Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serology
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Secchi, M.; Bazzigaluppi, E.; Brigatti, C.; Marzinotto, I.; Tresoldi, C.; Rovere-Querini, P.; Poli, A.; Castagna, A.; Scarlatti, G.; Zangrillo, A.; et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J. Clin. Investig. 2020, 130, 6366–6378. [Google Scholar] [CrossRef]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Cheng, Y.; Ling, R.; Dai, Y.; Huang, B.; Huang, W.; Zhang, S.; Jiang, Y. Antibody-dependent enhancement of coronavirus. Int. J. Infect. Dis. 2020, 100, 483–489. [Google Scholar] [CrossRef]
- Dijkstra, J.M.; Hashimoto, K. Expected immune recognition of COVID-19 virus by memory from earlier infections with common coronaviruses in a large part of the world population. F1000Research 2020, 9, 285. [Google Scholar] [CrossRef]
- WHO. R & D Blueprint—Novel Coronavirus COVID19 Therapeutic Trial Synopsis. 2020. Available online: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf?ua=1 (accessed on 1 October 2020).
- Barros, A.J.; Hirakata, V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef]
- Devarakonda, C.K.V.; Meredith, E.; Ghosh, M.; Shapiro, L.H. Coronavirus Receptors as Immune Modulators. J. Immunol. 2020, 206, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Loos, C.; Atyeo, C.; Fischinger, S.; Burke, J.; Slein, M.D.; Streeck, H.; Lauffenburger, D.; Ryan, E.T.; Charles, R.C.; Alter, G. Evolution of Early SARS-CoV-2 and Cross-Coronavirus Immunity. mSphere 2020, 5, e00622-20. [Google Scholar] [CrossRef]
- Hicks, J.; Klumpp-Thomas, C.; Kalish, H.; Shunmugavel, A.; Mehalko, J.; Denson, J.P.; Snead, K.; Drew, M.; Corbett, K.; Graham, B.; et al. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal Betacoronaviruses. medRxiv 2020. [Google Scholar]
- Poston, D.; Weisblum, Y.; Wise, H.; Templeton, K.; Jenks, S.; Hatziioannou, T.; Bieniasz, P. Absence of SARS-CoV-2 neutralizing activity in pre-pandemic sera from individuals with recent seasonal coronavirus infection. medRxiv 2020. medRxiv:2020.10.08.20209650. [Google Scholar]
- Legros, V.; Denolly, S.; Vogrig, M.; Boson, B.; Rigaill, J.; Pillet, S.; Grattard, F.; Gonzalo, S.; Verhoeven, P.; Allatif, O.; et al. A longitudinal study of SARS-CoV-2 Infected Patients Shows High Correlation between Neutralizing Antibodies and COVID-19 Severity. 2020. Available online: https://www.medrxiv.org/content/medrxiv/early/2020/09/01/2020.08.27.20182493.full.pdf (accessed on 5 February 2021).
- Ruetalo, N.; Businger, R.; Althaus, K.; Fink, S.; Ruoff, F.; Hamprecht, K.; Flehmig, B.; Bakchoul, T.; Templin, M.F.; Schindler, M. Neutralizing Antibody Response in Non-Hospitalized SARS-CoV-2 Patients. 2020. Available online: https://www.medrxiv.org/content/medrxiv/early/2020/09/22/2020.08.07.20169961.full.pdf (accessed on 5 February 2021).
