Beat Detection Recruits the Visual Cortex in Early Blind Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Equipment and Stimuli
2.3. Behavioral Procedure
2.4. 3D-MRI and fMRI Acquisition
2.5. fMRI Data Analysis
3. Results
3.1. Behavioral Results
3.2. fMRI Results from Whole Brain ANOVA
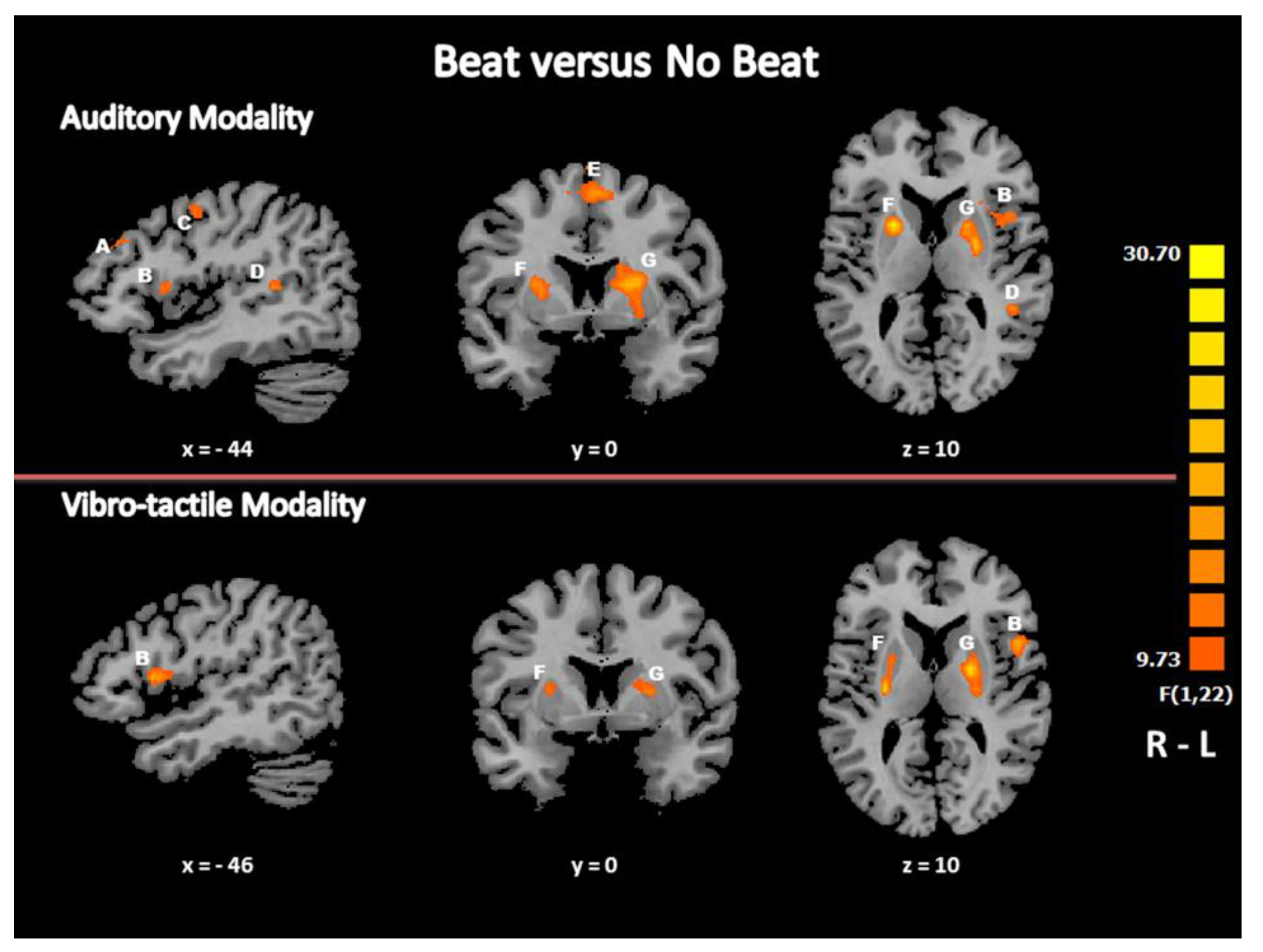
3.2.1. Main Effect of the Condition: Brain Activation Specific to Beat Detection
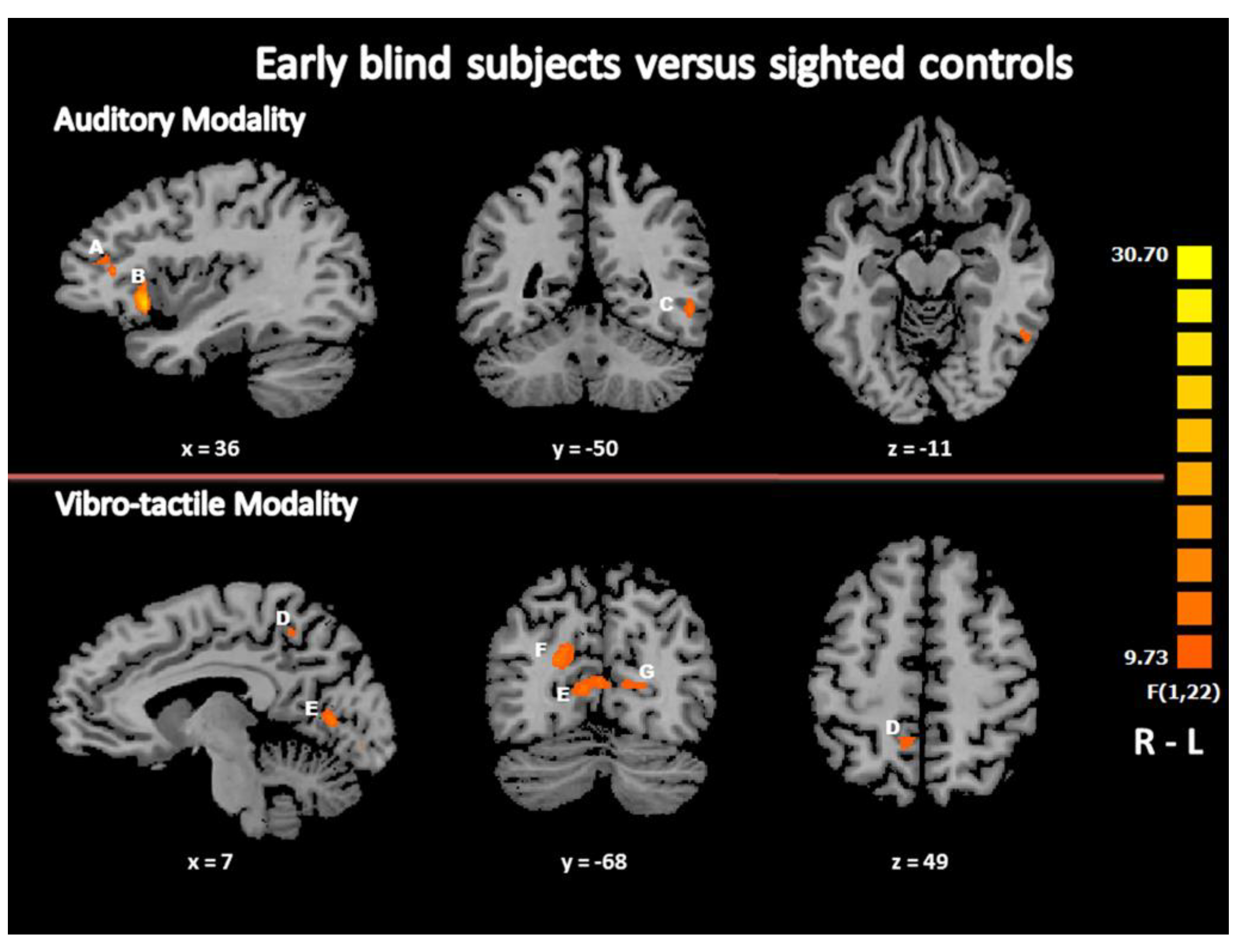
3.2.2. Main Effect of the Group: Differential Brain Activation in EB and Controls during Beat Processing
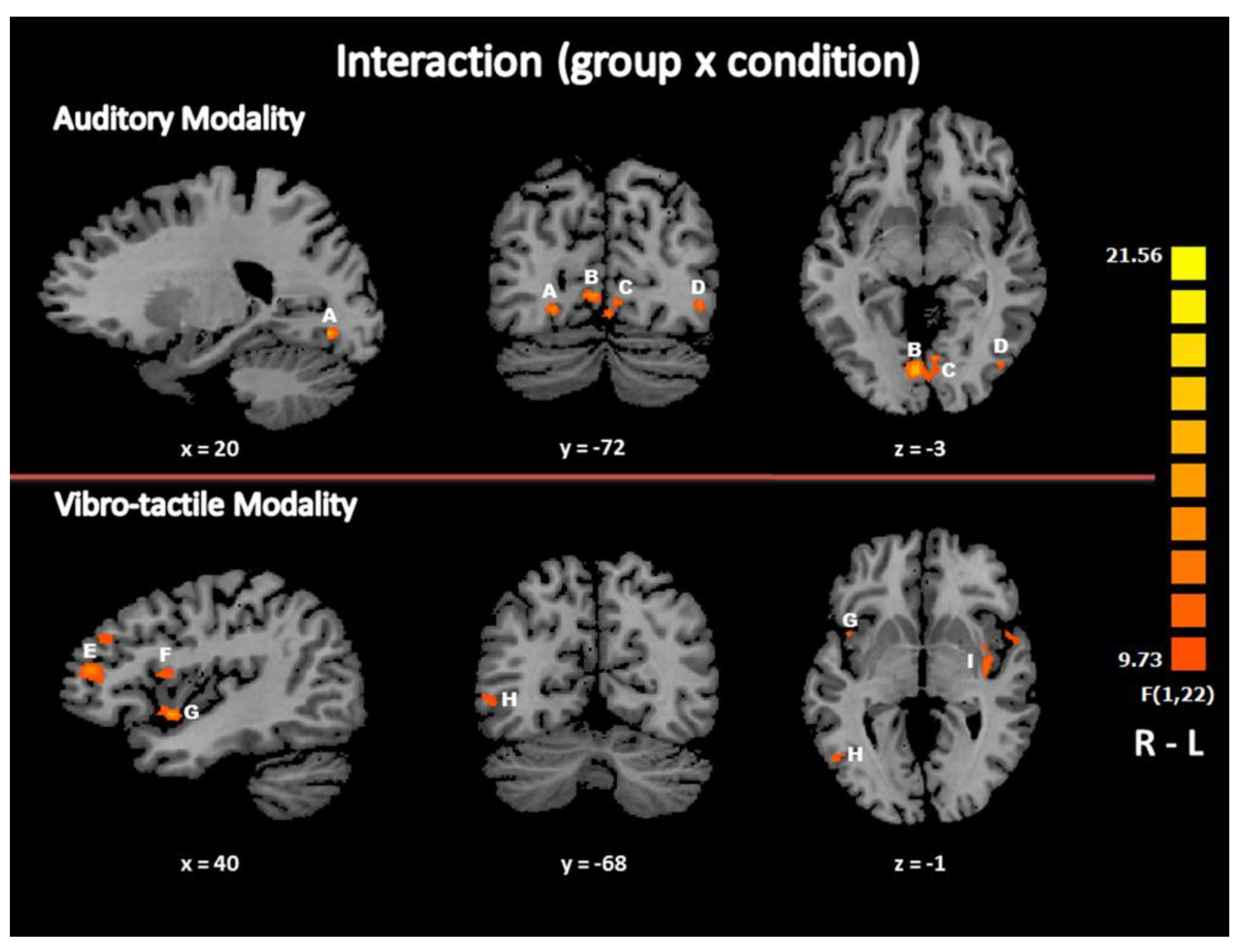
3.2.3. Interaction: Brain Activation Specific to Beat Detection in EB Subjects Compared to Controls
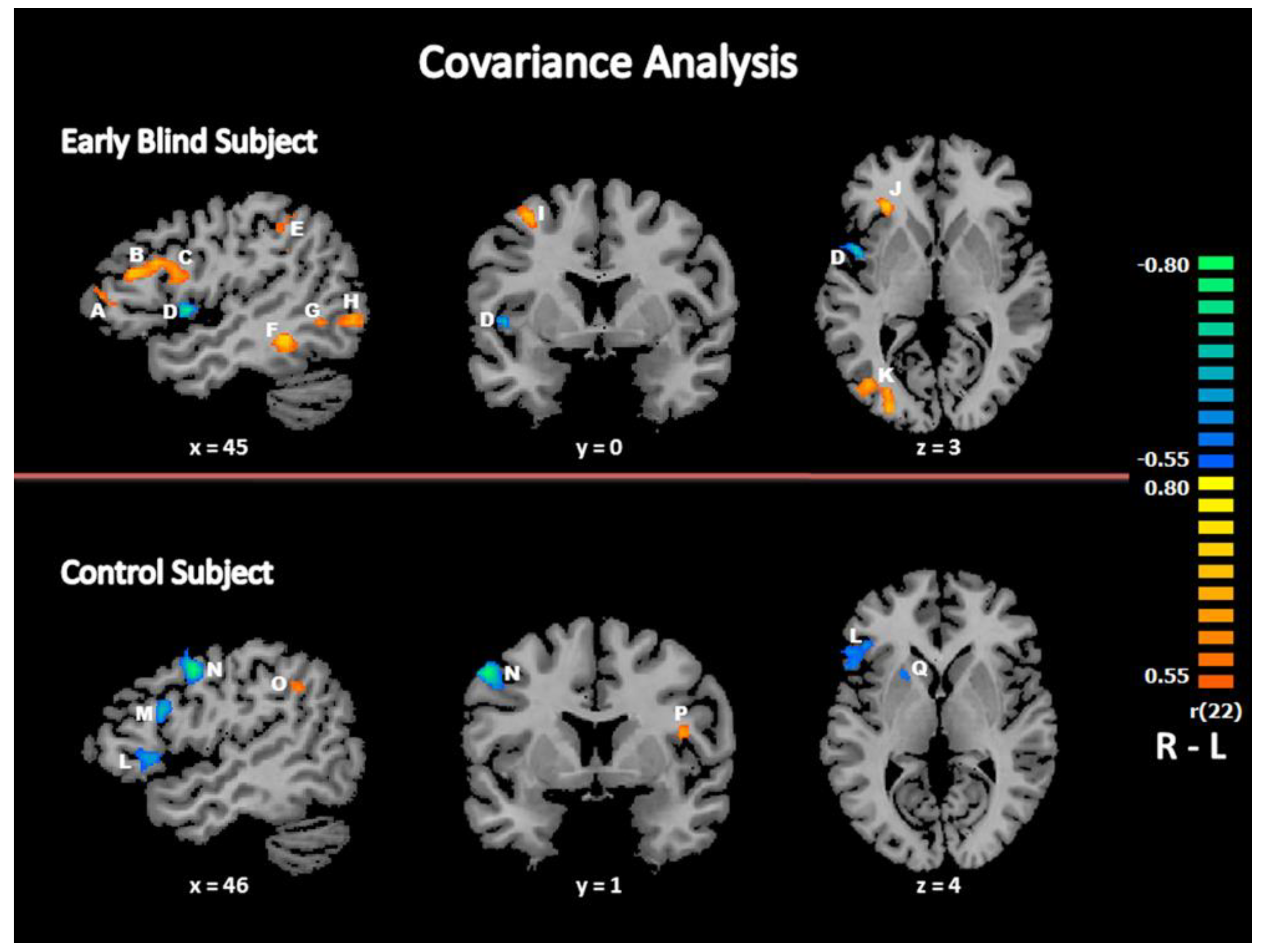
3.2.4. Covariance Analysis: Relationship between Response Times and Brain Activity
4. Discussion
4.1. Specific Neural Network for Beat Detection
4.2. Differences between Early Blind and Controls in Beat Processing
4.3. Differences between EB and SC Subjects in Beat (Minus No Beat) Detection
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Buchel, C.; Price, C.; Frackowiak, R.S.; Friston, K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain 1998, 121 Pt 3, 409–419. [Google Scholar] [CrossRef]
- Burton, H.; McLaren, D.G.; Sinclair, R.J. Reading embossed capital letters: An fMRI study in blind and sighted individuals. Hum. Brain Mapp. 2006, 27, 325–339. [Google Scholar] [CrossRef][Green Version]
- Kupers, R.; Beaulieu-Lefebvre, M.; Schneider, F.C.; Kassuba, T.; Paulson, O.B.; Siebner, H.R.; Ptito, M. Neural correlates of olfactory processing in congenital blindness. Neuropsychologia 2011, 49, 2037–2044. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Korte, M. Auditory compensation for early blindness in cat cerebral cortex. J. Neurosci. 1993, 13, 4538–4548. [Google Scholar] [CrossRef]
- Renier, L.; De Volder, A.G.; Rauschecker, J.P. Cortical plasticity and preserved function in early blindness. Neurosci. Biobehav. Rev. 2014, 41, 53–63. [Google Scholar] [CrossRef][Green Version]
- Renier, L.A.; Anurova, I.; De Volder, A.G.; Carlson, S.; VanMeter, J.; Rauschecker, J.P. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron 2010, 68, 138–148. [Google Scholar] [CrossRef]
- Sadato, N.; Pascual-Leone, A.; Grafman, J.; Ibanez, V.; Deiber, M.P.; Dold, G.; Hallett, M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature 1996, 380, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Amedi, A.; Raz, N.; Pianka, P.; Malach, R.; Zohary, E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci. 2003, 6, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Bedny, M.; Pascual-Leone, A.; Dodell-Feder, D.; Fedorenko, E.; Saxe, R. Language processing in the occipital cortex of congenitally blind adults. Proc. Natl. Acad. Sci. USA 2011, 108, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Bonino, D.; Ricciardi, E.; Sani, L.; Gentili, C.; Vanello, N.; Guazzelli, M.; Vecchi, T.; Pietrini, P. Tactile spatial working memory activates the dorsal extrastriate cortical pathway in congenitally blind individuals. Arch. Ital. Biol. 2008, 146, 133–146. [Google Scholar] [PubMed]
