Single Nucleotide Polymorphism in the IL17A Gene Is Associated with Interstitial Lung Disease Positive to Anti-Jo1 Antisynthetase Autoantibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects Included
2.1.1. Cases Groups
2.1.2. Control Group
2.2. Ethics Approval and Informed Consent
2.3. DNA Extraction
2.4. SNP Selection
2.5. SNP Genotyping
2.6. Hardy-Weinberg Equilibrium and Haplotypes
2.7. Statistical Analysis
3. Results
3.1. Demographic Variables in Case and Control Groups
3.2. Demographic Variables in Anti-Jo1 and Non-Anti-Jo1 Groups
3.3. Hardy–Weinberg Equilibrium
3.4. Allele and Genotype Frequencies
3.4.1. Case and Control Groups
3.4.2. Anti-Jo1 and Non-Anti-Jo1 Groups
3.4.3. Anti-Jo1 and HS Groups
3.4.4. Anti-Ro52+ versus Anti-Ro52- and HS Groups
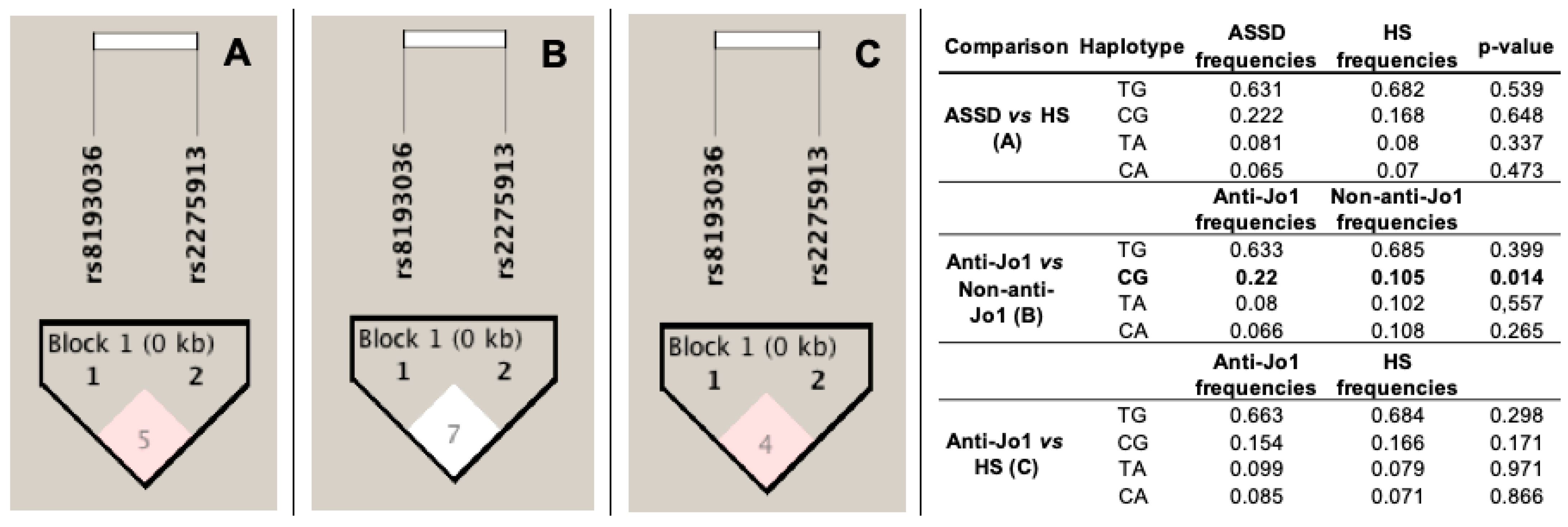
3.5. Linkage Disequilibrium (LD) and Haplotype Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imbert-Masseau, A.; Hamidou, M.; Agard, C.; Grolleau, J.Y.; Chérin, P. Antisynthetase syndrome. Jt. Bone Spine 2003, 70, 161–168. [Google Scholar] [CrossRef]
- Bernstein, R.M.; Morgan, S.H.; Chapman, J.; Bunn, C.C.; Mathews, M.B.; Turner-Warwick, M.; Hughes, G.R. Anti-Jo-1 antibody: A marker for myositis with interstitial lung disease. Br. Med. J. 1984, 289, 151–152. [Google Scholar] [CrossRef]
- Mahler, M.; Miller, F.W.; Fritzler, M.J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmun Rev. 2014, 13, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martinez, E.; Falfán-Valencia, R.; Pérez-Rubio, G.; Mejia, M.; Buendía-Roldán, I.; González-Pérez, M.I.; Mateos-Toledo, H.N.; Rojas-Serrano, J. Anti-Aminoacyl Transfer-RNA-Synthetases (Anti-tRNA) Autoantibodies Associated with Interstitial Lung Disease: Pulmonary Disease Progression has a Persistent Elevation of the Th17 Cytokine Profile. J. Clin. Med. 2020, 9, 1356. [Google Scholar] [CrossRef]
- Douglas, W.W.; Tazelaar, H.D.; Hartman, T.E.; Hartman, R.P.; Decker, P.A.; Schroeder, D.R.; Ryu, J.H. Polymyositis-dermatomyositis-associated interstitial lung disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1182–1185. [Google Scholar] [CrossRef]
- Scirè, C.A.; Gonzalez-Gay, M.A.; Selva-O’Callaghan, A.; Cavagna, L. Clinical spectrum time course of interstitial pneumonia with autoimmune features in patients positive for antisynthetase antibodies. Respir. Med. 2017, 132, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Mejía, M.; Herrera-Bringas, D.; Pérez-Román, D.I.; Rivero, H.; Mateos-Toledo, H.; Castorena-García, P.; Figueroa, J.E.; Rojas-Serrano, J. Interstitial lung disease and myositis-specific and associated autoantibodies: Clinical manifestations, survival and the performance of the new ATS/ERS criteria for interstitial pneumonia with autoimmune features (IPAF). Respir. Med. 2017, 123, 79–86. [Google Scholar] [CrossRef]
