Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good?
Abstract
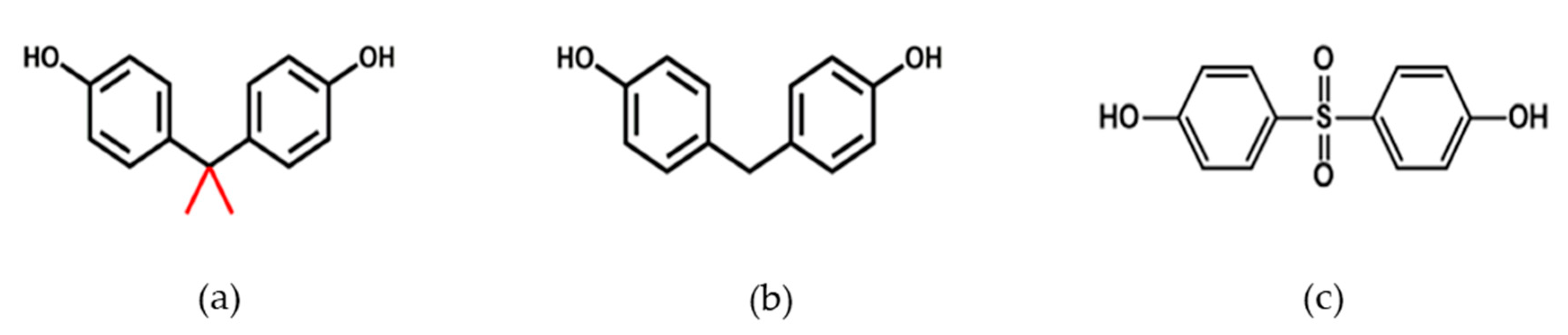
1. Introduction
2. Materials and Methods
3. Results
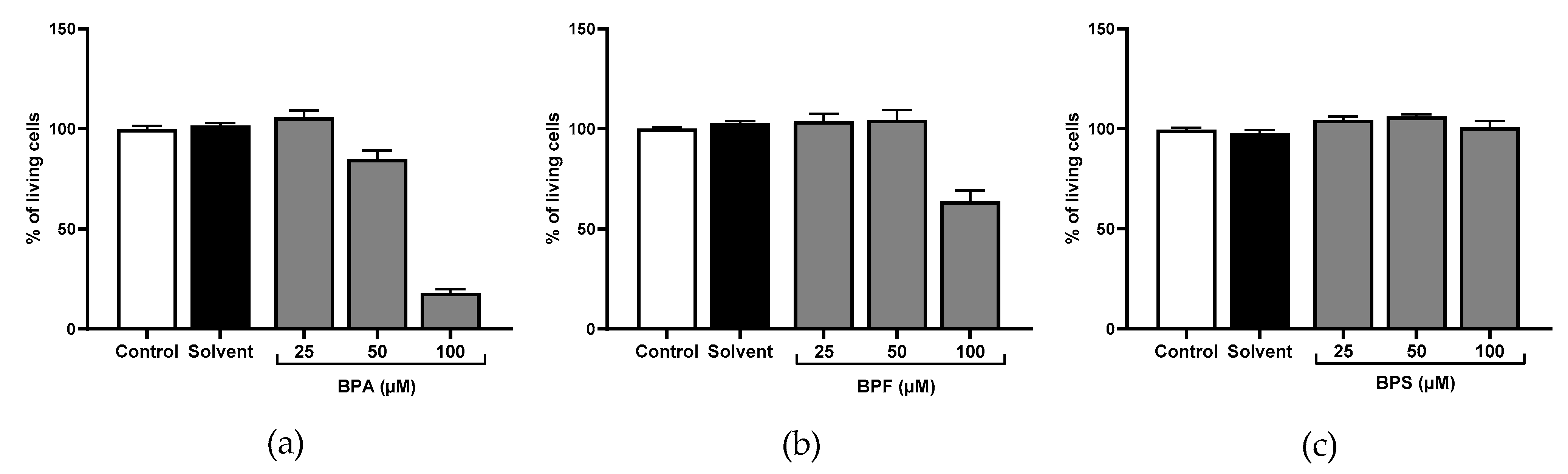
3.1. Cell Viability
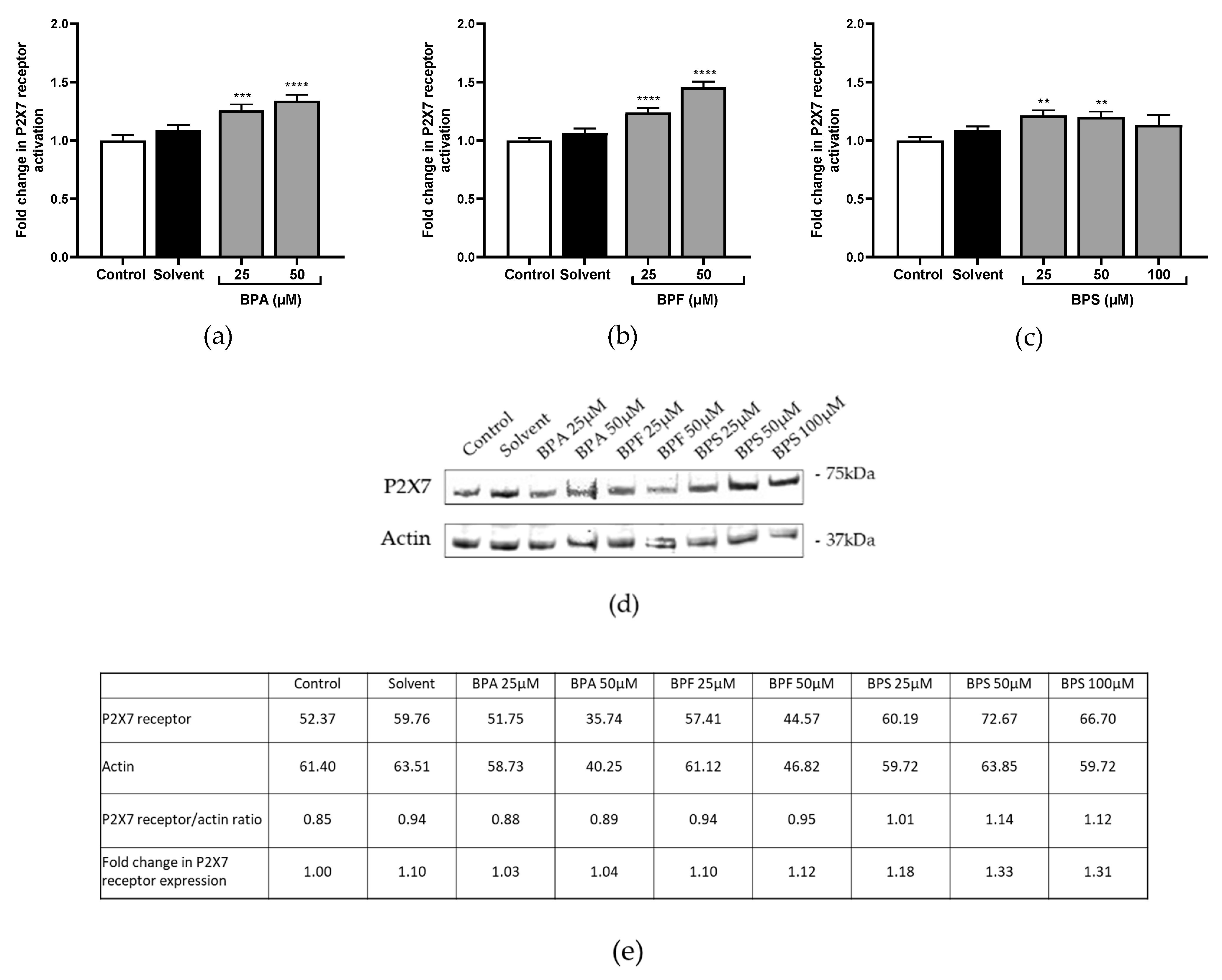
3.2. P2X7 Receptor Activation and Expression
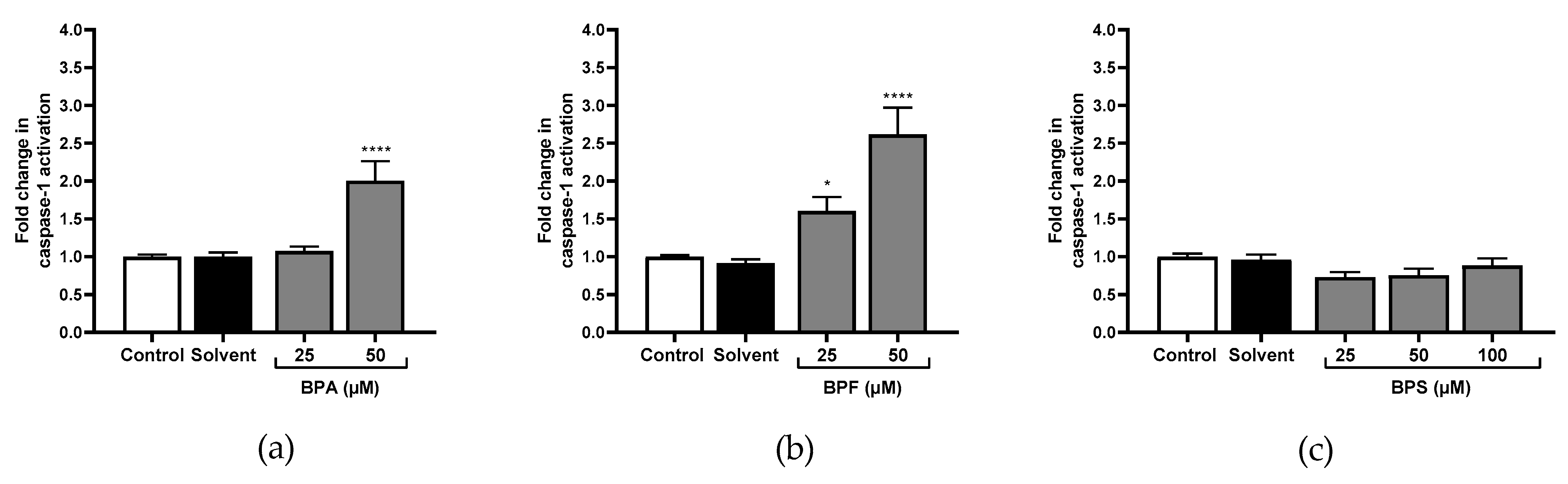
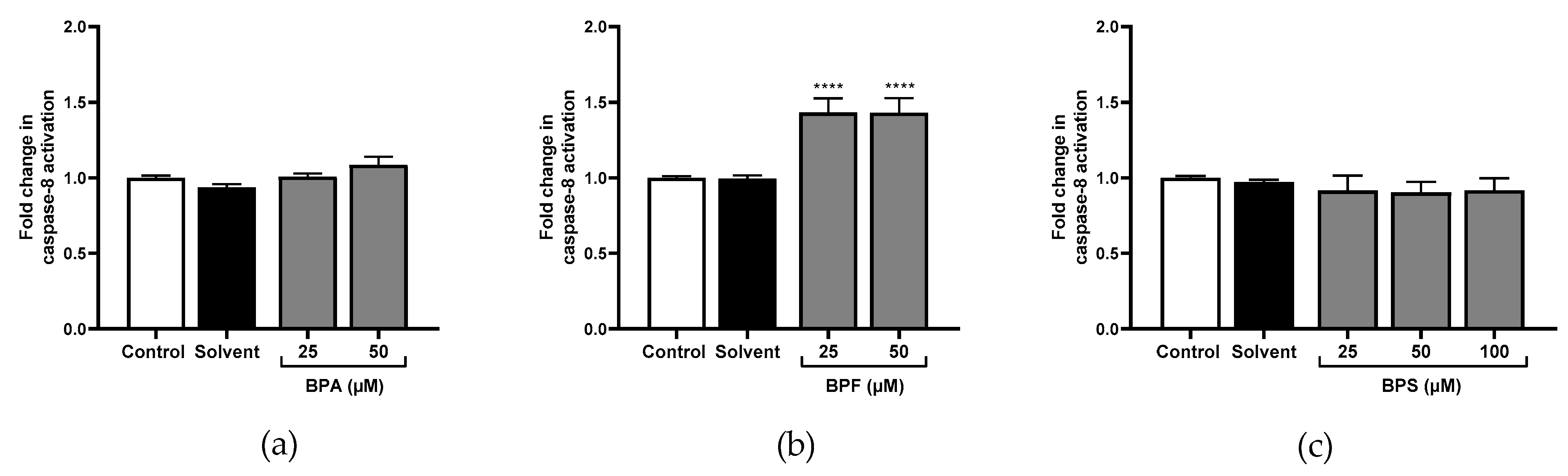
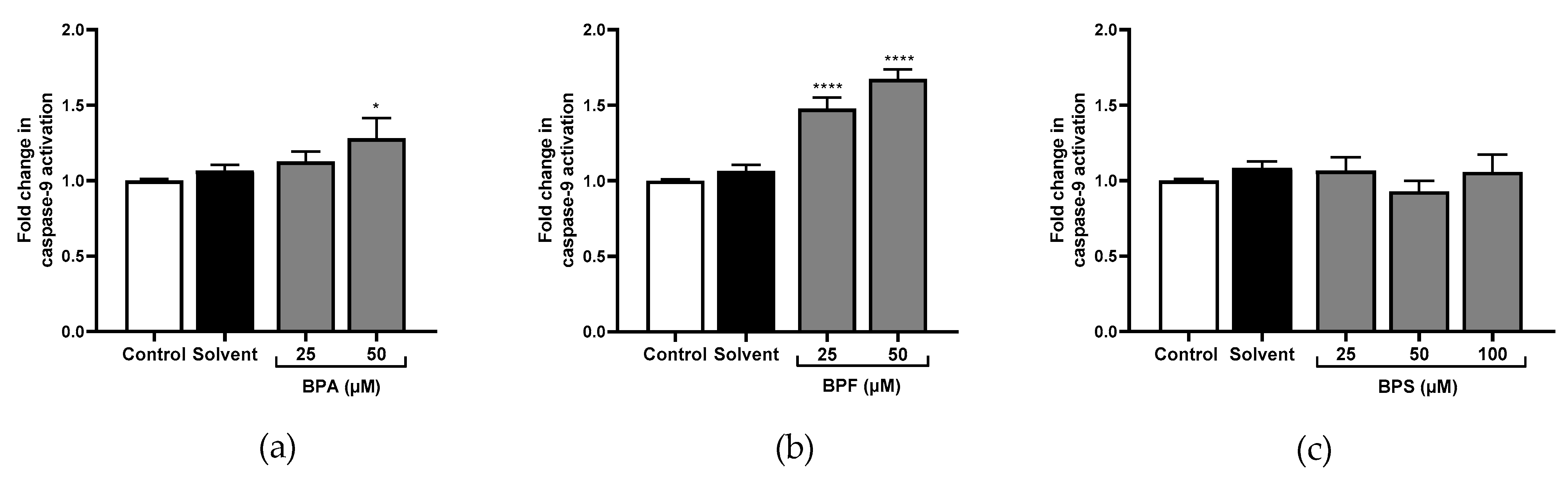
3.3. Caspase-1, Caspase-8, Caspase-9, and Caspase-3 Activity
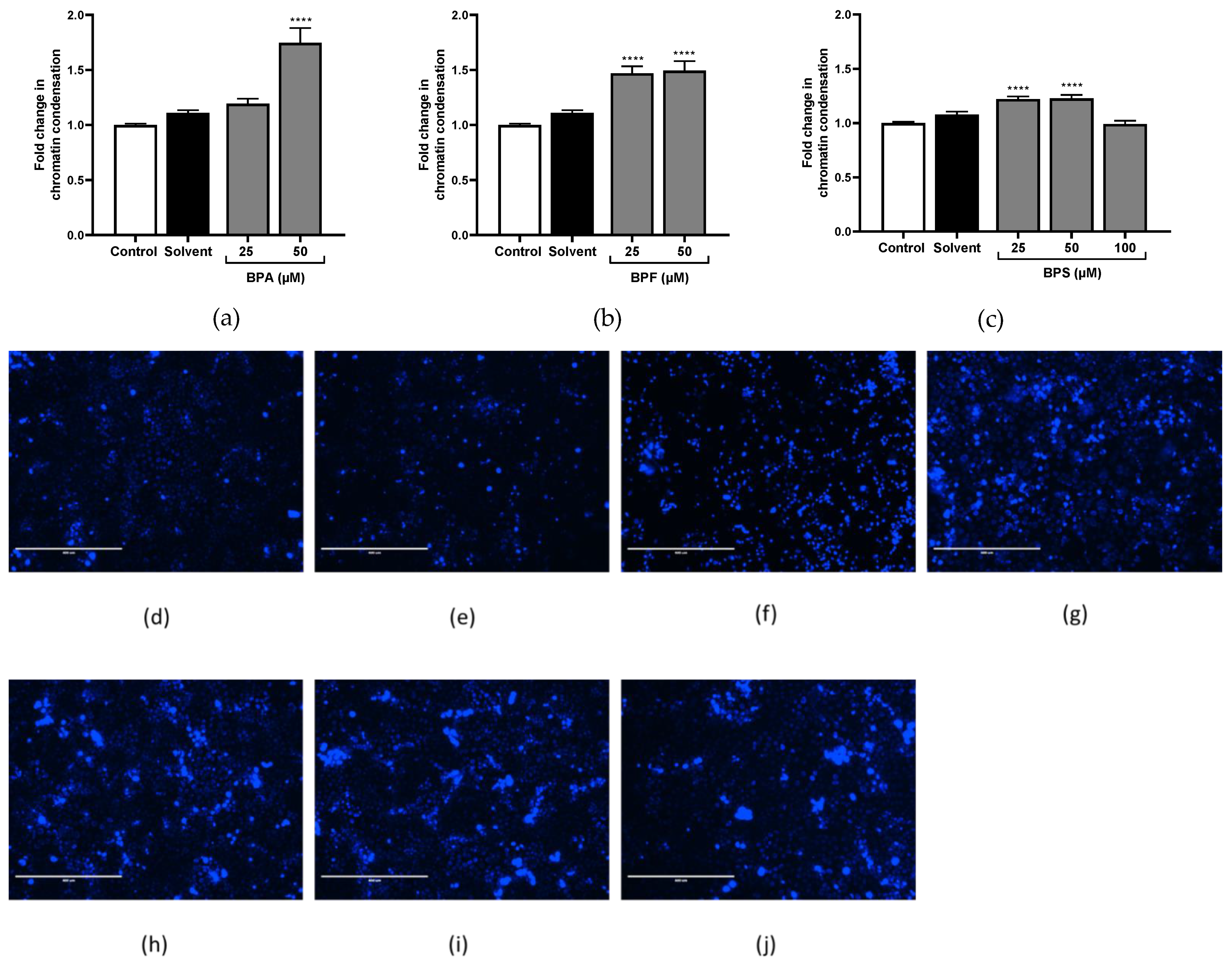
3.4. Chromatin Condensation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent Bisphenol A Accumulation in the Human Maternal-Fetal-Placental Unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.; Schoenfelder, G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, F.; Dubois, M.-F.; Aris, A. Maternal, Placental and Fetal Exposure to Bisphenol A in Women with and without Preeclampsia. Hypertens. Pregnancy 2014, 33, 341–348. [Google Scholar] [CrossRef]
- Goldman-Wohl, D.; Yagel, S. Regulation of Trophoblast Invasion: From Normal Implantation to Pre-Eclampsia. Mol. Cell Endocrinol. 2002, 187, 233–238. [Google Scholar] [CrossRef]
- Sebire, N.J.; Foskett, M.; Fisher, R.A.; Rees, H.; Seckl, M.; Newlands, E. Risk of Partial and Complete Hydatidiform Molar Pregnancy in Relation to Maternal Age. BJOG 2002, 109, 99–102. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Placental Oxidative Stress: From Miscarriage to Preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef]
- Ball, E.; Bulmer, J.N.; Ayis, S.; Lyall, F.; Robson, S.C. Late Sporadic Miscarriage Is Associated with Abnormalities in Spiral Artery Transformation and Trophoblast Invasion. J. Pathol. 2006, 208, 535–542. [Google Scholar] [CrossRef]