- Ladner, J.T.; Henson, S.N.; Boyle, A.S.; Engelbrektson, A.L.; Fink, Z.W.; Rahee, F.; D’Ambrozio, J.; Schaecher, K.E.; Stone, M.; Dong, W.; et al. Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with an endemic human CoV. Cell Rep. Med. 2021, 2, 100189. [Google Scholar] [CrossRef]
- Song, G.; He, W.T.; Callaghan, S.; Anzanello, F.; Huang, D.; Ricketts, J.; Torres, J.L.; Beutler, N.; Peng, L.; Vargas, S.; et al. Cross-reactive serum and memory B cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. bioRxiv 2020. [Google Scholar]
- Tso, F.Y.; Lidenge, S.J.; Peña, P.B.; Clegg, A.A.; Ngowi, J.R.; Mwaiselage, J.; Ngalamika, O.; Julius, P.; West, J.T.; Wood, C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int. J. Infect. Dis. 2021, 102, 577–583. [Google Scholar] [CrossRef]
- Simula, E.R.; Manca, M.A.; Jasemi, S.; Uzzau, S.; Rubino, S.; Manchia, P.; Bitti, A.; Palermo, M.; Sechi, L.A. HCoV-NL63 and SARS-CoV-2 Share Recognized Epitopes by the Humoral Response in Sera of People Collected Pre- and during CoV-2 Pandemic. Microorganisms 2020, 8, 1993. [Google Scholar] [CrossRef]
- Laing, E.; Sterling, S.; Richard, S.; Epsi, N.; Phogat, S.; Samuels, E.; Yan, L.; Moreno, N.; Coles, C.; Drew, M.; et al. A betacoronavirus multiplex microsphere immunoassay detects early SARS-CoV-2 seroconversion and antibody cross reactions. Res. Sq. 2020. [Google Scholar]
- Westerhuis, B.M.; Aguilar-Bretones, M.; Raadsen, M.P.; de Bruin, E.; Okba, N.M.A.; Haagmans, B.L.; Langerak, T.; Endeman, H.; van den Akker, J.P.C.; Gommers, D.A.M.P.J.; et al. Severe COVID-19 patients display a back boost of seasonal coronavirus-specific antibodies. medRxiv 2020. medRxiv:2020.10.10.20210070. [Google Scholar]