- Burton, H.; Sinclair, R.J.; Dixit, S. Working memory for vibrotactile frequencies: Comparison of cortical activity in blind and sighted individuals. Hum. Brain Mapp. 2010, 31, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Kitada, R.; Yoshihara, K.; Sasaki, A.T.; Hashiguchi, M.; Kochiyama, T.; Sadato, N. The brain network underlying the recognition of hand gestures in the blind: The supramodal role of the extrastriate body area. J. Neurosci. 2014, 34, 10096–10108. [Google Scholar] [CrossRef] [PubMed]
- Reich, L.; Szwed, M.; Cohen, L.; Amedi, A. A ventral visual stream reading center independent of visual experience. Curr. Biol. 2011, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Collignon, O.; Davare, M.; Olivier, E.; De Volder, A.G. Reorganisation of the right occipito-parietal stream for auditory spatial processing in early blind humans. A transcranial magnetic stimulation study. Brain Topogr. 2009, 21, 232–240. [Google Scholar] [CrossRef]
- Gougoux, F.; Zatorre, R.J.; Lassonde, M.; Voss, P.; Lepore, F. A functional neuroimaging study of sound localization: Visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005, 3, e27. [Google Scholar] [CrossRef]
- Raz, N.; Amedi, A.; Zohary, E. V1 activation in congenitally blind humans is associated with episodic retrieval. Cereb. Cortex 2005, 15, 1459–1468. [Google Scholar] [CrossRef]
- Cohen, L.G.; Celnik, P.; Pascual-Leone, A.; Corwell, B.; Falz, L.; Dambrosia, J.; Honda, M.; Sadato, N.; Gerloff, C.; Catala, M.D.; et al. Functional relevance of cross-modal plasticity in blind humans. Nature 1997, 389, 180–183. [Google Scholar] [CrossRef]
- Kupers, R.; Pappens, M.; de Noordhout, A.M.; Schoenen, J.; Ptito, M.; Fumal, A. rTMS of the occipital cortex abolishes Braille reading and repetition priming in blind subjects. Neurology 2007, 68, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Elbert, T.; Sterr, A.; Rockstroh, B.; Pantev, C.; Muller, M.M.; Taub, E. Expansion of the tonotopic area in the auditory cortex of the blind. J. Neurosci. 2002, 22, 9941–9944. [Google Scholar] [CrossRef] [PubMed]
- Fiehler, K.; Rosler, F. Plasticity of multisensory dorsal stream functions: Evidence from congenitally blind and sighted adults. Restor. Neurol. Neurosci. 2010, 28, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Gougoux, F.; Belin, P.; Voss, P.; Lepore, F.; Lassonde, M.; Zatorre, R.J. Voice perception in blind persons: A functional magnetic resonance imaging study. Neuropsychologia 2009, 47, 2967–2974. [Google Scholar] [CrossRef]
- Burton, H.; Sinclair, R.J.; McLaren, D.G. Cortical activity to vibrotactile stimulation: An fMRI study in blind and sighted individuals. Hum. Brain Mapp. 2004, 23, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Torres, F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain 1993, 116 Pt 1, 39–52. [Google Scholar] [CrossRef]
- Sadato, N.; Okada, T.; Honda, M.; Yonekura, Y. Critical period for cross-modal plasticity in blind humans: A functional MRI study. Neuroimage 2002, 16, 389–400. [Google Scholar] [CrossRef]
