Reproductive Potential and Outcomes in Patients with Hidradenitis Suppurativa: Clinical Profile and Therapeutic Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Design: Prospective Observational Study
- -
- HS women of childbearing age (15–49 years-old);
- -
- Women who were prescribed systemic therapy for the treatment of HS.
- -
- HS women who did not sign the written consent form or young women under 18 whose legal representative did not sign the informed consent;
- -
- Climacteric women.
2.2. Variables of Interest
2.2.1. Main Variables of Interest
2.2.2. Other Variables of Interest
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features of Women Based on the Fulfillment of Reproductive Desire
3.2. Treatment Patterns in HS Women of Childbearing Age
3.3. Demographic and Clinical Features of Women of Childbearing Age Receiving First-Line Biologic Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuenca-Barrales, C.; Molina-Leyva, A. Risk Factors of Sexual Dysfunction in Patients with Hidradenitis Suppurativa: A Cross-Sectional Study. Dermatology 2020, 236, 37–45. [Google Scholar] [CrossRef]
- Kimball, A.B.; Jemec, G.B. Hidradenitis Suppurativa. Hidradenitis Suppurativa 2017, 366, 158–164. [Google Scholar]
- Alikhan, A.; Lynch, P.J.; Eisen, D.B. Hidradenitis suppurativa: A comprehensive review. J. Am. Acad. Dermatol. 2009, 60, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Revuz, J.E.; Canoui-Poitrine, F.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Roujeau, J.C.; Bonnelye, G.; et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J. Am. Acad. Dermatol. 2008, 59, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Adelekun, A.A.; Micheletti, R.G.; Hsiao, J.L. Creation of a Registry to Address Knowledge Gaps in Hidradenitis Suppurativa and Pregnancy. JAMA Dermatol. 2020, 156, 353. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cantero, A.; Carretero, G.; Rivera, R.; Ferrándiz, C.; Daudén, E.; De La Cueva, P.; Gómez-García, F.; Belinchón, I.; Herrera-Ceballos, E.; Ruiz-Genao, D.; et al. Women with moderate-to-severe psoriasis in Spain (BIOBADADERM registry) show more than a 50% reduction in age-adjusted fertility rate when compared with the general population. Br. J. Dermatol. 2019, 181, 1085–1087. [Google Scholar] [CrossRef]
- Khizroeva, J.; Nalli, C.; Bitsadze, V.; Lojacono, A.; Zatti, S.; Andreoli, L.; Tincani, A.; Shoenfeld, Y.; Makatsariya, A. Infertility in women with systemic autoimmune diseases. Best Pr. Res. Clin. Endocrinol. Metab. 2019, 33, 101369. [Google Scholar] [CrossRef]
- Alavi, A.; Anooshirvani, N.; Kim, W.B.; Coutts, P.; Sibbald, R.G. Quality-of-Life Impairment in Patients with Hidradenitis Suppurativa: A Canadian Study. Am. J. Clin. Dermatol. 2014, 16, 61–65. [Google Scholar] [CrossRef]
- Matusiak, L.; Bieniek, A.; Szepietowski, J.C. Psychophysical Aspects of Hidradenitis Suppurativa. Acta Dermato-Venereol. 2010, 90, 264–268. [Google Scholar] [CrossRef]
- Zouboulis, C.; Desai, N.; Emtestam, L.; Hunger, R.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.; Revuz, J.; et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Ruiz-Villaverde, R.; Molina-Leyva, A. Sexual Distress in Patients with Hidradenitis Suppurativa: A Cross-Sectional Study. J. Clin. Med. 2019, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Jost, A.; Peters, E.M.J.; Witte-Haendel, E.; Sterry, W.; Sabat, R. Association of Hidradenitis Suppurativa With Body Image. JAMA Dermatol. 2018, 154, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, S.; Agnoli, C.; Silvestri, A.; Giari, S.; Bettoli, V.; Caracciolo, S. Anger, Emotional Fragility, Self-esteem, and Psychiatric Comorbidity in Patients with Hidradenitis Suppurativa/Acne Inversa. J. Clin. Psychol. Med. Settings 2019, 27, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.O.; Stergiopoulos, V.; Maes, M.; Kurdyak, P.A.; Lin, P.Y.; Wang, L.