- Nechipurenko, Y.D.; Anashkina, A.A.; Matveeva, O.V. Change of Antigenic Determinants of SARS-CoV-2 Virus S-Protein as a Possible Cause of Antibody-Dependent Enhancement of Virus Infection and Cytokine Storm. Biophysics 2020, 65, 703–709. [Google Scholar] [CrossRef]
- Beretta, A.; Cranage, M.; Zipeto, D. Is Cross-Reactive Immunity Triggering COVID-19 Immunopathogenesis? Front. Immunol. 2020, 11, 567710. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Nandi, M.; Ihsan, A.U.; Chamard, H.A.; Ilangumaran, S.; Ramanathan, S. ADE and hyperinflammation in SARS-CoV2 infection- comparison with dengue hemorrhagic fever and feline infectious peritonitis. Cytokine 2020, 136, 155256. [Google Scholar] [CrossRef] [PubMed]
- Karthik, K.; Senthilkumar, T.M.A.; Udhayavel, S.; Raj, G.D. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum. Vaccines Immunother. 2020, 16, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; So, R.T.Y.; Yuan, M.; Liu, H.; Lee, C.-C.D.; Yip, G.K.; Ng, W.W.; Wilson, I.A.; Peiris, M.; Wu, N.C.; et al. Evidence of antigenic imprinting in sequential Sarbecovirus immunization. bioRxiv 2020. bioRxiv:2020.10.14.339465. [Google Scholar]
- Klompus, S.; Leviatan, S.; Vogl, T.; Kalka, I.; Godneva, A.; Shinar, E.; Weinberger, A.; Segal, E. Cross-reactive antibody responses against SARS-CoV-2 and seasonal common cold coronaviruses. medRxiv 2020. medRxiv:2020.09.01.20182220. [Google Scholar]
- Dugas, M.; Grote-Westrick, T.; Vollenberg, R.; Lorentzen, E.; Brix, T.; Schmidt, H.; Tepasse, P.-R.; Kühn, J. Less severe course of COVID-19 is associated with elevated levels of antibodies against seasonal human coronaviruses OC43 and HKU1 (HCoV OC43, HCoV HKU1). medRxiv 2020. medRxiv:2020.10.12.20211599. [Google Scholar]
- Dugas, M.; Grote-Westrick, T.; Merle, U.; Fontenay, M.; Kremer, A.E.; Vollenberg, R.; Lorentzen, E.; Tiwari-Heckler, S.; Duchemin, J.; Ellouze, S.; et al. Lack of antibodies against seasonal coronavirus OC43 nucleocapsid protein identifies patients at risk of critical COVID-19. medRxiv 2020. medRxiv:2020.12.07.20245241. [Google Scholar]