- Sterr, A.; Muller, M.M.; Elbert, T.; Rockstroh, B.; Pantev, C.; Taub, E. Changed perceptions in Braille readers. Nature 1998, 391, 134–135. [Google Scholar] [CrossRef]
- Burton, H.; Snyder, A.Z.; Raichle, M.E. Resting state functional connectivity in early blind humans. Front. Syst. Neurosci. 2014, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, C.; Liang, M.; Li, J.; Tian, L.; Zhou, Y.; Qin, W.; Li, K.; Jiang, T. Whole brain functional connectivity in the early blind. Brain 2007, 130, 2085–2096. [Google Scholar] [CrossRef]
- Ortiz-Teran, L.; Ortiz, T.; Perez, D.L.; Aragon, J.I.; Diez, I.; Pascual-Leone, A.; Sepulcre, J. Brain Plasticity in Blind Subjects Centralizes Beyond the Modal Cortices. Front. Syst. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef]
- Proulx, M.J.; Brown, D.J.; Pasqualotto, A.; Meijer, P. Multisensory perceptual learning and sensory substitution. Neurosci. Biobehav. Rev. 2014, 41, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sepulcre, J.; Liu, H.; Talukdar, T.; Martincorena, I.; Yeo, B.T.; Buckner, R.L. The organization of local and distant functional connectivity in the human brain. PLoS Comput. Biol. 2010, 6, e1000808. [Google Scholar] [CrossRef]
- Grahn, J.A. See what I hear? Beat perception in auditory and visual rhythms. Exp. Brain Res. 2012, 220, 51–61. [Google Scholar] [CrossRef]
- Large, E.W.; Palmer, C. Perceiving temporal regularity in music. Cogn. Sci. 2002, 26, 1–37. [Google Scholar] [CrossRef]
- Patel, A.D.; Iversen, J.R. The evolutionary neuroscience of musical beat perception: The Action Simulation for Auditory Prediction (ASAP) hypothesis. Front. Syst. Neurosci. 2014, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Brochard, R.; Touzalin, P.; Despres, O.; Dufour, A. Evidence of beat perception via purely tactile stimulation. Brain Res. 2008, 1223, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Brett, M. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 2007, 19, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Henry, M.J.; McAuley, J.D. FMRI investigation of cross-modal interactions in beat perception: Audition primes vision, but not vice versa. Neuroimage 2011, 54, 1231–1243. [Google Scholar] [CrossRef]
- Grahn, J.A.; Rowe, J.B. Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 2009, 29, 7540–7548. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gamble, D.; Sarnlertsophon, K.; Wang, X.; Hsiao, S. Integration of auditory and tactile inputs in musical meter perception. Adv. Exp. Med. Biol. 2013, 787, 453–461. [Google Scholar]
- Lerens, E.; Araneda, R.; Renier, L.; De Volder, A.G. Improved beat asynchrony detection in early blind individuals. Perception 2014, 43, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.L.; Ullen, F.; Ehrsson, H.H.; Hashimoto, T.; Kito, T.; Naito, E.; Forssberg, H.; Sadato, N. Listening to rhythms activates motor and premotor cortices. Cortex 2009, 45, 62–71. [Google Scholar] [CrossRef]