- Huang, H.L.; Lin, W.C.; Yeh, C.C.; Sun, Y.T. Serological risk factors for concomitant interstitial lung disease in patients with idiopathic inflammatory myopathy. J. Clin. Neurosci. 2020, 74, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Serrano, J.; Herrera-Bringas, D.; Mejía, M.; Rivero, H.; Mateos-Toledo, H.; Figueroa, J.E. Prognostic factors in a cohort of antisynthetase syndrome (ASS): Serologic profile is associated with mortality in patients with interstitial lung disease (ILD). Clin. Rheumatol. 2015, 34, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Marie, I.; Josse, S.; Hatron, P.Y.; Dominique, S.; Hachulla, E.; Janvresse, A.; Cherin, P.; Mouthon, L.; Vittecoq, O.; Menard, J.F.; et al. Interstitial lung disease in anti-Jo-1 patients with antisynthetase syndrome. Arthritis Care Res. 2013, 65, 800–808. [Google Scholar] [CrossRef]
- Kang, E.H.; Go, D.J.; Mimori, T.; Lee, S.J.; Kwon, H.M.; Park, J.W.; Park, M.H.; Song, E.Y.; Ha, Y.J.; Lee, E.Y.; et al. Novel susceptibility alleles in HLA region for myositis and myositis specific autoantibodies in Korean patients. Semin. Arthritis Rheum. 2019, 49, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Chinoy, H.; Salway, F.; Fertig, N.; Shephard, N.; Tait, B.D.; Thomson, W.; Isenberg, D.A.; Oddis, C.V.; Silman, A.J.; Ollier, W.E.R.; et al. In adult onset myositis, the presence of interstitial lung disease and myositis specific/associated antibodies are governed by HLA class II haplotype, rather than by myositis subtype. Arthritis Res. Ther. 2006, 8, R13. [Google Scholar] [CrossRef]
- Eskandari-Nasab, E.; Moghadampour, M.; Tahmasebi, A. Meta-Analysis of Risk Association Between Interleukin-17A and F Gene Polymorphisms and Inflammatory Diseases. J. Interferon Cytokine Res. 2017, 37, 165–174. [Google Scholar] [CrossRef]
- Marwa, O.S.; Kalthoum, T.; Wajih, K.; Kamel, H. Association of IL17A and IL17F genes with rheumatoid arthritis disease and the impact of genetic polymorphisms on response to treatment. Immunol. Lett. 2017, 183, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.; Mosaad, Y.M.; Hammad, E.M.; Elhanbly, S.; El-Bassiony, S.R.; Al-Harrass, M.F.; Eid, R.; Eldein, O.A.S.; Alsawah, G.A.; Yahia, S.; et al. Interleukin-17A rs2275913, Interleukin-17F rs763780 and rs2397084 gene polymorphisms as possible risk factors in Juvenile lupus and lupus related nephritis. Autoimmunity. 2016, 49, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Montúfar-Robles, I.; Barbosa-Cobos, R.E.; Alemán-Ávila, I.; Ramírez-Bello, J. IL-17A haplotype confers susceptibility to systemic lupus erythematosus but not to rheumatoid arthritis in Mexican patients. Int. J. Rheum Dis. 2019, 22, 473–479. [Google Scholar] [CrossRef]
- Ensembl Genome Browser 89 [Internet]. Available online: http://www.ensembl.org/index.html (accessed on 8 September 2020).