- Morgenlander, W.R.; Henson, S.; Monaco, D.; Chen, A.; Littlefield, K.; Bloch, E.M.; Fujimura, E.; Ruczinski, I.; Crowley, A.R.; Natarajan, H.; et al. Antibody responses to endemic coronaviruses modulate COVID-19 convalescent plasma functionality. J. clin. Investing. 2021, 146927, Online ahead of print. [Google Scholar]
- Greenbaum, U.; Klein, K.; Martinez, F.; Song, J.; Thall, P.F.; Ramdial, J.L.; Knape, C.; Aung, F.M.; Scroggins, J.; Knopfelmacher, A.; et al. High levels of common cold coronavirus antibodies in convalescent plasma are associated with improved survival in COVID-19 patients. medRxiv 2021. medRxiv:2021.03.08.21252775. [Google Scholar]
| Category | No. | % |
|---|---|---|
| Gender | ||
| Female | 41 | 52.6 |
| Male | 37 | 47.4 |
| Age, years | ||
| <65 | 39 | 50.0 |
| 65–75 | 20 | 25.6 |
| >75 | 19 | 24.4 |
| Cardiological comorbidities | ||
| No | 55 | 70.5 |
| Yes | 17 | 21.8 |
| NA | 6 | 7.7 |
| Infections | ||
| No | 44 | 56.4 |
| Yes | 25 | 32.1 |
| NA | 9 | 11.5 |
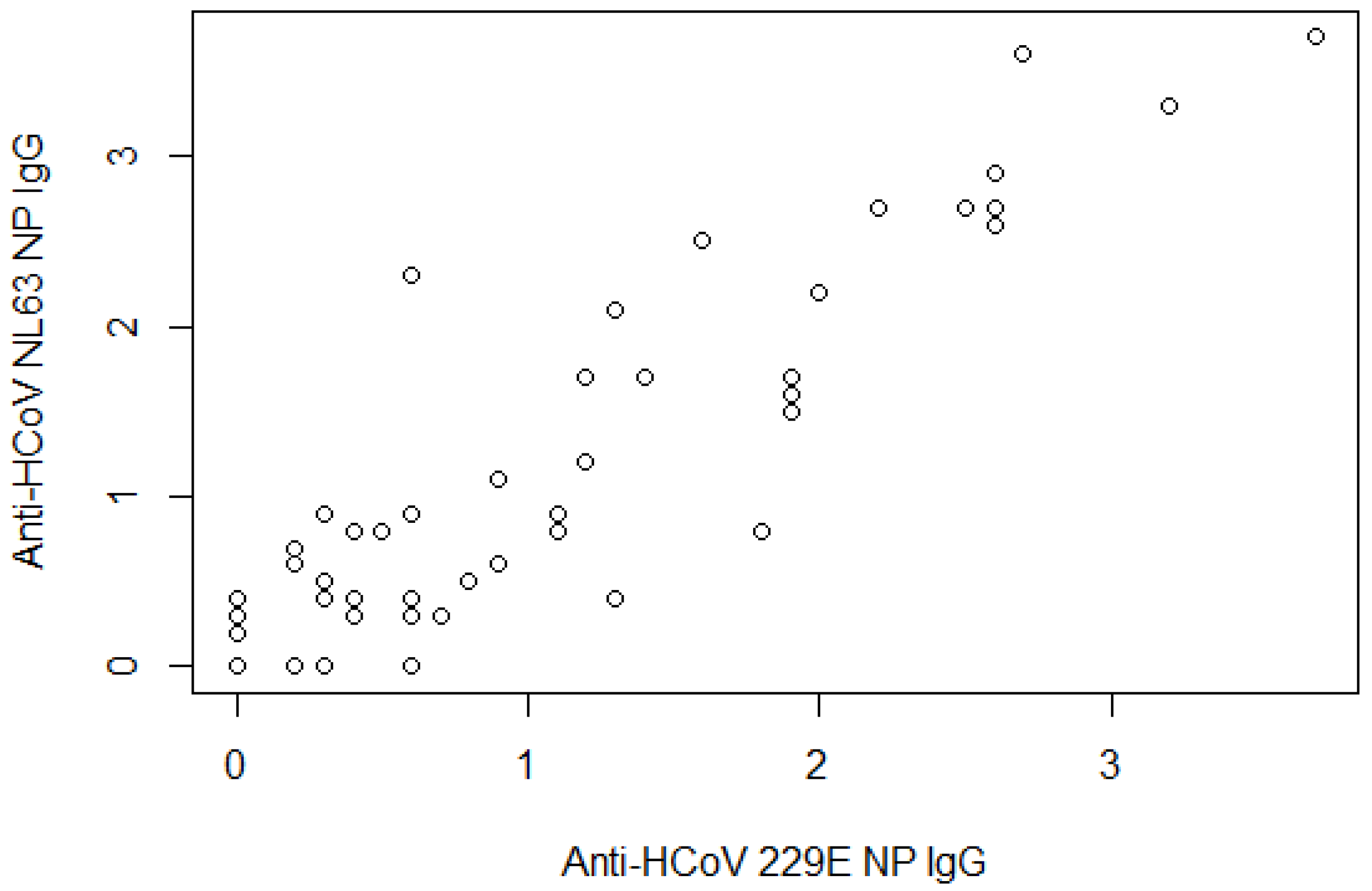
| Anti-HCoV-229E NP IgG | ||
| Mean readings ± SD | 0.8 | 0.9 |
| Negative | 53 | 67.9 |
| Positive | 25 | 32.1 |
| Anti-HCoV-NL63 NP IgG | ||
| Mean readings ± SD | 0.9 | 1.0 |
| Negative | 53 | 67.9 |
| Positive | 25 | 32.1 |
| Anti-HCoV-229E NP IgG + anti-NL63 NP IgG | ||
| None | 49 | 62.8 |
| One | 8 | 10.3 |
| Both | 21 | 26.9 |
| Anti-HCoV-229E NP IgG + anti-HCoV-NL63 NP IgG | ||
| Negative | 49 | 62.8 |
| Positive | 29 | 37.2 |
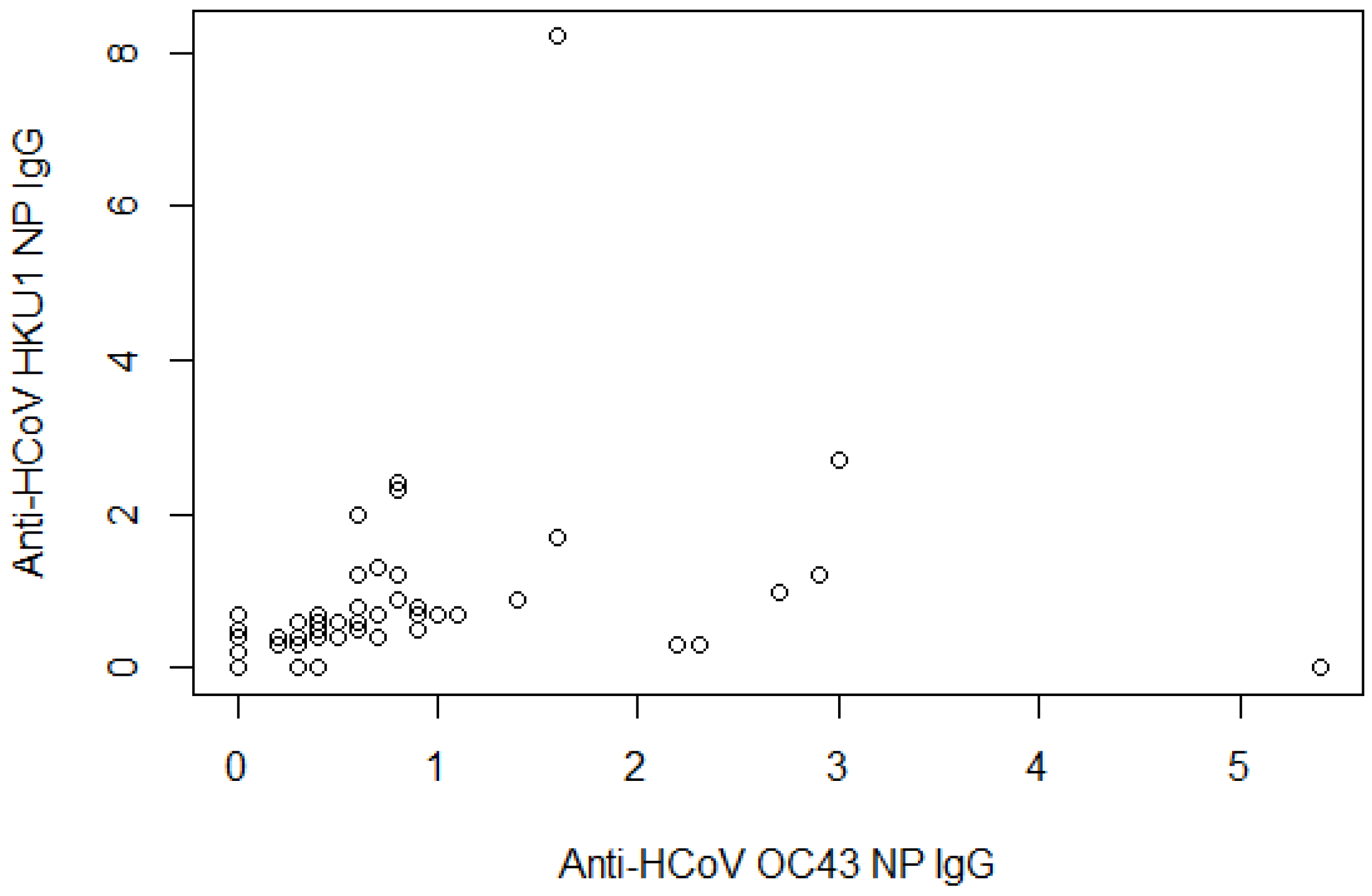
| Anti-HCoV-OC43 NP IgG | ||
| Mean readings ± SD | 0.6 | 0.9 |
| Negative | 66 | 84.6 |
| Positive | 12 | 15.4 |
| Anti-HCoV-HKU1 NP IgG | ||