-J.; Shyu, Y.-C.; Firth, J.; Koyanagi, A.; Solmi, M.; et al. Depression and Anxiety in Adults With Hidradenitis Suppurativa: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, L.; Cohen, A.D.; Gislason, G.H.; Jemec, G.B.; Egeberg, A. Increased Suicide Risk in Patients with Hidradenitis Suppurativa. J. Investig. Dermatol. 2018, 138, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.; Bechara, F.; Dickinson-Blok, J.; Gulliver, W.; Horváth, B.; Hughes, R.; Kimball, A.; Kirby, B.; Martorell, A.; Podda, M.; et al. Hidradenitis suppurativa/acne inversa: A practical framework for treatment optimization—Systematic review and recommendations from the HS ALLIANCE working group. J. Eur. Acad. Dermatol. Venereol. 2018, 33, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Garruti, G.; De Palo, R.; De Angelisc, M. Weighing the Impact of Diet and Lifestyle on Female Reproductive Function. Curr. Med. Chem. 2019, 26, 3584–3592. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Fabbrocini, G.; Annunziata, G.; Muscogiuri, G.; Donnarumma, M.; Marasca, C.; Colao, A.; Savastano, S. Role of Nutrition and Adherence to the Mediterranean Diet in the Multidisciplinary Approach of Hidradenitis Suppurativa: Evaluation of Nutritional Status and Its Association with Severity of Disease. Nutrients 2018, 11, 57. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef]
- Kelly, G.; Prens, E.P. Inflammatory Mechanisms in Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 51–58. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Llurba, E.; Gris, J.M. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Perng, P.; Zampella, J.G.; Okoye, G.A. Management of hidradenitis suppurativa in pregnancy. J. Am. Acad. Dermatol. 2017, 76, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Leachman, S.A.; Reed, B.R. The Use of Dermatologic Drugs in Pregnancy and Lactation. Dermatol. Clin. 2006, 24, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Cush, J.J.; Ahmed, M.S.; Bermas, B.L.; Chakravarty, E.; Chambers, C.; Clowse, M.; Curtis, J.R.; Dao, K.; Hankins, G.D.V.; et al. Proceedings From the American College of Rheumatology Reproductive Health Summit: The Management of Fertility, Pregnancy, and Lactation in Women With Autoimmune and Systemic Inflammatory Diseases. Arthritis Rheum. 2014, 67, 313–325. [Google Scholar] [CrossRef]
- Van de Bor, M. Fetal toxicology. Handb. Clin. Neurol. 2019, 162, 31–55. [Google Scholar]
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.B.; Gottlieb, A.B.; Hamzavi, I.; et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J. Am. Acad. Dermatol. 2019, 81, 76–90. [Google Scholar] [CrossRef]
- US Food and Drug Administration. KENALOG®-10 INJECTION (Triamcinolone Acetonide Injectable Suspension, USP). 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/012041s045lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. ORACEA® (Doxycycline) Capsules. 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050805s002lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. YASMIN (Drospirenone/Ethinyl Estradiol) Tablets. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021098s019lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. CLEOCIN HCl® Clindamycin Hydrochloride Capsules. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050162s092s093lbl.pdf (accessed on 28 April 2020).
- Deckers, I.E.; Prens, E.P. An Update on Medical Treatment Options for Hidradenitis Suppurativa. Drugs 2015, 76, 215–229. [Google Scholar] [CrossRef]
- US Food and Drug Administration. HUMIRA® (Adalimumab) Injection, for Subcutaneous Use. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s0110lbl.pdf (accessed on 8 April 2020).