- Duley, L. The Global Impact of Pre-Eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Carty, D.M.; Delles, C.; Dominiczak, A.F. Preeclampsia and Future Maternal Health. J. Hypertens. 2010, 28, 1349–1355. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” Are Associated with Disorders of Deep Placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.E.; Meeker, J.D.; Ferguson, K.K.; Mukherjee, B.; Hauser, R.; McElrath, T.F. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ. Health Perspect. 2016, 124, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Tassinari, R.; Maranghi, F.; Mantovani, A. Bisphenol A Affects Placental Layers Morphology and Angiogenesis during Early Pregnancy Phase in Mice. J. Appl. Toxicol. 2015, 35, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Benachour, N.; Aris, A. Toxic Effects of Low Doses of Bisphenol-A on Human Placental Cells. Toxicol. Appl. Pharmacol. 2009, 241, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tsimis, M.E.; Lei, J.; Rosenzweig, J.M.; Arif, H.; Shabi, Y.; Alshehri, W.; Talbot, C.C.; Baig-Ward, K.M.; Segars, J.; Graham, E.M.; et al. P2X7 Receptor Blockade Prevents Preterm Birth and Perinatal Brain Injury in a Mouse Model of Intrauterine Inflammation. Biol. Reprod. 2017, 97, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Fodor, P.; White, B.; Khan, R. Inflammation-The Role of ATP in Pre-Eclampsia. Microcirculation 2020, 27, e12585. [Google Scholar] [CrossRef] [PubMed]
- Roberts, V.H.J.; Greenwood, S.L.; Elliott, A.C.; Sibley, C.P.; Waters, L.H. Purinergic Receptors in Human Placenta: Evidence for Functionally Active P2X4, P2X7, P2Y2, and P2Y6. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1374–R1386. [Google Scholar] [CrossRef]