| Mean readings ± SD | 0.6 | 1.1 |
| Negative | 67 | 85.9 |
| Positive | 11 | 14.1 |
| Anti-HCoV-OC43 NP IgG + anti-HCoV-HKU1 NP IgG | ||
| None | 60 | 76.9 |
| One | 13 | 16.7 |
| Both | 5 | 6.4 |
| Anti-HCoV-OC43 IgG+ Anti-HCoV-HKU1 IgG | ||
| Negative | 60 | 76.9 |
| Positive | 18 | 23.1 |
| Anti-SARS-CoV-2 RBD IgG (Mikrogen), mean readings ± SD | 2.7 | 3.2 |
| Anti-SARS-CoV-2 S1 IgG (Mikrogen), mean readings ± SD | 3.4 | 4.0 |
| Cumulative number of anti-SARS-CoV-2 positive IgG specificities (Mikrogen) | ||
| 0 | 40 | 51.3 |
| 1 | 2 | 2.6 |
| 2 | 4 | 5.1 |
| 3 | 32 | 41.0 |
| Final anti-SARS-CoV-2 serological diagnosis (Mikrogen) | ||
| Negative | 40 | 51.3 |
| Positive | 38 | 48.7 |
| Anti-S1/S2 IgG (DiaSorin), mean readings ± SD | 109.0 | 82.2 |
| Anti-S1/S2 IgG (DiaSorin) | ||
| Negative | 6 | 7.7 |
| Positive | 24 | 30.8 |
| NA | 48 | 61.5 |
| WHO Score < 5 | WHO Score ≥ 5 | IRR | (95% CI) | IRR Adjusted * | (95% CI) | |
|---|---|---|---|---|---|---|
| Infections | ||||||
| No | 33 (82.5) | 11 (37.9) | 1 | (ref.) | 1 | (ref.) |
| Yes | 7 (17.5) | 18 (62.1) | 2.88 | (1.63, 5.10) | 2.75 | (1.56, 4.83) |
| Anti-HCoV 229E NP IgG | ||||||
| No | 34 (81.0) | 19 (52.8) | 1 | (ref.) | 1 | (ref.) |
| Yes | 8 (19.0) | 17 (47.2) | 1.90 | (1.21, 2.98) | 1.87 | (1.22, 2.87) |
| Anti-HCoV NL63 NP IgG | ||||||
| No | 33 (78.6) | 20 (55.6) | 1 | (ref.) | 1 | (ref.) |
| Yes | 9 (21.4) | 16 (44.4) | 1.70 | (1.07, 2.68) | 1.80 | (1.18, 2.74) |
| Anti-HCoV 229E NP + Anti-HCoV NL63 NP IgG | ||||||
| None | 31 (73,8) | 18 (50.0) | 1 | (ref.) | 1 | (ref.) |
| One | 5 (11.9) | 3 (8.3) | 1.02 | (0.39, 2.70) | 1.34 | (0.49, 3.63) |
| Both | 6 (14.3) | 15 (41.7) | 1.94 | (1.23, 3.08) | 1.94 | (1.27, 2.98) |
| Anti-HCoV 229E NP + Anti-HCoV NL63 NP IgG | ||||||
| None | 31 (73.8) | 18 (50.0) | 1 | (ref.) | 1 | (ref.) |
