In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cytotoxicity of Compounds
2.3. Efficacy of Compounds—Cell Pretreatment
2.4. Efficacy of Compounds—Virus Pretreatment
2.5. Efficacy of Compounds—Post-Adsorption
2.6. XTT-Based Viability Assay
3. Results
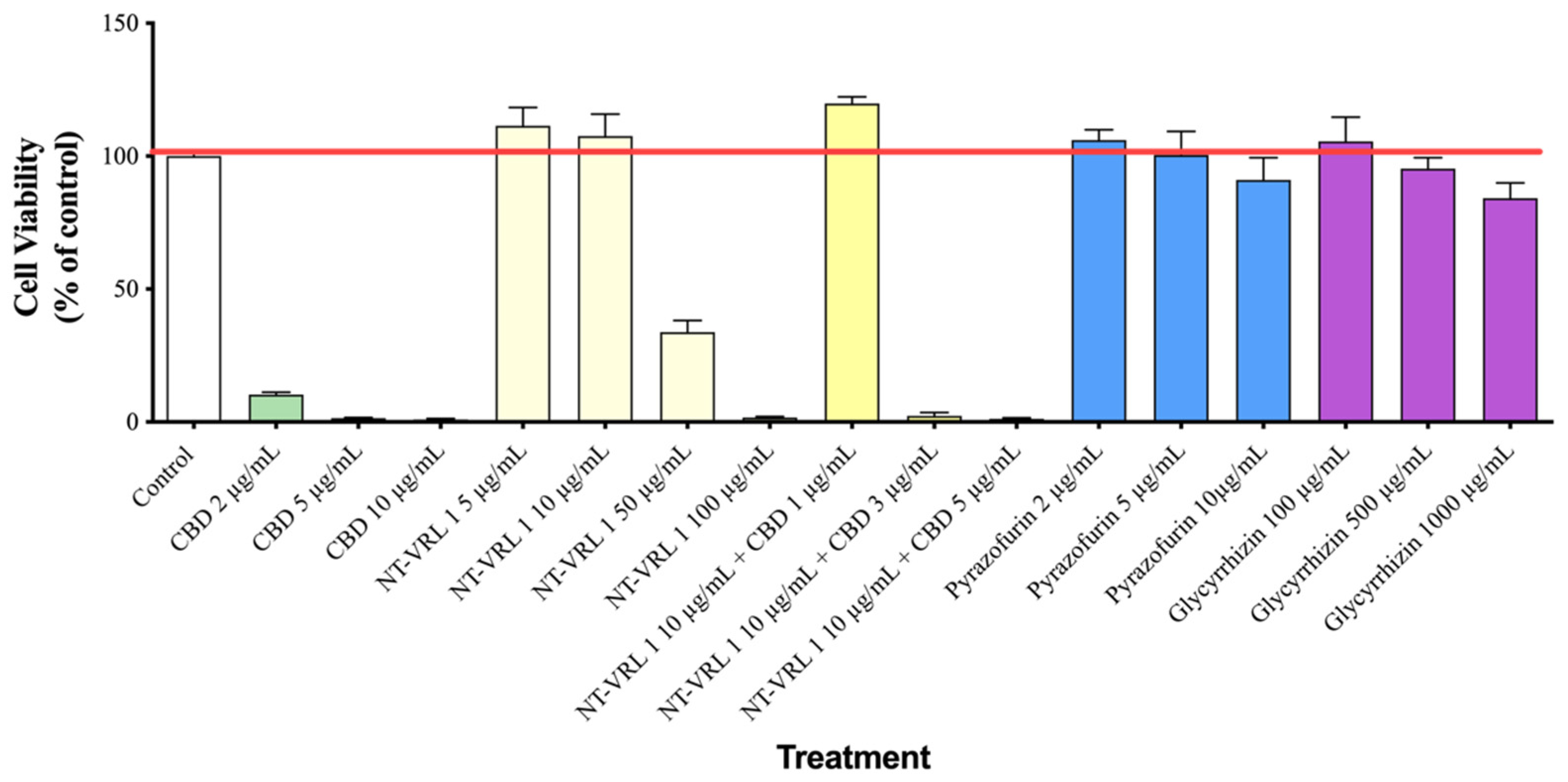