- Barakonyi, A.; Miko, E.; Szereday, L.; Polgar, P.D.; Nemeth, T.; Szekeres-Bartho, J.; Engels, G.L. Cell Death Mechanisms and Potentially Cytotoxic Natural Immune Cells in Human Pregnancies Complicated by Preeclampsia. Reprod. Sci. 2014, 21, 155–166. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Takahashi, M. Role of the NLRP3 Inflammasome in Preeclampsia. Front. Endocrinol. (Lausanne) 2020, 11, 80. [Google Scholar] [CrossRef]
- Wakx, A.; Regazzetti, A.; Dargère, D.; Auzeil, N.; Gil, S.; Evain-Brion, D.; Laprévote, O.; Rat, P. New in Vitro Biomarkers to Detect Toxicity in Human Placental Cells: The Example of Benzo[A]Pyrene. Toxicol. In Vitro 2016, 32, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Wakx, A.; Fouyet, S.; Dutot, M.; Rat, P. JEG-3 Placental Cells in Toxicology Studies: A Promising Tool to Reveal Pregnancy Disorders. Anat. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Daoud, G.; Barrak, J.; Abou-Kheir, W. Assessment Of Different Trophoblast Cell Lines As In Vitro Models For Placental Development. FASEB J. 2016, 30, 1247.18. [Google Scholar] [CrossRef]
- Rat, P.; Olivier, E.; Tanter, C.; Wakx, A.; Dutot, M. A Fast and Reproducible Cell- and 96-Well Plate-Based Method for the Evaluation of P2X7 Receptor Activation Using YO-PRO-1 Fluorescent Dye. J. Biol. Methods 2017, 4, e64. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling Nuclear DNA with Hoechst 33342. Cold Spring Harb. Protoc. 2011, 2011, pdb.prot5557. [Google Scholar] [CrossRef]