| At least one | 11 (26.2) | 18 (50.0) | 1.69 | (1.06, 2.70) | 1.82 | (1.17, 2.81) |
| Anti-HCoV OC43 NP IgG | ||||||
| No | 36 (85.7) | 30 (83.3) | 1 | (ref.) | 1 | (ref.) |
| Yes | 6 (14.3) | 6 (16.7) | 1.10 | (0.59, 2.06) | 0.97 | (0.53, 1.79) |
| Anti-HCoV HKU1 NP IgG | ||||||
| No | 37 (88.1) | 30 (83.3) | 1 | (ref.) | 1 | (ref.) |
| Yes | 5 (11.9) | 6 (16.7) | 1.22 | (0.66, 2.23) | 1.05 | (0.54, 2.03) |
| Anti-HCoV OC43 NP IgG + Anti-HCoV HKU1 NP IgG | ||||||
| None | 34 (81.0) | 26 (72.2) | 1 | (ref.) | 1 | (ref.) |
| One | 5 (11.9) | 8 (22.2) | 1.42 | (0.84, 2.39) | 1.34 | (0.80, 2.25) |
| Both | 3 (7.1) | 2 (5.6) | 0.92 | (0.30, 2.83) | 0.74 | (0.24, 2.31) |
| Anti-HCoV-OC43 NP IgG + anti-HCoV-HKU1 NP IgG | ||||||
| None | 34 (81.0) | 26 (72.2) | 1 | (ref.) | 1 | (ref.) |
| At least one | 8 (19.0) | 10 (27.8) | 1.28 | (0.77, 2.13) | 1.16 | (0.69, 1.94) |
| Number of positive IgGs against HCoV | ||||||
| 0 | 27 (64.3) | 16 (44.4) | 1 | (ref.) | 1 | (ref.) |
| 1 | 7 (16.7) | 3 (8.3) | 0.81 | (0.29, 2.26) | 0.92 | (0.36, 2.38) |
| 2 | 5 (11.9) | 10 (27.8) | 1.79 | (1.05, 3.04) | 1.78 | (1.05, 3.03) |
| ≥3 | 3 (7.2) | 7 (19.5) | 1.88 | (1.07, 3.31) | 1.76 | (1.02, 3.04) |
| Number of positive IgGs against HCoV | ||||||
| 0 | 27 (64.3) | 16 (44.4) | 1 | (ref.) | 1 | (ref.) |
| >0 | 15 (35.7) | 20 (55.6) | 1.54 | (0.94, 2.50) | 1.56 | (0.99, 2.47) |
| Number of positive IgG specificities against SARS CoV-2 | ||||||
| 0 | 27 (64.3) | 13 (36.1) | 1 | (ref.) | 1 | (ref.) |
| 1–2 | 5 (11.9) | 1 (2.8) | 0.51 | (0.08, 3.28) | 0.47 | (0.08, 2.67) |
| 3 | 10 (23.8) | 22 (61.1) | 2.11 | (1.27, 3.51) | 1.91 | (1.14, 3.18) |
| Number of positive IgG specificities against SARS CoV-2 | ||||||
| 0 | 27 (64.3) | 13 (36.1) | 1 | (ref.) | 1 | (ref.) |
| >0 | 15 (35.7) | 23 (63.9) | 1.86 | (1.11, 3.13) | 1.65 | (0.98, 2.79) |
