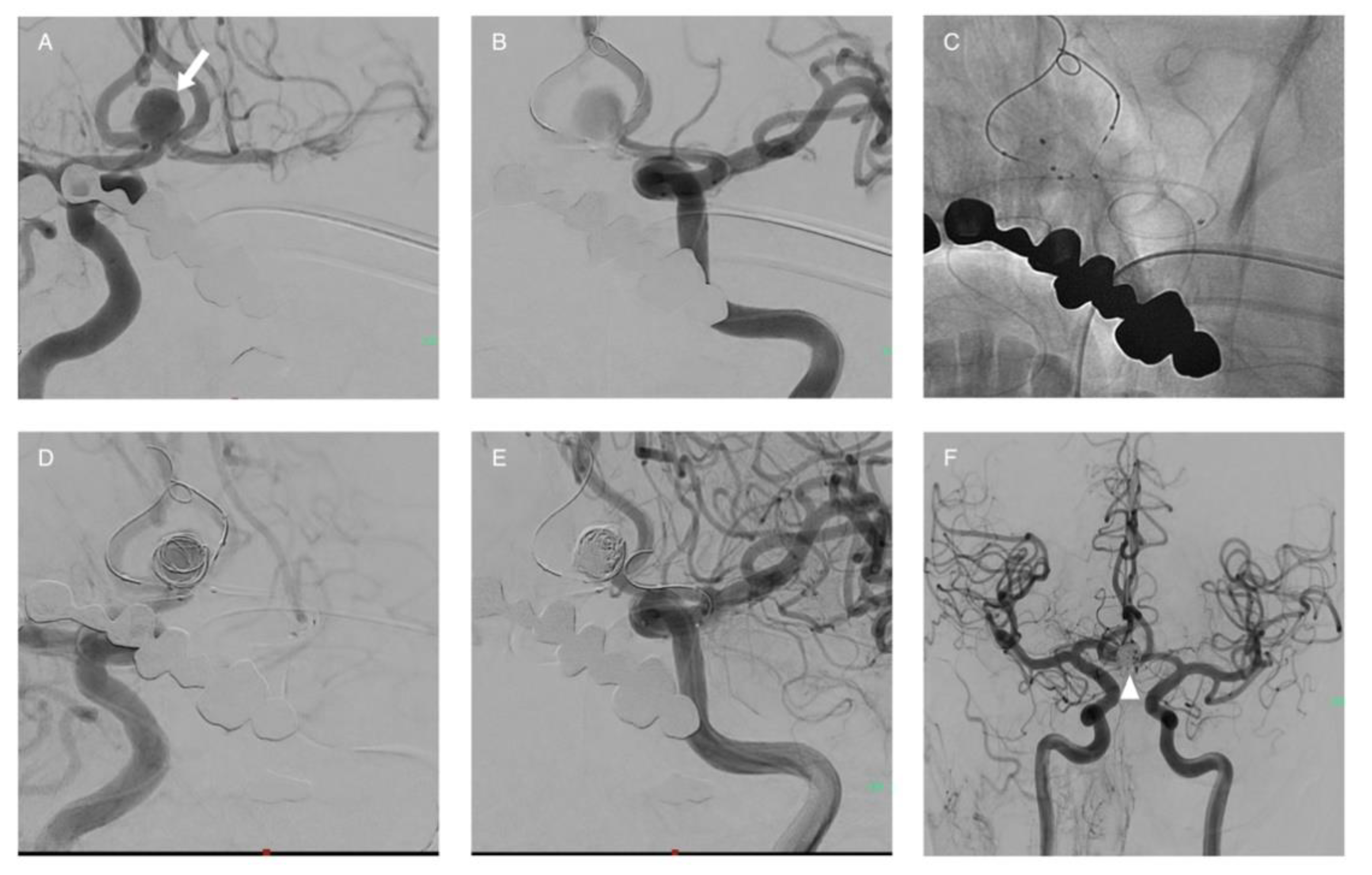
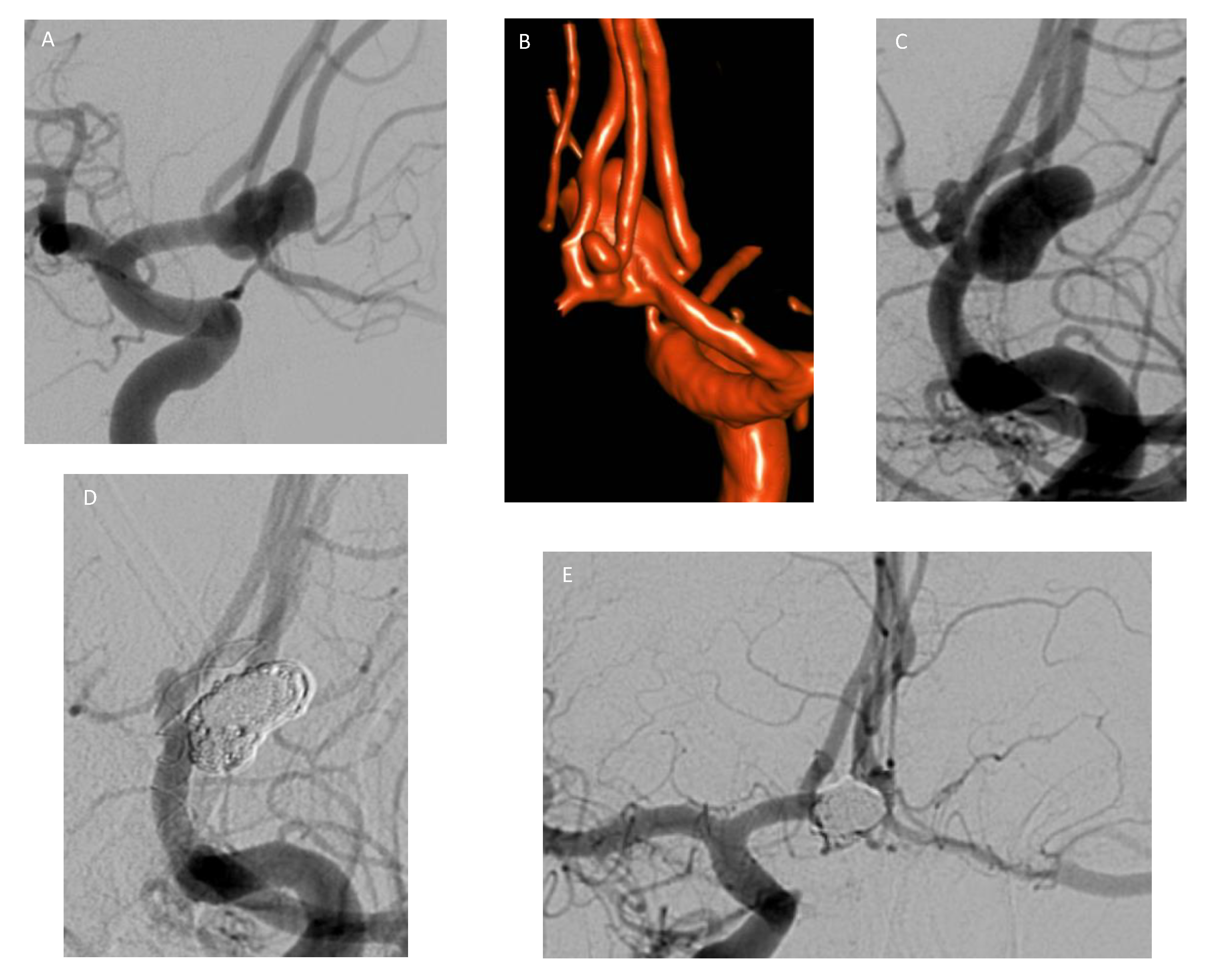
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamou, A.; Alexandrou, M.; Roth, C.; Chatziioannou, A.; Papanagiotou, P. Endovascular Treatment of Intracranial Aneurysms. Life 2021, 11, 335. https://doi.org/10.3390/life11040335
Adamou A, Alexandrou M, Roth C, Chatziioannou A, Papanagiotou P. Endovascular Treatment of Intracranial Aneurysms. Life. 2021; 11(4):335. https://doi.org/10.3390/life11040335
Chicago/Turabian StyleAdamou, Antonis, Maria Alexandrou, Christian Roth, Achilles Chatziioannou, and Panagiotis Papanagiotou. 2021. "Endovascular Treatment of Intracranial Aneurysms" Life 11, no. 4: 335. https://doi.org/10.3390/life11040335
APA StyleAdamou, A., Alexandrou, M., Roth, C., Chatziioannou, A., & Papanagiotou, P. (2021). Endovascular Treatment of Intracranial Aneurysms. Life, 11(4), 335. https://doi.org/10.3390/life11040335