- Chapin, H.L.; Zanto, T.; Jantzen, K.J.; Kelso, S.J.; Steinberg, F.; Large, E.W. Neural responses to complex auditory rhythms: The role of attending. Front. Psychol. 2010, 1, 224. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A. The role of the basal ganglia in beat perception: Neuroimaging and neuropsychological investigations. Ann. N. Y. Acad. Sci. 2009, 1169, 35–45. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.D.; Henry, M.J.; Tkach, J. Tempo mediates the involvement of motor areas in beat perception. Ann. N. Y. Acad. Sci. 2012, 1252, 77–84. [Google Scholar] [CrossRef]
- Teki, S.; Grube, M.; Griffiths, T.D. A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Front. Integr. Neurosci. 2011, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Teki, S.; Grube, M.; Kumar, S.; Griffiths, T.D. Distinct neural substrates of duration-based and beat-based auditory timing. J. Neurosci. 2011, 31, 3805–3812. [Google Scholar] [CrossRef]
- Wiener, M.; Turkeltaub, P.; Coslett, H.B. The image of time: A voxel-wise meta-analysis. Neuroimage 2010, 49, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.J.; Chen, J.L.; Zatorre, R.J.; Penhune, V.B. Interacting cortical and basal ganglia networks underlying finding and tapping to the musical beat. J. Cogn. Neurosci. 2013, 25, 401–420. [Google Scholar] [CrossRef]
- Araneda, R.; Renier, L.; Ebner-Karestinos, D.; Dricot, L.; De Volder, A.G. Hearing, feeling or seeing a beat recruits a supramodal network in the auditory dorsal stream. Eur. J. Neurosci. 2017, 45, 1439–1450. [Google Scholar] [CrossRef]
- Weeks, R.; Horwitz, B.; Aziz-Sultan, A.; Tian, B.; Wessinger, C.M.; Cohen, L.G.; Hallett, M.; Rauschecker, J.P. A positron emission tomographic study of auditory localization in the congenitally blind. J. Neurosci. 2000, 20, 2664–2672. [Google Scholar] [CrossRef]
- Drake, C.; Palmer, C. Skill acquisition in music performance: Relations between planning and temporal control. Cognition 2000, 74, 1–32. [Google Scholar] [CrossRef]
- Repp, B.H. Sensorimotor synchronization: A review of the tapping literature. Psychon. Bull. Rev. 2005, 12, 969–992. [Google Scholar] [CrossRef]
- Strait, D.L.; Kraus, N.; Parbery-Clark, A.; Ashley, R. Musical experience shapes top-down auditory mechanisms: Evidence from masking and auditory attention performance. Hear Res. 2010, 261, 22–29. [Google Scholar] [CrossRef]
- Grondin, S. Duration discrimination of empty and filled intervals marked by auditory and visual signals. Percept Psychophys. 1993, 54, 383–394. [Google Scholar] [CrossRef]
- Essens, P.J.; Povel, D.J. Metrical and nonmetrical representations of temporal patterns. Percept Psychophys. 1985, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging; Georg Thieme: Stuttgart, Germany; New York, NY, USA, 1988. [Google Scholar]
- Friston, K.J.; Jezzard, P.; Turner, R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1994, 1, 153–171. [Google Scholar] [CrossRef]
- Boynton, G.M.; Engel, S.A.; Glover, G.H.; Heeger, D.J. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 1996, 16, 4207–4221. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.; Zeger, S.L. Non-linear Fourier Time Series Analysis for Human Brain Mapping by Functional Magnetic Resonance Imaging. J. R. Stat. Soc. 1997, 46, 1–29. [Google Scholar] [CrossRef]