| Final serological SARS-CoV-2 diagnosis (Mikrogen) | ||||||
| No | 27 (64.3) | 13 (36.1) | 1 | (ref.) | 1 | (ref.) |
| Yes | 15 (35.7) | 23 (63.9) | 1.86 | (1.11, 3.13) | 1.65 | (0.98, 2.79) |
| Final serological SARS-CoV-2 diagnosis (DiaSorin) | ||||||
| No | 5 (27.8) | 1 (8.3) | 1 | (ref.) | 1 | (ref.) |
| Yes | 13 (72.2) | 11 (91.7) | 2.75 | (0.42, 17.89) | 2.49 | (0.39, 15.93) |
| Genus | Species | Cellular Receptor | Sequence Identity to SARS CoV-2 |
|---|---|---|---|
| Alpha | NL63 | ACE2 | 49% |
| 229E | Aminopeptidase N | 48% | |
| Beta | SARS CoV-2 | ACE2 | 100% |
| SARS CoV | 80% | ||
| MERS CoV | DPP-IV | 54% | |
| HKU-1 | sialoglycan-based receptors with 9-O-acetylated sialic acid (9-O-Ac-Sia) | 52% | |
| OC43 | 51% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Focosi, D.; Genoni, A.; Lucenteforte, E.; Tillati, S.; Tamborini, A.; Spezia, P.G.; Azzi, L.; Baj, A.; Maggi, F. Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin. Life 2021, 11, 298. https://doi.org/10.3390/life11040298
Focosi D, Genoni A, Lucenteforte E, Tillati S, Tamborini A, Spezia PG, Azzi L, Baj A, Maggi F. Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin. Life. 2021; 11(4):298. https://doi.org/10.3390/life11040298
Chicago/Turabian StyleFocosi, Daniele, Angelo Genoni, Ersilia Lucenteforte, Silvia Tillati, Antonio Tamborini, Pietro Giorgio Spezia, Lorenzo Azzi, Andreina Baj, and Fabrizio Maggi. 2021. "Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin" Life 11, no. 4: 298. https://doi.org/10.3390/life11040298
APA StyleFocosi, D., Genoni, A., Lucenteforte, E., Tillati, S., Tamborini, A., Spezia, P. G., Azzi, L., Baj, A., & Maggi, F. (2021). Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin. Life, 11(4), 298. https://doi.org/10.3390/life11040298