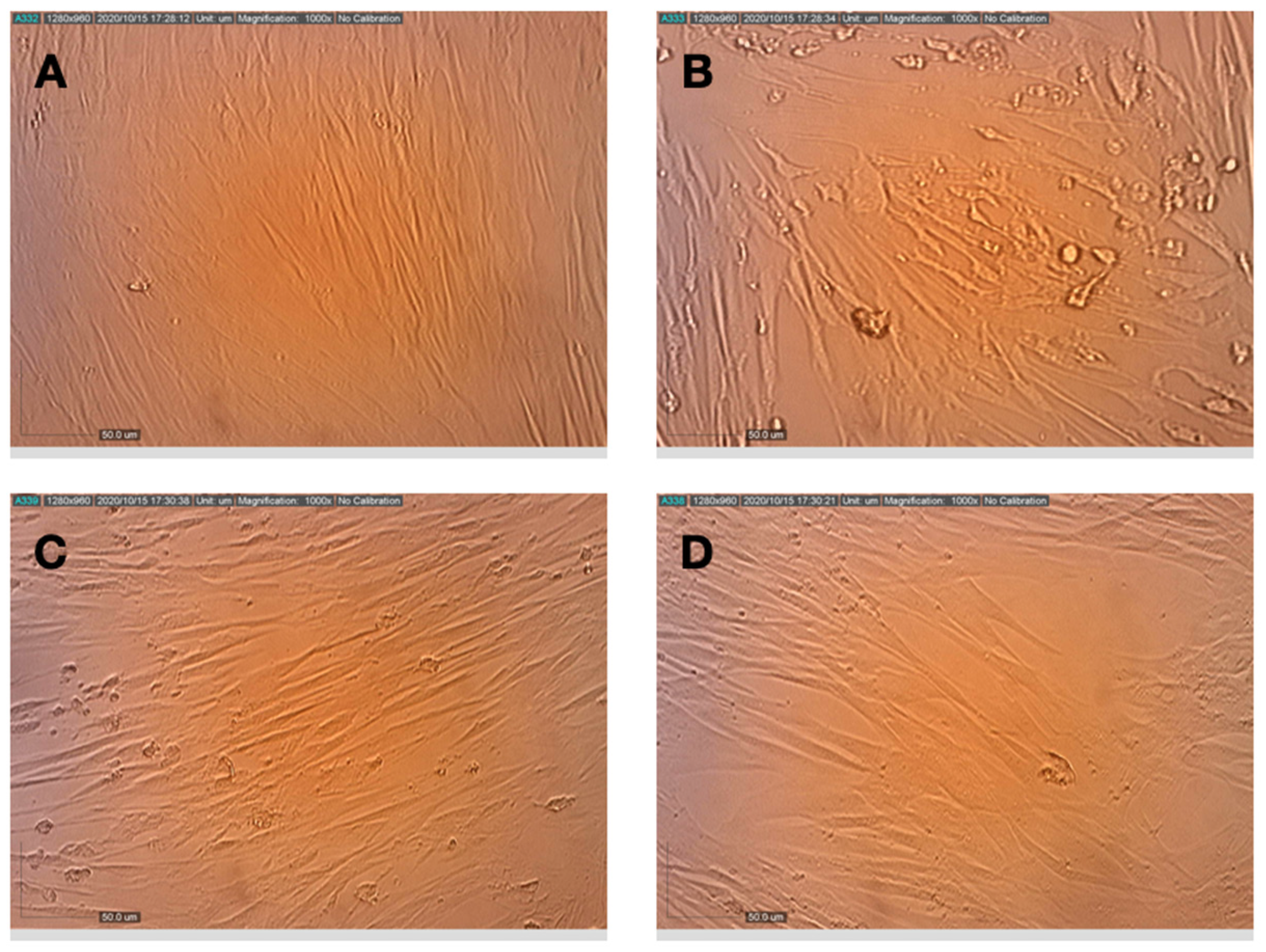
3.1. Cytotoxicity of Compounds
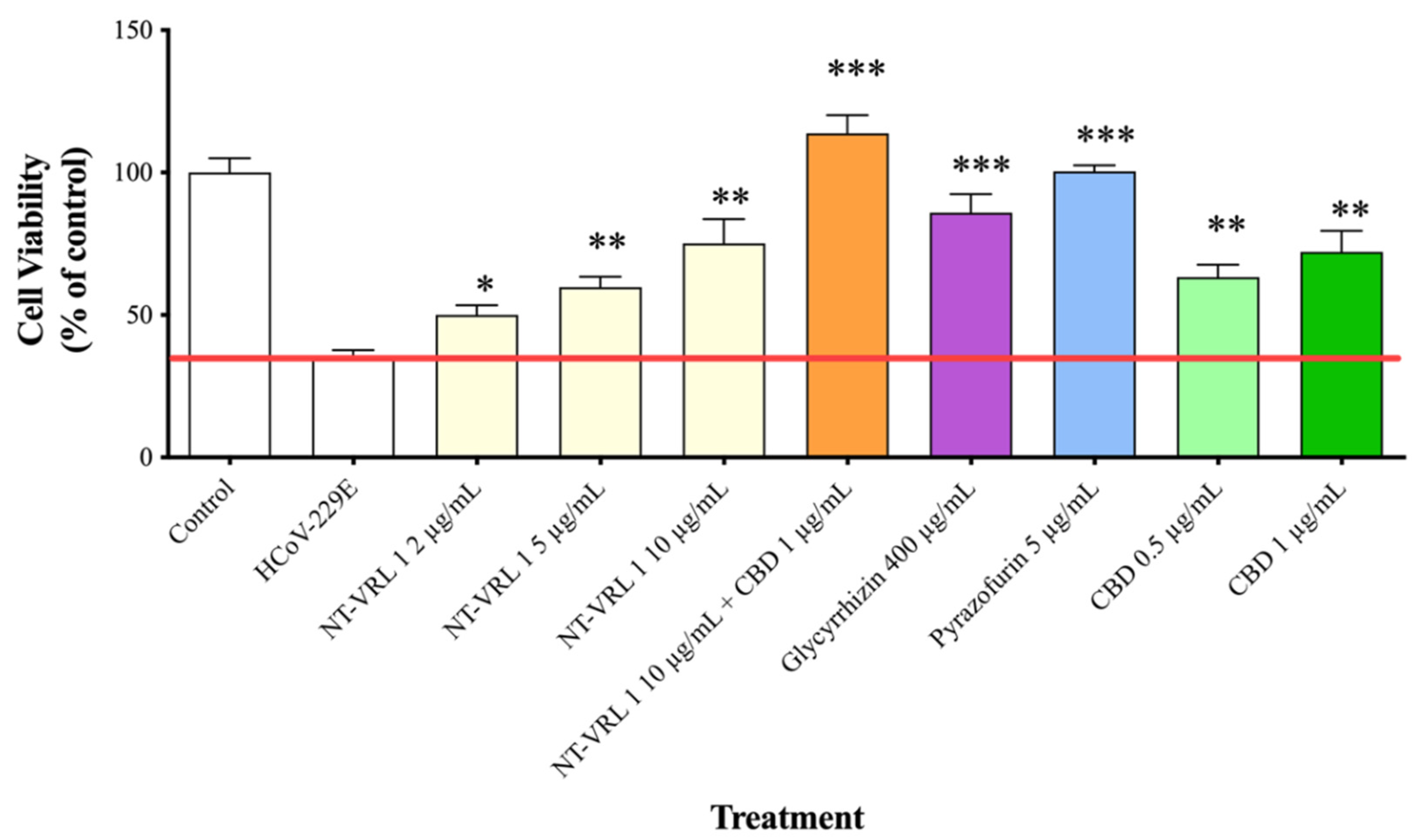