- Ponce-Gallegos, M.A.; Pérez-Rubio, G.; Ambrocio-Ortiz, E.; Partida-Zavala, N.; Hernández-Zenteno, R.; Flores-Trujillo, F.; García-Gómez, L.; Hernández-Pérez, A.; Ramírez-Venegas, A.; Falfán-Valencia, R. Genetic variants in IL17A and serum levels of IL-17A are associated with COPD related to tobacco smoking and biomass burning. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Little, J.; Higgins, J.P.T.; Ioannidis, J.P.A.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. STrengthening the REporting of Genetic Association studies (STREGA)--an extension of the STROBE statement. Eur. J. Clin. Investig. 2009, 39, 247–266. [Google Scholar] [CrossRef]
- SNPStats: A Web Tool for the Analysis of Association Studies—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/16720584/ (accessed on 8 September 2020).
- Haploview: Analysis and Visualization of LD and Haplotype Maps—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/15297300/ (accessed on 8 September 2020).
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The Structure of Haplotype Blocks in the Human Genome. Science (80- ) [Internet]. 2002. Available online: https://pubmed.ncbi.nlm.nih.gov/12029063/ (accessed on 8 September 2020).
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2004.
- González-Pérez, M.I.; Mejía-Hurtado, J.G.; Pérez-Román, D.I.; Buendía-Roldán, I.; Mejía, M.; Falfán-Valencia, R.; Mateos-Toledo, H.N.; Rojas-Serrano, J. Evolution of Pulmonary Function in a Cohort of Interstitial Lung Disease Patients Positive to Antisynthetase Antibodies (ASAB). J. Rheumatol. 2019. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Huapaya, J.A.; Albayda, J.; Paik, J.J.; Johnson, C.; Silhan, L.; Christopher-Stine, L.; Mammen, A.L.; Danoff, S.K. A longitudinal cohort study of the anti-synthetase syndrome: Increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatol 2017, 56, 999–1007. [Google Scholar] [CrossRef]
- Trallero-Araguás, C.; Cavazzana, M.; Feist, R.-S.; Cavazzana, I.; Rojas-Serrano, J.; Feist, E.; Zanframundo, G.; Morandi, V.; Meyer, A.; da Silva, J.A.P.; et al. Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course. J. Clin. Med. 2019, 8, 2013. [Google Scholar]
- Hervier, B.; Devilliers, H.; Stanciu, R.; Meyer, A.; Uzunhan, Y.; Masseau, A.; Dubucquoi, S.; Hatron, P.Y.; Musset, L.; Wallaert, B.; et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 2012, 12, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Waseda, Y.; Johkoh, T.; Egashira, R.; Sumikawa, H.; Saeki, K.; Watanabe, S.; Matsunama, R.; Takato, H.; Ichikawa, Y.; Hamaguchi, Y.; et al. Antisynthetase syndrome: Pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur. J. Radiol. 2016, 85, 1421–1426. [Google Scholar] [CrossRef]
- Jensen, M.L.; Løkke, A.; Hilberg, O.; Hyldgaard, C.; Bendstrup, E.; Tran, D. Clinical characteristics and outcome in patients with antisynthetase syndrome associated interstitial lung disease: A retrospective cohort study Clinical characteristics and outcome in patients with antisynthetase syndrome associated interstitial lung disease: A retrospective cohort study. Eur. Clin. Respir. J. 2019, 6. [Google Scholar] [CrossRef]
- Ponce-Gallegos, M.A.; Ramos-Martínez, E.; García-Carmona, A.; Mejía, M.; Nava-Quiroz, K.J.; Pérez-Rubio, G.; Ambrocio-Ortiz, E.; González-Pérez, M.I.; Buendía-Roldán, I.; Rojas-Serrano, J.; et al. Genetic Susceptibility to Antisynthetase Syndrome Associated With Single-Nucleotide Variants in the IL1B Gene That Lead Variation in IL-1β Serum Levels. Front. Med. 2020, 7, 547186. [Google Scholar] [CrossRef]
- Sugiura, T.; Kawaguchi, Y.; Goto, K.; Hayashi, Y.; Tsuburaya, R.; Furuya, T.; Gono, T.; Nishino, I.; Yamanaka, H. Positive association between STAT4 polymorphisms, and polymyositis/ dermatomyositis in a Japanese population. Ann. Rheum Dis. 2012, 71, 1646–1650. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Wen, X.; Wang, Q.; Lv, G.; Li, J.; Yang, F.; Zhang, F.; Li, Y. Positive association between ankrd55 polymorphism 7731626 and dermatomyositis/polymyositis with interstitial lung disease in Chinese han population. Biomed. Res. Int. 2017, 2017, 2905987. [Google Scholar]
- López-Mejías, R.; Remuzgo-Martínez, S.; Genre, F.; Pulito-Cueto, V.; Rozas, S.M.F.; Llorca, J.; Fernández, D.I.; Cuesta, V.M.M.; Ortego-Centeno, N.; Gómez, N.P.; et al. Influence of MUC5B gene on antisynthetase syndrome. Sci. Rep. 2020, 10, 1415. [Google Scholar] [CrossRef] [PubMed]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Juge, P.A.; Lee, J.S.; Ebstein, E.; Furukawa, H.; