A Small Molecule Targeting Human MEK1/2 Enhances ERK and p38 Phosphorylation under Oxidative Stress or with Phenothiazines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Treatment of Cells with INR119 and Oxidizing Agents
2.3. Western Blot Analysis
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Statistical Analysis
3. Results
3.1. Phosphorylation of ERK1/2 and p38 under H2O2-Induced Oxidative Stress Is Enhanced by INR119
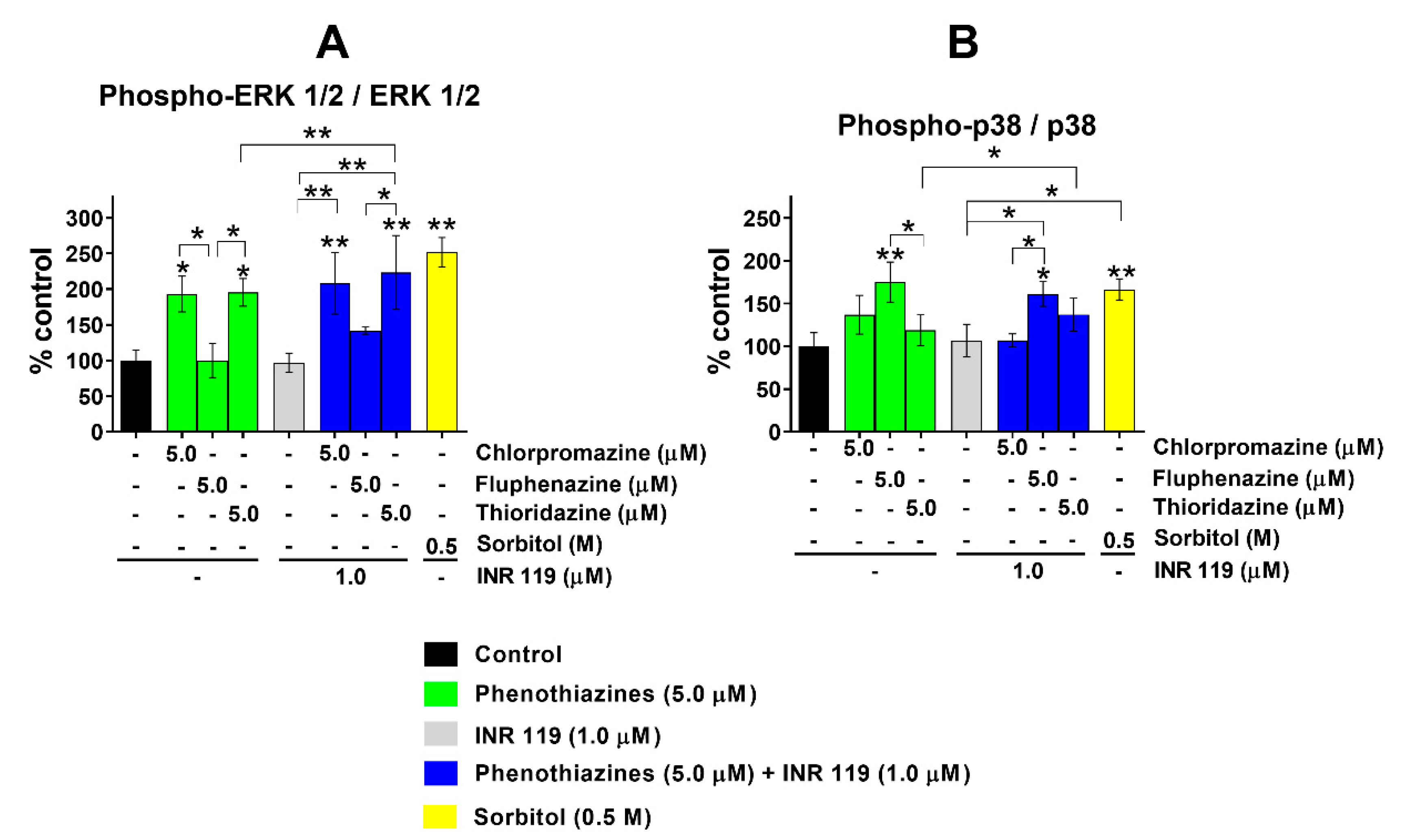
3.2. Phosphorylation of ERK1/2 and p38 under Phenothiazine-Induced Oxidative Stress Is Enhanced by INR119
3.3. TP53 and BAX Expression under Phenothiazine-Induced Oxidative Stress Is Enhanced by INR119
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauch, N.; Rukhlenko, O.S.; Kolch, W.; Kholodenko, B.N. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr. Opin. Struct. Biol. 2016, 41, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Peng, W.; Calvo, E. Rational Approaches for Combination Therapy Strategies Targeting the MAP Kinase Pathway in Solid Tumors. Mol. Cancer Ther. 2018, 17, 3–16. [Google Scholar] [CrossRef]
- Huhn, M.; Engel, R.R.; Leucht, S. Fluphenazine versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Hendouei, N.; Saghafi, F.; Shadfar, F.; Hosseinimehr, S.J. Molecular mechanisms of anti-psychotic drugs for improvement of cancer treatment. Eur. J. Pharmacol. 2019, 856, 172402. [Google Scholar] [CrossRef]
- Varga, B.; Csonka, Á.; Molnár, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer. Res. 2017, 37, 5983–5993. [Google Scholar] [CrossRef]
- Otręba, M.; Kośmider, L.; Rzepecka-Stojko, A. Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur. J. Pharmacol. 2020, 887, 173553. [Google Scholar] [CrossRef] [PubMed]
- Otręba, M.; Kośmider, L. In vitro anticancer activity of fluphenazine, perphenazine and prochlorperazine. A review. J. Appl. Toxicol. 2021, 41, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Antherieu, S.; Azzi, P.B.-E.; Dumont, J.; Fromenty, B.; Robin, M.-A.; Guillouzo, A.; Abdel-Razzak, Z.; Guguen-Guillouzo, C. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 2013, 57, 1518–1529. [Google Scholar] [CrossRef]
- Otręba, M.; Beberok, A.; Wrześniok, D.; Rok, J.; Buszman, E. Effect of thioridazine on antioxidant status of HEMn-DP melanocytes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xia, Y.; Feng, Z.; Lin, W.; Xue, Q.; Jiang, J.; Yu, X.; Peng, C.; Luo, M.; Yang, Y.; et al. Repositioning antipsychotic fluphenazine hydrochloride for treating triple negative breast cancer with brain metastases and lung metastases. Am. J. Cancer Res. 2019, 9, 459–478. [Google Scholar]