3.2. Efficacy of Compounds—Cell Pretreatment
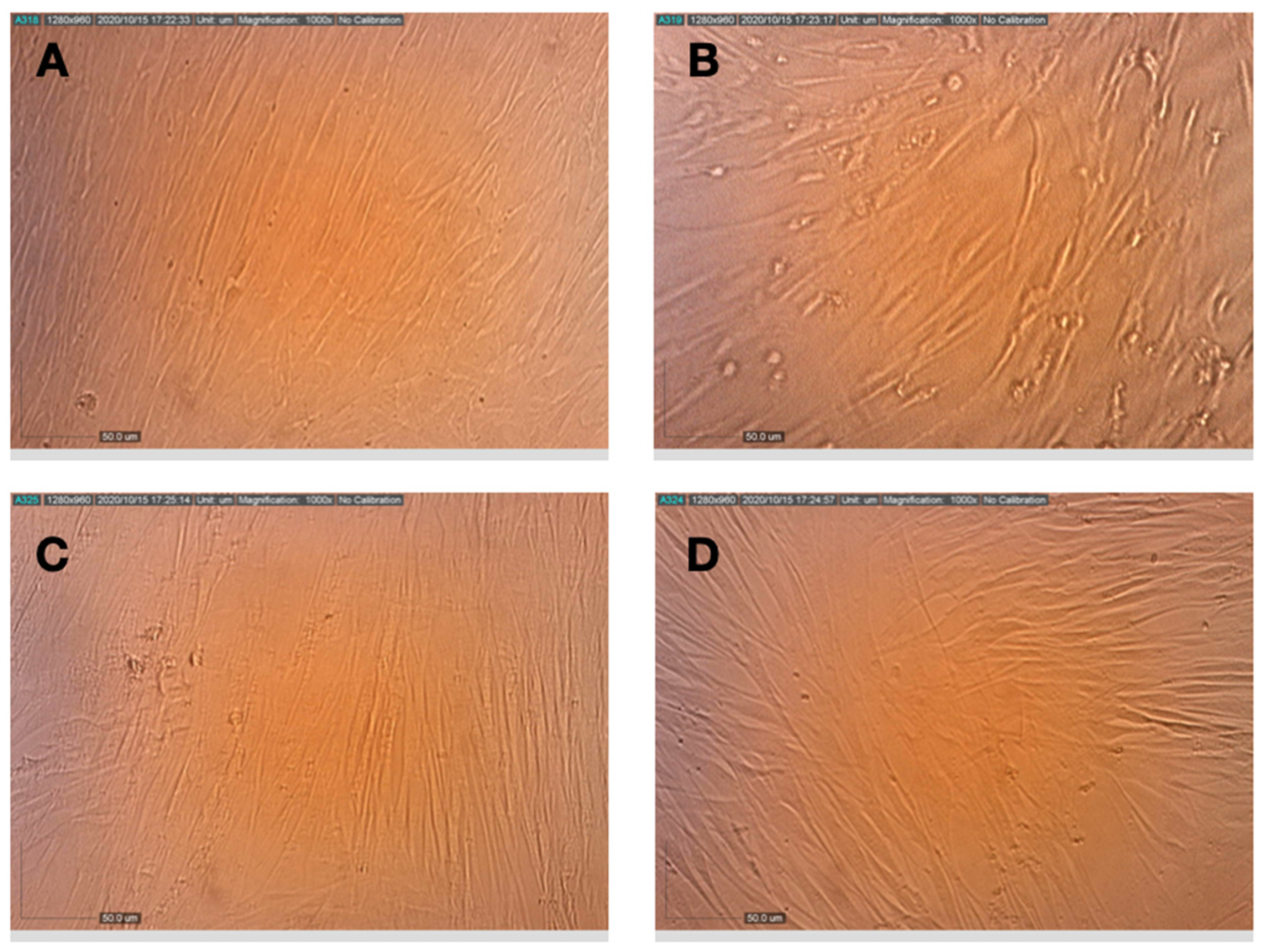
3.3. Efficacy of Compounds—Virus Pretreatment