- US Food and Drug Administration. MIRENA (Levonorgestrel-Releasing Intrauterine System). 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021225s031lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. Xylocaine (Lidocaine HCl and Epinephrine Injection, USP). 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/006488s074lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. ALDACTONE® (Spironolactone). 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/012151s075lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. SORIATANE (Acitretin) Capsules. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. COLCRYSTM (Colchicine, USP) Tablets for Oral Use. 2009. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022352lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. RIFADIN® (Rifampin Capsules USP). 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/050420s080,050627s023lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. GLUCOPHAGE® (Metformin Hydrochloride) Tablets 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020357s037s039,021202s021s023lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. RAYOS (Prednisone). 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf (accessed on 28 April 2020).
- US Food and Drug Administration. REMICADE (Infliximab). 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf (accessed on 28 April 2020).
- Cooke, C.-L.M.; Davidge, S.T. Advanced maternal age and the impact on maternal and offspring cardiovascular health. Am. J. Physiol. Circ. Physiol. 2019, 317, H387–H394. [Google Scholar] [CrossRef]
- Finer, L.B.; Zolna, M.R. Shifts in Intended and Unintended Pregnancies in the United States, 2001–2008. Am. J. Public Health 2014, 104, S43–S48. [Google Scholar] [CrossRef]
- Rendall, M.; Couet, C.; Lappegard, T.; Robert-Bobée, I.; Rønsen, M.; Smallwood, S. First births by age and education in Britain, France and Norway. Popul. Trends 2005, 2005, 27–34. [Google Scholar]
- Ibarra-Nava, I.; Choudhry, V.; Agardh, A. Desire to delay the first childbirth among young, married women in India: A cross-sectional study based on national survey data. BMC Public Health 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Gearon, E.; Backholer, K.; Bauman, A.; Peeters, A. Age-specific changes in BMI and BMI distribution among Australian adults using cross-sectional surveys from 1980 to 2008. Int. J. Obes. 2015, 39, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Houlihan, C.; Shub, A.; Churilov, L.; Pritchard, N.; Price, S.; Ekinci, E.; Proietto, J.; Permezel, M. How common is substantial weight gain after pregnancy? Obes. Res. Clin. Pr. 2018, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Collier, E.; Shi, V.Y.; Parvataneni, R.K.; Lowes, M.A.; Hsiao, J.L. Special considerations for women with hidradenitis suppurativa. Int. J. Women’s Dermatol. 2020, 6, 85–88. [Google Scholar] [CrossRef]
- Perng, P.; Zampella, J.; Okoye, G. Considering the impact of pregnancy on the natural history of hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.; Peacock, A.; McKenzie, S.; Jacobsen, G.; Naik, H.; Shi, V.; Hamzavi, I.; Hsiao, J. Retrospective cohort study of pregnancy outcomes in hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 945–947. [Google Scholar] [CrossRef]
- Lyons, A.B.; Peacock, A.; McKenzie, S.A.; Jacobsen, G.; Naik, H.B.; Shi, V.Y.; Hamzavi, I.H.; Hsiao, J.L. Evaluation of Hidradenitis Suppurativa Disease Course During Pregnancy and Postpartum. JAMA Dermatol. 2020, 156, 681. [Google Scholar] [CrossRef]
- INE. Instituto Nacional de Estadistica. Población de 16 y Más Años por Estado Civil, Sexo y Grupo de Edad; 2020. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=4031#!tabs-tabla (accessed on 10 May 2020).