- Otręba, M.; Wrześniok, D.; Beberok, A.; Rok, J.; Buszman, E. Melanogenesis and antioxidant defense system in normal human melanocytes cultured in the presence of chlorpromazine. Toxicol. Vitr. 2015, 29, 221–227. [Google Scholar] [CrossRef]
- Otreba, M.; Wrześniok, D.; Beberok, A.; Rok, J.; Buszman, E. Fluphenazine and perphenazine impact on melanogenesis and antioxidant enzymes activity in normal human melanocytes. Acta Pol. Pharm. Drug Res. 2016, 73, 903–911. [Google Scholar]
- Redwan, I.N.; Dyrager, C.; Solano, C.; De Troconiz, G.F.; Voisin, L.; Bliman, D.; Meloche, S.; Grøtli, M. Towards the development of chromone-based MEK1/2 modulators. Eur. J. Med. Chem. 2014, 85, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.T.; Pang, L.; Decker, S.J.; Bridges, A.J.; Saltiel, A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 1995, 92, 7686–7689. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, J.J.; Tarczykowska, A.; Picazo, C.; Cossio, I.; Redwan, I.N.; Gao, C.; Solano, C.; Toledano, M.B.; Grøtli, M.; Molin, M.; et al. A Redox-Sensitive Thiol in Wis1 Modulates the Fission Yeast Mitogen-Activated Protein Kinase Response to H2O2 and Is the Target of a Small Molecule. Mol. Cell. Biol. 2020, 40, e00346-19. [Google Scholar] [CrossRef]
- Ohren, J.F.; Chen, H.; Pavlovsky, A.; Whitehead, C.; Zhang, E.; Kuffa, P.; Yan, C.; McConnell, P.; Spessard, C.; Banotai, C.; et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004, 11, 1192–1197. [Google Scholar] [CrossRef]
- Otręba, M.; Pajor, M.; Warncke, J.D. Antimelanoma activity of perphenazine and prochlorperazine in human COLO829 and C32 cell lines. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1257–1264. [Google Scholar] [CrossRef]
- Siebel, A.; Cubillos-Rojas, M.; Santos, R.C.; Schneider, T.; Bonan, C.D.; Bartrons, R.; Ventura, F.; De Oliveira, J.R.; Rosa, J.L. Contribution of S6K1/MAPK Signaling Pathways in the Response to Oxidative Stress: Activation of RSK and MSK by Hydrogen Peroxide. PLoS ONE 2013, 8, e75523. [Google Scholar] [CrossRef]
- Barata, A.G.; Dick, T.P. A role for peroxiredoxins in H2O2- and MEKK-dependent activation of the p38 signaling pathway. Redox Biol. 2020, 28, 101340. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liang, J.; Qian, J.; Jin, L.; Du, M.; Li, M.; Li, D. Opposing role of JNK-p38 kinase and ERK1/2 in hydrogen perox-ide-induced oxidative damage of human trophoblast-like JEG-3 cells. Int. J. Clin. Exp. Pathol. 2014, 7, 959–968. [Google Scholar]
- Ruisong, M.; Xiaorong, H.; Gangying, H.; Chunfeng, Y.; Changjiang, Z.; Xuefei, L.; Yuanhong, L.; Hong, J. The Protective Role of Interleukin-33 in Myocardial Ischemia and Reperfusion Is Associated with Decreased HMGB1 Expression and Up-Regulation of the P38 MAPK Signaling Pathway. PLoS ONE 2015, 10, e0143064. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Li, Y.; Li, N.; Shi, X.; Ding, H.; Zhang, Y.; Li, X.; Liu, G.; Wang, Z. Non-esterified fatty acids activate the ROS–p38–p53/Nrf2 signaling pathway to induce bovine hepatocyte apoptosis in vitro. Apoptosis 2014, 19, 984–997. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, L.-R. Turning Death to Growth: Hematopoietic Growth Factors Promote Neurite Outgrowth through MEK/ERK/p53 Pathway. Mol. Neurobiol. 2017, 55, 5913–5925. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, R.; Feng, C.; Zhang, J.; Liu, N.; Xu, K.; Wang, X.; Zhang, S.; Li, Z.; Liu, X.; et al. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncol. Rep. 2014, 32, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Majorini, M.T.; Elliott, R.; Ashworth, A.; Lord, C.J.; Cancelliere, C.; Bardelli, A.; Seneci, P.; Walczak, H.; Delia, D.; et al. Oncogenic KRAS sensitizes premalignant, but not malignant cells, to Noxa-dependent apoptosis through the activation of the MEK/ERK pathway. Oncotarget 2015, 6, 10994–11008. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otręba, M.; Sjölander, J.J.; Grøtli, M.; Sunnerhagen, P. A Small Molecule Targeting Human MEK1/2 Enhances ERK and p38 Phosphorylation under Oxidative Stress or with Phenothiazines. Life 2021, 11, 297. https://doi.org/10.3390/life11040297
Otręba M, Sjölander JJ, Grøtli M, Sunnerhagen P. A Small Molecule Targeting Human MEK1/2 Enhances ERK and p38 Phosphorylation under Oxidative Stress or with Phenothiazines. Life. 2021; 11(4):297. https://doi.org/10.3390/life11040297
Chicago/Turabian StyleOtręba, Michał, Johanna Johansson Sjölander, Morten Grøtli, and Per Sunnerhagen. 2021. "A Small Molecule Targeting Human MEK1/2 Enhances ERK and p38 Phosphorylation under Oxidative Stress or with Phenothiazines" Life 11, no. 4: 297. https://doi.org/10.3390/life11040297
APA StyleOtręba, M., Sjölander, J. J., Grøtli, M., & Sunnerhagen, P. (2021). A Small Molecule Targeting Human MEK1/2 Enhances ERK and p38 Phosphorylation under Oxidative Stress or with Phenothiazines. Life, 11(4), 297. https://doi.org/10.3390/life11040297