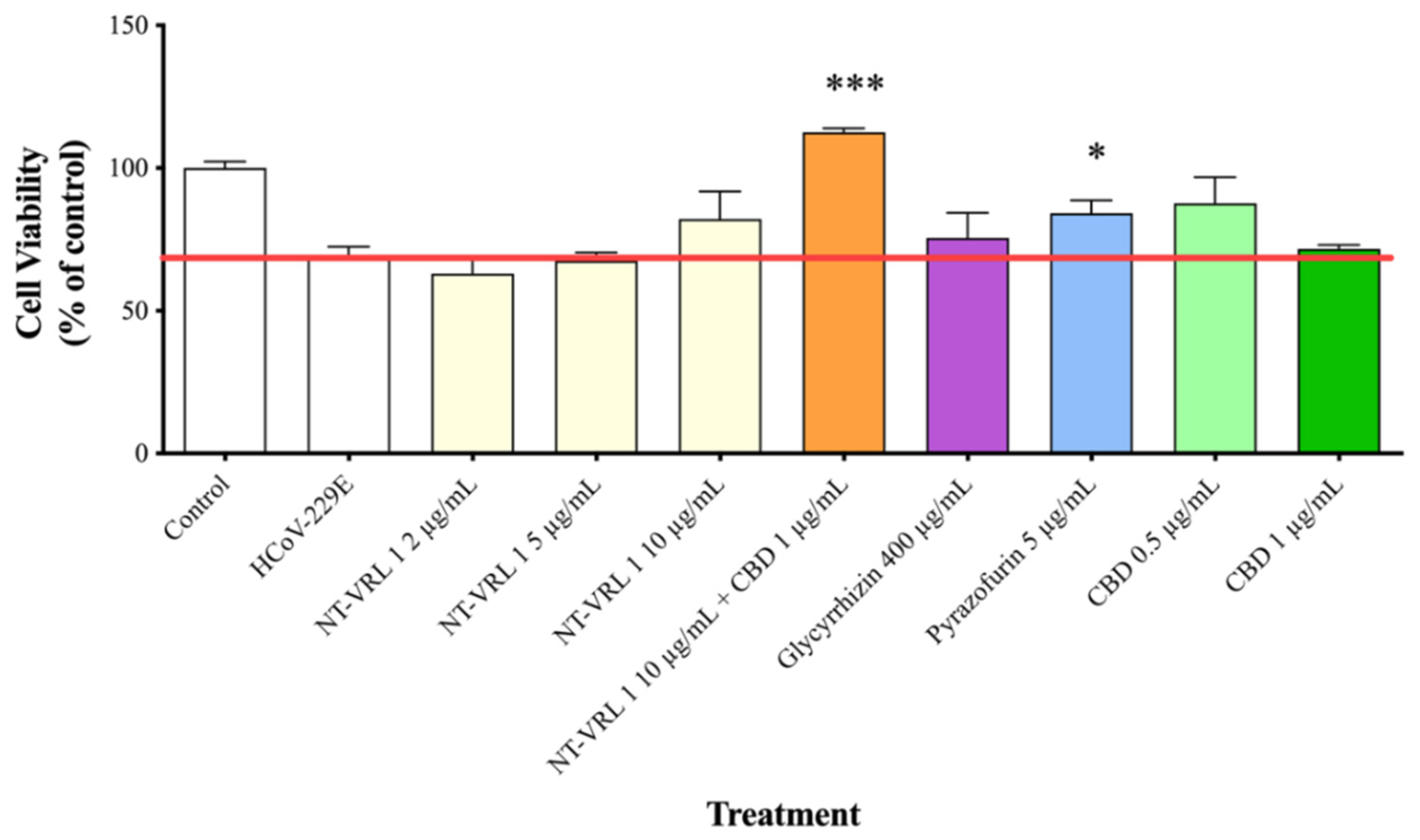
3.4. Efficacy of Compounds—Post-Adsorption
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 29 March 2021).
- Ma, Q.; Li, R.; Pan, W.; Huang, W.; Liu, B.; Xie, Y.; Wang, Z.; Li, C.; Jiang, H.; Huang, J.; et al. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine 2020, 78, 153296. [Google Scholar] [CrossRef] [PubMed]
- Parang, K.; El-Sayed, N.S.; Kazeminy, A.J.; Tiwari, R.K. Comparative antiviral activity of remdesivir and anti-HIV nucleoside analogs against human coronavirus 229E (HCoV-229E). Molecules 2020, 25, 2343. [Google Scholar] [CrossRef] [PubMed]
- Pene, F.; Merlat, A.; Vabret, A.; Rozenberg, F.; Buzyn, A.; Dreyfus, F.; Cariou, A.; Freymuth, F.; Lebon, P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003, 37, 929–932. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar]
- Prakash, V. Terpenoids as source of anti-inflammatory compounds. Asian J. Pharm. Clin. Res. 2017, 10, 68–76. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Quintans, J.S.; Quintans, L.J. Monoterpenes with analgesic activity—A systematic review. Phytother. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran J. Microbiol. 2014, 6, 149–155. [Google Scholar]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative anti-infectious bronchitis virus (IBV) activity of (−)-pinene: Effect on nucleocapsid (N) protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef]
- Zamora, A.; Edmonds, J.H.; Reynolds, M.J.; Khromykh, A.A.; Ralph, S.J. The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology 2016, 495, 18–32. [Google Scholar] [CrossRef]
- Bicchi, C.; Rubiolo, P.; Ballero, M.; Sanna, C.; Matteodo, M.; Esposito, F.; Zinzula, L.; Tramontano, E. HIV-1-inhibiting activity of the essential oil of Ridolfia segetum and Oenanthe crocata. Planta Med. 2009, 75, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Haiying, L.; Na, H.; Xiaoyuan, X. The curative effects of glycyrrhizin on patients with SARS. In Proceedings of the Annual Meeting of the Society of Infectious and Parasitic Diseases, Wuhan, China, 18‒22 October 2003. [Google Scholar]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.A.; Bynoe, M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet 1966, 1, 76–77. [Google Scholar] [CrossRef]
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.K.; Dey, A. Moringa oleifera Lam and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2020, 129, 272–282. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Wang, B.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, S.; Kovalchuk, I.; Kovalchuk, O. Fighting the storm: Could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19? Aging 2021, 13, 1571–1590. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J. Antiviral activity of glycyrrhizic acid derivatives against SARS coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatow, L.; Nudel, A.; Nesher, I.; Hayo Hemo, D.; Rozenberg, P.; Voropaev, H.; Winkler, I.; Levy, R.; Kerem, Z.; Yaniv, Z.; et al. In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229. Life 2021, 11, 290. https://doi.org/10.3390/life11040290
Chatow L, Nudel A, Nesher I, Hayo Hemo D, Rozenberg P, Voropaev H, Winkler I, Levy R, Kerem Z, Yaniv Z, et al. In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229. Life. 2021; 11(4):290. https://doi.org/10.3390/life11040290
Chicago/Turabian StyleChatow, Lior, Adi Nudel, Iris Nesher, David Hayo Hemo, Perri Rozenberg, Hanna Voropaev, Ilan Winkler, Ronnie Levy, Zohar Kerem, Zohara Yaniv, and et al. 2021. "In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229" Life 11, no. 4: 290. https://doi.org/10.3390/life11040290
APA StyleChatow, L., Nudel, A., Nesher, I., Hayo Hemo, D., Rozenberg, P., Voropaev, H., Winkler, I., Levy, R., Kerem, Z., Yaniv, Z., & Eyal, N. (2021). In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229. Life, 11(4), 290. https://doi.org/10.3390/life11040290