- Killasli, H.; Sartorius, K.; Emtestam, L.; Svensson, Å. Hidradenitis Suppurativa in Sweden: A Registry-Based Cross-Sectional Study of 13,538 Patients. Dermatology 2020, 236, 281–288. [Google Scholar] [CrossRef]
- Bhatti, Z.; Finlay, A.Y.; Bolton, C.; George, L.; Halcox, J.; Jones, S.; Ketchell, R.; Moore, R.; Salek, M. Chronic disease influences over 40 major life-changing decisions (MLCDs): A qualitative study in dermatology and general medicine. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1344–1355. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Montero-Vílchez, T.; Szepietowski, J.; Matusiak, L.; Molina-Leyva, A. Sexual impairment in patients with hidradenitis suppurativa: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 345–352. [Google Scholar] [CrossRef]
- Bhatti, Z.; Salek, M.; Finlay, A.Y. Chronic diseases influence major life changing decisions: A new domain in quality of life research. J. R. Soc. Med. 2011, 104, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, K.; Hassard, J.; De Silva, R.; Johnson, L. Acceptability of screening for pregnancy intention in general practice: A population survey of people of reproductive age. BMC Fam. Pr. 2020, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Manfredini, M.; Massoli, L.; Carillo, C.; Barozzi, A.; Amendolagine, G.; Ruina, G.; Musmeci, D.; Libanore, M.; Curtolo, A.; et al. Rates of antibiotic resistance/sensitivity in bacterial cultures of hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. 2018, 33, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Seow, C.H.; Leung, Y.; Casteele, N.V.; Afshar, E.E.; Tanyingoh, D.; Bindra, G.; Stewart, M.J.; Beck, P.L.; Kaplan, G.G.; Ghosh, S.; et al. The effects of pregnancy on the pharmacokinetics of infliximab and adalimumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
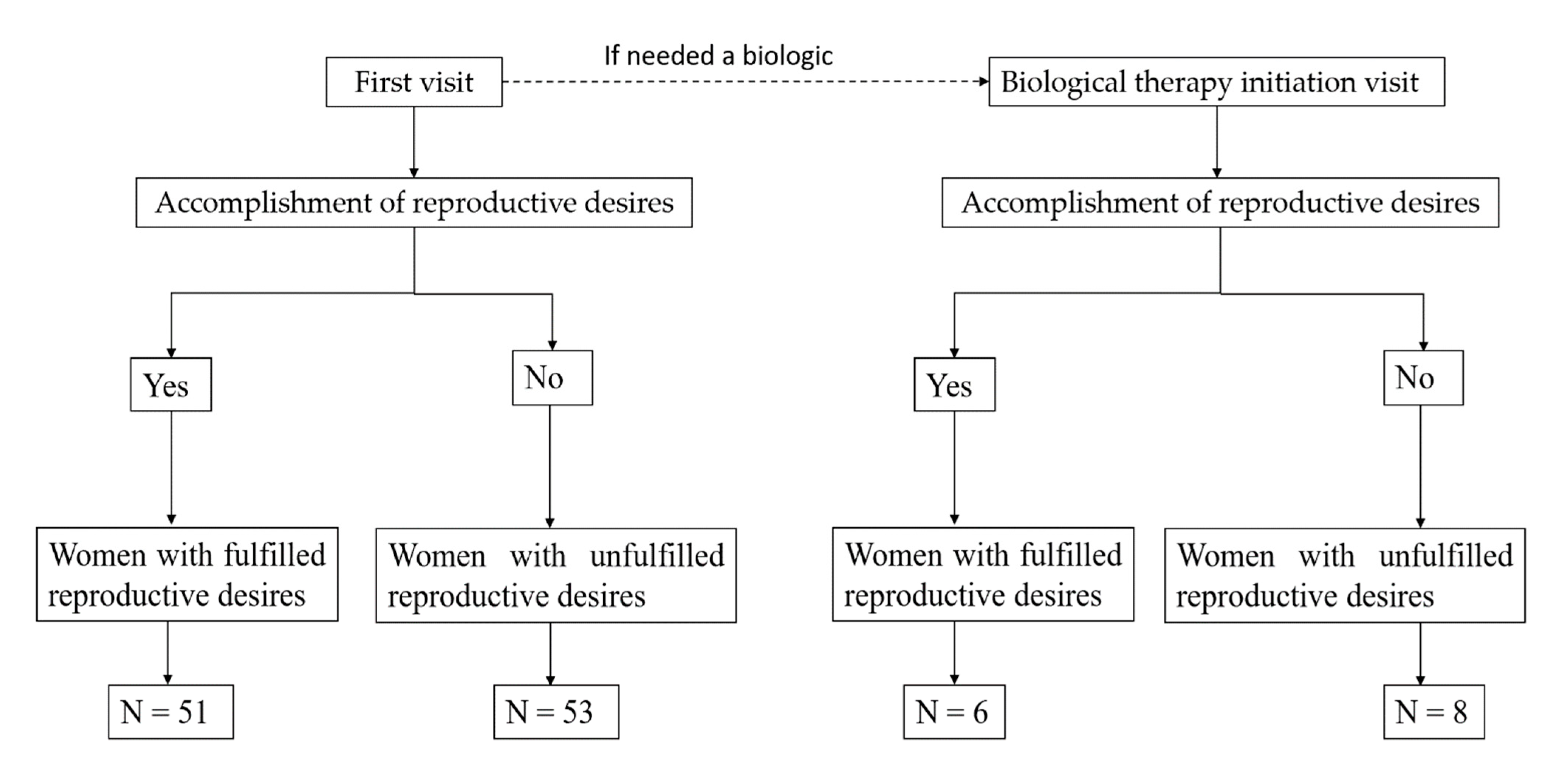
| Variables | Women with Fulfilled Reproductive Desires (N = 51) | Women with Unfulfilled Reproductive Desires (N = 53) | p |
|---|---|---|---|
| Age (years) | 42.06 (SD 6.46) | 29.08 (SD 8.90) | <0.001 * |
Civil status
| 35 (69.63%) 2 (3.92%) 14 (27.45%) | 12 (22.64%) 4 (7.55%) 37 (69.81%) | <0.001 * |
| Mandatory education level (yes) | 30 (58.82%) | 44 (83.02%) | 0.007 * |
| Unemployed (yes) | 26 (50.98%) | 19 (35.85%) | 0.120 |