- Friston, K.J.; Holmes, A.P.; Price, C.J.; Buchel, C.; Worsley, K.J. Multisubject fMRI studies and conjunction analyses. Neuroimage 1999, 10, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Goebel, R.; Esposito, F.; Formisano, E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006, 27, 392–401. [Google Scholar] [CrossRef]
- Chen, J.L.; Penhune, V.B.; Zatorre, R.J. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 2008, 18, 2844–2854. [Google Scholar] [CrossRef]
- Schubotz, R.I.; Friederici, A.D.; von Cramon, D.Y. Time perception and motor timing: A common cortical and subcortical basis revealed by fMRI. Neuroimage 2000, 11, 1–12. [Google Scholar] [CrossRef]
- Schubotz, R.I. Prediction of external events with our motor system: Towards a new framework. Trends Cogn. Sci. 2007, 11, 211–218. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Chen, J.L.; Penhune, V.B. When the brain plays music: Auditory-motor interactions in music perception and production. Nat. Rev. Neurosci. 2007, 8, 547–558. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Scott, S.K. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat. Neurosci. 2009, 12, 718–724. [Google Scholar] [CrossRef]
- Mendoza, G.; Merchant, H. Motor system evolution and the emergence of high cognitive functions. Prog. Neurobiol. 2014, 122, 73–93. [Google Scholar] [CrossRef]
- Slutsky, D.A.; Recanzone, G.H. Temporal and spatial dependency of the ventriloquism effect. Neuroreport 2001, 12, 7–10. [Google Scholar] [CrossRef]
- Burr, D.; Banks, M.S.; Morrone, M.C. Auditory dominance over vision in the perception of interval duration. Exp. Brain Res. 2009, 198, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.M.; Christiansen, M.H. Modality-constrained statistical learning of tactile, visual, and auditory sequences. J. Exp. Psychol. Learn Mem. Cogn. 2005, 31, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, A.O. Seeing slow and seeing fast: Two limits on perception. Trends Cogn. Sci. 2009, 13, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Shams, L.; Kamitani, Y.; Shimojo, S. Illusions. What you see is what you hear. Nature 2000, 408, 788. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu. Rev. Neurosci. 2005, 28, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Bola, L.; Zimmermann, M.; Mostowski, P.; Jednorog, K.; Marchewka, A.; Rutkowski, P.; Szwed, M. Task-specific reorganization of the auditory cortex in deaf humans. Proc. Natl. Acad. Sci. USA 2017, 114, E600–E609. [Google Scholar] [CrossRef] [PubMed]
- Fadiga, L.; Craighero, L.; D’Ausilio, A. Broca’s area in language, action, and music. Ann. N. Y. Acad. Sci. 2009, 1169, 448–458. [Google Scholar] [CrossRef]
- Maess, B.; Koelsch, S.; Gunter, T.C.; Friederici, A.D. Musical syntax is processed in Broca’s area: An MEG study. Nat. Neurosci. 2001, 4, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D. Language, music, syntax and the brain. Nat. Neurosci. 2003, 6, 674–681. [Google Scholar] [CrossRef]