- Spagnol, S.T.; Dahl, K.N. Spatially Resolved Quantification of Chromatin Condensation through Differential Local Rheology in Cell Nuclei Fluorescence Lifetime Imaging. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of Bisphenol A Concentrations in Human Biological Fluids Reveals Significant Early Prenatal Exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef]
- Caserta, D.; Di Segni, N.; Mallozzi, M.; Giovanale, V.; Mantovani, A.; Marci, R.; Moscarini, M. Bisphenol a and the Female Reproductive Tract: An Overview of Recent Laboratory Evidence and Epidemiological Studies. Reprod. Biol. Endocrinol. 2014, 12, 37. [Google Scholar] [CrossRef]
- Feng, M.J.; Wu, X.Q.; Li, J.; Ding, L.; Wang, Z.Q.; Shen, Y.; Song, Z.C.; Wang, L.; Yang, Q.; Wang, X.P.; et al. [Relationship between daily exposure to bisphenol A and male sexual function-a study from the reproductive center]. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 836–840. [Google Scholar] [CrossRef]
- Legeay, S.; Faure, S. Is Bisphenol A an Environmental Obesogen? Fundam Clin. Pharmacol. 2017, 31, 594–609. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A Substitutes and Obesity in US Adults: Analysis of a Population-Based, Cross-Sectional Study. Lancet Planet Health 2017, 1, e114–e122. [Google Scholar] [CrossRef]
- Roen, E.L.; Wang, Y.; Calafat, A.M.; Wang, S.; Margolis, A.; Herbstman, J.; Hoepner, L.A.; Rauh, V.; Perera, F.P. Bisphenol A Exposure and Behavioral Problems among Inner City Children at 7–9 Years of Age. Environ. Res. 2015, 142, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Davis, K.; Boylan, K.; Fisher, M.; Fu, J. Bisphenol A, Phthalates and Lead and Learning and Behavioral Problems in Canadian Children 6–11 Years of Age: CHMS 2007–2009. Neurotoxicology 2016, 54, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.