| BMI (kg/cm2) | 32.64 (SD 7.61) | 28.56 (SD 5.29) | 0.002 * |
| Smoking habit (yes) | 31 (60.78%) | 23 (43.40%) | 0.076 |
| Alcohol consumption (yes) | 9 (17.65%) | 12 (22.64%) | 0.526 |
| Age of onset (years) | 24.63 (SD 10.96) | 18.23 (SD 7.16) | 0.006 * |
| Disease duration (years) | 17.43 (SD 9.29) | 10.85 (SD 6.36) | <0.001 * |
| Family history (yes) | 28 (54.90%) | 27 (50.94%) | 0.686 |
Hurley stage
| 13 (25.49%) 23 (45.10%) 15 (29.41%) | 26 (49.06%) 22 (41.51%) 5 (9.43%) | 0.010 * |
| AN count | 1.86 (SD 2.07) | 2.68 (SD 3.24) | 0.130 |
| Draining tunnels count | 1.14 (SD 1.58) | 0.49 (SD 0.80) | 0.009 * |
| IHS4 | 7.33 (SD 6.75) | 5.51 (SD 5.19) | 0.124 |
| Number of affected areas | 1.90 (SD 1.06) | 1.60 (SD 1.00) | 0.145 |
| Number of previous medical treatments | 1.96 (SD 1.57) | 1.68 (SD 1.36) | 0.330 |
| Number of previous surgeries | 0.98 (SD 1.21) | 1.04 (SD 1.81) | 0.850 |
| Treatment | Total (N = 104) | Women with Fulfilled Reproductive Desires (N = 51) | Women with Unfulfilled Reproductive Desires (N = 53) | p | Pregnancy Category ABCDX | Does It Make Pregnancy Impossible? | Recommended Washing Time to Get Pregnant (Weeks) | Fertility Comments |
|---|---|---|---|---|---|---|---|---|
| Intralesional Corticosteroids [22,27] | 59 (56.73%) | 28 (54.90%) | 31 (58.49%) | 0.136 | C | No. Safe in pregnancy | Safe in pregnancy | Not reported in women |
| Doxycycline [28] | 36 (34.62%) | 16 (31.3%) | 20 (37.7%) | 0.49 | D | Yes. Teratogenic, risk of dental staining, poor bone growth. | 1 # | Unknown. Possible risk of contraceptive failure |
| Oral contraceptives [29] - Combined oral contraceptives - Desogestrel | 33 (31.73%) | 13 (25.49) | 20 (37.74%) | 0.10 | B | No. Little or no increased risk of birth defects in women who inadvertently use during early pregnancy | 2 # | Reversible loss of fertility |
| 21 (20.19%) | 5 (9.80%) | 16 (30.19%) | 0.013 * | |||||
| 12 (11.54%) | 8 (15.69%) | 4 (7.55%) | 0.231 | |||||
| Oral clindamycin [30] | 15 (14.42%) | 10 (19.61%) | 5 (9.43%) | 0.140 | B | No. Not contraindicated during pregnancy, no evidence of teratogenicity | Safe in pregnancy | No effects on fertility or mating ability reported. |
| Resorcinol [22,31] | 8 (7.69%) | 4 (7.84%) | 4 (7.55%) | 1.00 | D | No. Insufficient safety data | One day # | May not affect the reproductive performance and fertility |
| Adalimumab [32] | 8 (7.69%) | 6 (11.76%) | 2 (3.77%) | 0.156 | B | No. Safety unclear no increased risk of adverse birth outcomes to date | 24 | No fertility rate reported to date |
| Levonorgestrel-releasing intrauterine system [33] | 6 (5.77%) | 4 (7.84%) | 2 (3.77%) | 0.432 | D | Yes. Ectopic pregnancy, pregnancy loss, septic abortion | 1 # | Reversible loss of fertility |
| Surgery [22,34] | 5 (4.81%) | 2 (3.92%) | 3 (5.66%) | 0.679 | B | No. Lidocaine epinephrine is local anesthetic of choice. Procedures requiring general anesthesia should be deferred | One day | No effect |
| Spironolactone [35] | 4 (3.85%) | 3 (5.88%) | 1 (1.89%) | 0.358 | D | No. May affect sex differentiation for the male during embryogenesis due to anti-androgenic properties | 1 # | May impair mating, fertility, and fecundity |
| Acitretin [36] | 4 (3.85%) | 3 (5.88%) | 1 (1.89%) | 0.358 | X | Yes. Embryotoxic and/or teratogenic | 144 | Reversible mild to moderate spermatogenic/Not fertility impairment reported in women |
| Colchicine [37] | 4 (3.85%) | 3 (5.88%) | 1 (1.89%) | 0.358 | C | No. Risk of teratogenicity is discussed. | One day # | Not established |
| Rifampin [38] | 3 (2.88%) | 2 (3.92%) | 1 (1.89%) | 0.614 | C | No. Insufficient data. May increase the risk for maternal postpartum hemorrhage and bleeding in the exposed infant when administered during the last few weeks of pregnancy | 1 # | Fertility not affected. Possible risk of contraceptive failure concomitant with combined oral contraceptives |
| Metformin [39] | 2 (1.92%) | 0 (0.00%) | 2 (3.77%) | 0.495 | B | No | Safe in pregnancy | No effect |
| Systemic Corticosteroids [22,40] | 2 (1.92%) | 1 (1.96%) | 1 (1.89%) | 1.00 | D | No. Fetal harm can occur with first trimester use. Small risk of oral cleft deformity, no risk of major anomalies | One day # | Not formally evaluated Menstrual irregularities. |
| Infliximab [41] | 1 (0.96%) | 1 (1.96%) | 0 (0.00%) | 0.49 | B | No. Safety unclear no increased risk of adverse birth outcomes to date | 24 | Unknown |
| Variable | Women with Fulfilled Reproductive Desires (N = 6) | Women with Unfulfilled Reproductive Desires (N = 8) | p |
|---|---|---|---|
| Age (years) | 46.50 (SD 4.28) | 30.13 (SD 11.06) | 0.005 * |
Civil status
| 6 (100%) 0 0 | 4 (50.00%) 0 4 (50.00%) | 0.084 |
Educational level
| 6 (100.00%) | 8 (100.00%) | 1 |
| BMI (kg/cm2) | 34.39 (SD 5.72) | 33.11 (SD 6.68) | 0.71 |
| Smoking habit (yes) | 3 (50.0%) | 3 (37.50%) | 0.64 |
| Alcohol consumption (yes) | 1 (16.7%) | 3 (37.50%) | 0.804 |
| Family history (yes) | 4 (66.67%) | 4 (50.00%) | 0.627 |
| Age of onset (years) | 26.83 (SD 9.41) | 15.50 (SD 5.73) | 0.016 * |
| Disease duration (years) | 19.67 (SD 9.58) | 14.63 (SD 5.49) | 0.38 |
Hurley stage
| 0 (0.00%) 1 (16.67%) 5 (83.33%) | 2 (25.00%) 2 (25.00%) 4 (50.00%) | 0.332 |
| AN count | 1.83 (SD 1.72) | 4.13 (SD 5.49) | 0.347 |
| Draining tunnels count | 2.60 (1.75) | 2.00 (SD 1.77) | 0.497 |
| IHS4 | 13.83 (SD 6.88) | 14.38 (SD 11.51) | 0.921 |
| Number of affected areas | 3.33 (SD 1.86) | 3.13 (SD 1.13) | 0.799 |
| Number of previous medical treatments | 4.50 (SD 2.74) | 4.25 (SD 1.28) | 0.822 |
| Number of previous surgeries | 1.00 (SD 0.89) | 2.75 (SD 2.25) | 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Vilchez, T.; Salvador-Rodriguez, L.; Rodriguez-Tejero, A.; Sanchez-Diaz, M.; Arias-Santiago, S.; Molina-Leyva, A. Reproductive Potential and Outcomes in Patients with Hidradenitis Suppurativa: Clinical Profile and Therapeutic Implications. Life 2021, 11, 277. https://doi.org/10.3390/life11040277
Montero-Vilchez T, Salvador-Rodriguez L, Rodriguez-Tejero A, Sanchez-Diaz M, Arias-Santiago S, Molina-Leyva A. Reproductive Potential and Outcomes in Patients with Hidradenitis Suppurativa: Clinical Profile and Therapeutic Implications. Life. 2021; 11(4):277. https://doi.org/10.3390/life11040277
Chicago/Turabian StyleMontero-Vilchez, Trinidad, Luis Salvador-Rodriguez, Andrea Rodriguez-Tejero, Manuel Sanchez-Diaz, Salvador Arias-Santiago, and Alejandro Molina-Leyva. 2021. "Reproductive Potential and Outcomes in Patients with Hidradenitis Suppurativa: Clinical Profile and Therapeutic Implications" Life 11, no. 4: 277. https://doi.org/10.3390/life11040277
APA StyleMontero-Vilchez, T., Salvador-Rodriguez, L., Rodriguez-Tejero, A., Sanchez-Diaz, M., Arias-Santiago, S., & Molina-Leyva, A. (2021). Reproductive Potential and Outcomes in Patients with Hidradenitis Suppurativa: Clinical Profile and Therapeutic Implications. Life, 11(4), 277. https://doi.org/10.3390/life11040277