- Grahn, J.A.; McAuley, J.D. Neural bases of individual differences in beat perception. Neuroimage 2009, 47, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Bedny, M. Evidence from Blindness for a Cognitively Pluripotent Cortex. Trends Cogn. Sci. 2017, 21, 637–648. [Google Scholar] [CrossRef]
| Subjects | Age | Sex | Handedness (**) | Onset of Blindness | Diagnosis | Musical Experience (***) |
|---|---|---|---|---|---|---|
| EB 1 | 37 | M | R | Birth | Leber congenital amaurosis | 7 |
| EB 2 | 40 | F | A | Birth | Retinopathy of prematurity | 7° |
| EB 3 | 29 | M | R | Birth | Persistent hyperplastic primary vitreous involving both eyes | 6 |
| EB 4 | 57 | M | R | Birth | Severe retinal dystrophy (*) | 13 |
| EB 5 | 46 | M | R | Birth | Leber congenital amaurosis | 3 |
| EB 6 | 37 | M | R | Birth | Anterior chamber cleavage syndrome (Peters syndrome) | 14° |
| EB 7 | 32 | F | R | Birth | Severe corneal dystrophy (*) | 1° |
| EB 8 | 62 | M | R | <18 months | Bilateral retinoblastoma | 15 |
| EB 9 | 28 | M | L | Birth | Hereditary retinal dysplasia (*) | 7 |
| EB 10 | 35 | F | R | Birth | Retinopathy of prematurity | 2 |
| EB 11 | 24 | M | R | Birth | Severe optic nerve dystrophy (*) | none |
| EB 12 | 32 | M | R | Birth | Genetic eye disorder (*) | 8° |
| F-Test (within) “Beat” Minus “No Beat” | |||||||
|---|---|---|---|---|---|---|---|
| Brain region | Brodmann area | Coordinates | Cluster size | F-value | p-value | ||
| x | y | z | |||||
| R Inferior Parietal Lobule | BA 40 | 57 | −29 | 22 | 262 | 18.0404 | 0.000330 |
| R_Cerebellum | 27 | −55 | −23 | 1384 | 38.9461 | 0.000003 | |
| R Putamen | 21 | 5 | 10 | 1113 | 31.3233 | 0.000013 | |
| R/L Medial Frontal Gyrus | BA 6 | −9 | −13 | 55 | 4156 | 38.9245 | 0.000003 |
| L Cingulate Gyrus | BA 24/32 | −12 | 14 | 34 | 314 | 24.8991 | 0.000054 |
| L_Putamen | −27 | −4 | 16 | 5197 | 33.9139 | 0.000007 | |
| L Inferior Parietal Lobule | BA 40 | −30 | −52 | 40 | 405 | 17.0245 | 0.000443 |
| L Middle Frontal Gyrus | BA 9 | −39 | 35 | 31 | 1363 | 24.7331 | 0.000056 |
| L Precentral /Middle Frontal Gyrus | BA 6 | −48 | −7 | 46 | 377 | 18.9000 | 0.000258 |
| L Inferior frontal/Middle Frontal Gyrus | BA 44/6 | −41 | 8 | 10 | 277 | 24.2598 | 0.000063 |
| L Superior Temporal Gyrus | BA 22 | −48 | −43 | 19 | 370 | 15.2869 | 0.000751 |
| F-test (between) EB Minus SC | |||||||
| Brain region | Brodmann area | Coordinates | Cluster size | F-value | p-value | ||
| x | y | z | |||||
| R Superior Temporal Gyrus | BA 22 | 57 | −10 | 4 | 249 | 15.8472 | 0.000632 |
| R Insula | BA 13 | 36 | 17 | −2 | 719 | 21.9237 | 0.000114 |
| R Inferior Frontal Gyrus | BA 46 | 36 | 32 | 13 | 207 | 14.8069 | 0.000573 |
| L Parahippocampal Gyrus/Hippocampus | BA 30 | −6 | −40 | 1 | 256 | 17.8974 | 0.000344 |
| L Inferior Parietal Lobule | BA 40 | −42 | −37 | 37 | 197 | 18.0088 | 0.000333 |
| L Inferior Temporal Gyrus/Fusiform Gyrus | BA 20/37 | −51 | −49 | −5 | 414 | 13.2791 | 0.000431 |
| L Middle Occipital Gyrus (*) | BA 19 | −52 | −67 | −2 | 102 | 11.8650 | 0.000612 |
| F-Test (Interaction) within “Beat” vs “No Beat” and between EB vs SC | |||||||