-M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A Review of the Carcinogenic Potential of Bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.-K.; Govindarajah, V.; Cheong, A.; Veevers, J.; Song, D.; Gear, R.; Zhu, X.; Ying, J.; Kendler, A.; Medvedovic, M.; et al. Gestational High-Fat Diet and Bisphenol A Exposure Heightens Mammary Cancer Risk. Endocr. Relat. Cancer 2017, 24, 365–378. [Google Scholar] [CrossRef]
- Xu, F.; Wang, X.; Wu, N.; He, S.; Yi, W.; Xiang, S.; Zhang, P.; Xie, X.; Ying, C. Bisphenol A Induces Proliferative Effects on Both Breast Cancer Cells and Vascular Endothelial Cells through a Shared GPER-Dependent Pathway in Hypoxia. Environ. Pollut. 2017, 231, 1609–1620. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Kotrogianni, P.; Christopoulos-Timogiannakis, E.; Koutaki, D.; Daskalakis, G.; Papantoniou, N. Bisphenol A and Adverse Pregnancy Outcomes: A Systematic Review of the Literature. J. Matern. Fetal Neonatal Med. 2018, 31, 3320–3327. [Google Scholar] [CrossRef]
- Cabaton, N.; Chagnon, M.-C.; Lhuguenot, J.-C.; Cravedi, J.-P.; Zalko, D. Disposition and Metabolic Profiling of Bisphenol F in Pregnant and Nonpregnant Rats. J. Agric. Food Chem. 2006, 54, 10307–10314. [Google Scholar] [CrossRef]
- Lehmler, H.-J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Liu, C.; Shen, Y.; Wang, Q.; Pan, A.; Yang, P.; Chen, Y.-J.; Deng, Y.-L.; Lu, Q.; Cheng, L.-M.; et al. Urinary Levels of Bisphenol A, F and S and Markers of Oxidative Stress among Healthy Adult Men: Variability and Association Analysis. Environ. Int. 2019, 123, 301–309. [Google Scholar] [CrossRef]