| Brain region | Brodmann area | Coordinates | Cluster size | F-value | p-value | ||
| x | y | z | |||||
| R Lingual Gyrus | BA 18 | 21 | −73 | −8 | 249 | 19.8387 | 0.000199 |
| R Lingual Gyrus | BA 18 | 6 | −79 | −5 | 899 | 20.5595 | 0.000164 |
| R Inferior Temporal Gyrus/Middle Occipital Gyrus (*) | BA 37/19 | 42 | −64 | −5 | 84 | 12.4729 | 0.002651 |
| L Lingual/Fusiform Gyrus | BA 18/19 | −3 | −70 | −11 | 677 | 16.5092 | 0.000517 |
| L Middle Temporal Gyrus /Middle Occipital Gyrus (*) | BA 37/19 | −42 | −70 | 7 | 123 | 14.2863 | 0.001031 |
| L Middle Occipital Gyrus (*) | BA 19 | −42 | −73 | −5 | 123 | 16.3655 | 0.000540 |
| F-Test (within) “Beat” Minus “No Beat” | ||||||||
| Brain region | Brodmann area | Coordinates | Cluster size | F-value | p-value | |||
| x | y | z | ||||||
| R Inferior Parietal Lobule | BA 40 | 28 | −46 | 55 | 213 | 17.6941 | 0.000364 | |
| R Putamen | 27 | −16 | 10 | 537 | 29.3930 | 0.000019 | ||
| R Middle Frontal Gyrus | BA 9 | 24 | 38 | 22 | 300 | 15.1124 | 0.000793 | |
| L Putamen | −21 | −7 | 12 | 1480 | 30.7040 | 0.000014 | ||
| L Insula | BA 13 | −36 | −25 | 25 | 739 | 30.5384 | 0.000015 | |
| L Precentral Gyrus/Inferior Frontal Gyrus | BA 6/44 | −48 | 11 | 10 | 699 | 25.8972 | 0.000042 | |
| F-Test (between) EB Minus SC | ||||||||
| Brain region | Brodmann area | Coordinates | Cluster size | F-value | p-value | |||
| x | y | z | ||||||
| R Parahippocampal gyrus/Hippocampus | BA 35/30 | 15 | −40 | −8 | 975 | 22.3237 | 0.000103 | |
| R Cuneus | BA 18 | 21 | −67 | 19 | 682 | 17.1111 | 0.000432 | |
| R Precuneus | BA 7 | 6 | −49 | 49 | 249 | 14.4397 | 0.000981 | |
| L Cuneus | BA 17/18 | −2 | −75 | 7 | 1073 | 17.0310 | 0.000443 | |
| L Lingual Gyrus | BA 19 | −27 | −58 | 0 | 498 | 17.7397 | 0.000360 | |
| L Parahippocampal gyrus/Hippocampus | BA 35/30 | −27 | −40 | −8 | 989 | 22.8442 | 0.000090 | |
| F-Test (Interaction) within “Beat” vs “No Beat” and between EB vs SC | ||||||||
| Brain region | Brodmann area | Coordinates | Cluster size | F-value | p-value | |||
| x | y | z | ||||||
| R Precentral Gyrus /Inferior Frontal Gyrus | BA 6/44 | 57 | 8 | 13 | 2208 | 23.1492 | 0.000083 | |
| R Middle Frontal Gyrus | BA 46 | 39 | 41 | 16 | 979 | 21.4826 | 0.000128 | |
| R Insula | BA 13 | 39 | 2 | −5 | 243 | 27.3261 | 0.000030 | |
| R Insula | BA 13 | 27 | 20 | 13 | 529 | 28.1927 | 0.000025 | |
| R Cingulate Gyrus | BA 24 | 12 | −7 | 43 | 214 | 14.0374 | 0.001117 | |
| R Medial Frontal Gyrus | BA 6 | 3 | −7 | 64 | 234 | 13.8985 | 0.001168 | |
| R Middle Temporal Gyrus/ Middle Occipital Gyrus (*) | BA 37/19 | 51 | −64 | 0 | 121 | 13.6849 | 0.001252 | |
| L Medial Frontal Gyrus | BA 6/8 | −9 | 20 | 49 | 235 | 17.6665 | 0.000367 | |
| L Insula | BA 13 | −39 | −7 | −2 | 473 | 17.4133 | 0.000396 | |
| L Middle Frontal Gyrus | BA 46 | −49 | 29 | 22 | 2838 | 28.3219 | 0.000024 | |
| L Inferior Frontal Gyrus/Precentral Gyrus | BA 44/6 | −48 | 7 | 7 | 1082 | 21.6273 | 0.000123 | |
| “Beat” Minus “No Beat”, Early Blind Subjects | |||||||
|---|---|---|---|---|---|---|---|
| Brain region | Brodmann area | Coordinates | Cluster size | R | p-value | ||
| x | y | z | |||||
| R Middle Frontal Gyrus | BA 9/46 | 52 | 26 | 25 | 549 | 0.7585 | 0.000017 |
| R Inferior Frontal Gyrus | BA 44 | 54 | 11 | 25 | 1777 | 0.7548 | 0.000020 |
| R Inferior Parietal Lobule | BA 40 | 36 | −46 | 46 | 2477 | 0.7501 | 0.000024 |
| R Superior Occipital Gyrus /Precuneus | BA 19/7 | 27 | −67 | 31 | 1290 | 0.8035 | 0.000002 |
| R Insula | BA 13 | 45 | 2 | 4 | 414 | −0.7936 | 0.000004 |
| R Middle Temporal Gyrus | BA 21 | 42 | −40 | −8 | 549 | 0.7613 | 0.000016 |
| R Inferior Temporal Gyrus/Middle Occipital Gyrus | BA 37/19 | 36 | −58 | −5 | 379 | 0.7276 | 0.000056 |
| R Lingual Gyrus | BA 19 | 30 | −52 | −5 | 376 | 0.7364 | 0.000041 |
| R Inferior Occipital Gyrus/ Inferior Temporal Gyrus | BA 18/19 | 39 | −76 | −2 | 1627 | 0.7854 | 0.000005 |
| R Inferior Frontal Gyrus | BA 45/46 | 27 | 29 | 7 | 514 | 0.8045 | 0.000002 |
| R Precentral/Middle Frontal Gyrus | BA 6 | 33 | −1 | 52 | 600 | 0.7613 | 0.000016 |
| R Cuneus | BA 17/18 | 18 | −85 | 13 | 236 | 0.7449 | 0.000030 |
| R Medial Frontal Gyrus | BA 10 | 6 | 62 | 12 | 597 | −0.7508 | 0.000024 |
| L Superior Frontal Gyrus | BA 6 | −9 | 17 | 52 | 1093 | −0.8134 | 0.000001 |
| L Cuneus | BA 18 | −15 | −73 | 25 | 592 | −0.7620 | 0.000015 |
| L Cuneus | BA 17/18 | −18 | −70 | 13 | 429 | −0.7578 | 0.000018 |
| L Putamen | −18 | 5 | 7 | 200 | −0.7038 | 0.000124 | |
| L Middle Frontal Gyrus | BA 46 | −36 | 44 | 19 | 474 | −0.7487 | 0.000026 |
| L Insula | BA 13 | −48 | −19 | 22 | 238 | 0.8065 | 0.000002 |
| L Superior Temporal Gyrus | BA 22 | −60 | −53 | 13 | 725 | −0.8415 | 0.000000 |
| “Beat” Minus “No Beat”, Control Subjects | |||||||
| Brain region | Brodmann area | Coordinates | Cluster size | R | p-value | ||
| x | y | z | |||||
| R Middle Frontal Gyrus | BA 9 | 48 | 11 | 25 | 1642 | −0.7310 | 0.000050 |
| R Inferior Frontal Gyrus | BA 45/46 | 54 | 14 | 6 | 953 | −0.7040 | 0.000123 |
| R Middle Frontal /Precentral Gyrus | BA 6 | 48 | −1 | 43 | 874 | −0.8603 | 0.000000 |
| R Inferior Parietal Lobule | BA 40 | 48 | −49 | 34 | 252 | 0.6797 | 0.000259 |
| R Putamen | 21 | 11 | 7 | 291 | −0.6559 | 0.000502 | |
| L Tail of Caudate Nucleus | −21 | −28 | 25 | 341 | 0.7355 | 0.000042 | |
| L Insula | BA 13 | −39 | 2 | 16 | 265 | 0.6710 | 0.000332 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araneda, R.; Silva Moura, S.; Dricot, L.; De Volder, A.G. Beat Detection Recruits the Visual Cortex in Early Blind Subjects. Life 2021, 11, 296. https://doi.org/10.3390/life11040296
Araneda R, Silva Moura S, Dricot L, De Volder AG. Beat Detection Recruits the Visual Cortex in Early Blind Subjects. Life. 2021; 11(4):296. https://doi.org/10.3390/life11040296
Chicago/Turabian StyleAraneda, Rodrigo, Sandra Silva Moura, Laurence Dricot, and Anne G. De Volder. 2021. "Beat Detection Recruits the Visual Cortex in Early Blind Subjects" Life 11, no. 4: 296. https://doi.org/10.3390/life11040296
APA StyleAraneda, R., Silva Moura, S., Dricot, L., & De Volder, A. G. (2021). Beat Detection Recruits the Visual Cortex in Early Blind Subjects. Life, 11(4), 296. https://doi.org/10.3390/life11040296