- Ihde, E.S.; Zamudio, S.; Loh, J.M.; Zhu, Y.; Woytanowski, J.; Rosen, L.; Liu, M.; Buckley, B. Application of a Novel Mass Spectrometric (MS) Method to Examine Exposure to Bisphenol-A and Common Substitutes in a Maternal Fetal Cohort. Hum. Ecol. Risk Assess. 2018, 24, 331–346. [Google Scholar] [CrossRef]
- Wu, L.-H.; Zhang, X.-M.; Wang, F.; Gao, C.-J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of Bisphenol S in the Environment and Implications for Human Exposure: A Short Review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, M.; Li, J.; Du, B.; Lan, S.; Liang, X.; Zeng, L. Occurrence and Maternal Transfer of Multiple Bisphenols, Including an Emerging Derivative with Unexpectedly High Concentrations, in the Human Maternal-Fetal-Placental Unit. Environ. Sci. Technol. 2020, 54, 3476–3486. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, R. Placental Toxicity. In Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 68; pp. 1301–1325. [Google Scholar]
- Liu, J.; Li, J.; Wu, Y.; Zhao, Y.; Luo, F.; Li, S.; Yang, L.; Moez, E.K.; Dinu, I.; Martin, J.W. Bisphenol A Metabolites and Bisphenol S in Paired Maternal and Cord Serum. Environ. Sci. Technol. 2017, 51, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Grandin, F.C.; Lacroix, M.Z.; Gayrard, V.; Viguié, C.; Mila, H.; de Place, A.; Vayssière, C.; Morin, M.; Corbett, J.; Gayrard, C.; et al. Is Bisphenol S a Safer Alternative to Bisphenol A in Terms of Potential Fetal Exposure? Placental Transfer across the Perfused Human Placenta. Chemosphere 2019, 221, 471–478. [Google Scholar] [CrossRef]
- Gingrich, J.; Pu, Y.; Roberts, J.; Karthikraj, R.; Kannan, K.; Ehrhardt, R.; Veiga-Lopez, A. Gestational Bisphenol S Impairs Placental Endocrine Function and the Fusogenic Trophoblast Signaling Pathway. Arch. Toxicol. 2018, 92, 1861–1876. [Google Scholar] [CrossRef]
- Da Silva, B.S.; Pietrobon, C.B.; Bertasso, I.M.; Lopes, B.P.; Carvalho, J.C.; Peixoto-Silva, N.; Santos, T.R.; Claudio-Neto, S.; Manhães, A.C.; Oliveira, E.; et al. Short and Long-Term Effects of Bisphenol S (BPS) Exposure during Pregnancy and Lactation on Plasma Lipids, Hormones, and Behavior in Rats. Environ. Pollut. 2019, 250, 312–322. [Google Scholar] [CrossRef]
- Mao, J.; Jain, A.; Denslow, N.D.; Nouri, M.-Z.; Chen, S.; Wang, T.; Zhu, N.; Koh, J.; Sarma, S.J.; Sumner, B.W.; et al. Bisphenol A and Bisphenol S Disruptions of the Mouse Placenta and Potential Effects on the Placenta-Brain Axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4642–4652. [Google Scholar] [CrossRef]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only Humans Have Human Placentas: Molecular Differences between Mice and Humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-Beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Ogura, Y.; Sutterwala, F.S.; Flavell, R.A. The Inflammasome: First Line of the Immune Response to Cell Stress. Cell 2006, 126, 659–662. [Google Scholar] [CrossRef]
- Zheng, L.M.; Zychlinsky, A.; Liu, C.C.; Ojcius, D.M.; Young, J.D. Extracellular ATP as a Trigger for Apoptosis or Programmed Cell Death. J. Cell Biol. 1991, 112, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.B.; Young, M.T.; Adinolfi, E.; Surprenant, A. Pseudoapoptosis Induced by Brief Activation of ATP-Gated P2X7 Receptors. J. Biol. Chem. 2005, 280, 33968–33976. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Los, M.; Bauer, M.K.; Vandenabeele, P.; Wesselborg, S.; Schulze-Osthoff, K. P2Z Purinoreceptor Ligation Induces Activation of Caspases with Distinct Roles in Apoptotic and Necrotic Alterations of Cell Death. FEBS Lett. 1999, 447, 71–75. [Google Scholar] [CrossRef]
- Aguirre, A.; Shoji, K.F.; Sáez, J.C.; Henríquez, M.; Quest, A.F.G. FasL-Triggered Death of Jurkat Cells Requires Caspase 8-Induced, ATP-Dependent Cross-Talk between Fas and the Purinergic Receptor P2X(7). J. Cell Physiol. 2013, 228, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.; Feng, Y.-H.; Li, X.; Zeng, R.; Gorodeski, G.I. P2X7 Receptor-Mediated Apoptosis of Human Cervical Epithelial Cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1349–C1358. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging Roles of Caspase-3 in Apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Sunilkumar, D.; Drishya, G.; Chandrasekharan, A.; Shaji, S.K.; Bose, C.; Jossart, J.; Perry, J.J.P.; Mishra, N.; Kumar, G.B.; Nair, B.G. Oxyresveratrol Drives Caspase-Independent Apoptosis-like Cell Death in MDA-MB-231 Breast Cancer Cells through the Induction of ROS. Biochem. Pharmacol. 2020, 173, 113724. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life 2021, 11, 314. https://doi.org/10.3390/life11040314
Fouyet S, Olivier E, Leproux P, Dutot M, Rat P. Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life. 2021; 11(4):314. https://doi.org/10.3390/life11040314
Chicago/Turabian StyleFouyet, Sophie, Elodie Olivier, Pascale Leproux, Mélody Dutot, and Patrice Rat. 2021. "Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good?" Life 11, no. 4: 314. https://doi.org/10.3390/life11040314
APA StyleFouyet, S., Olivier, E., Leproux, P., Dutot, M., & Rat, P. (2021). Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life, 11(4), 314. https://doi.org/10.3390/life11040314





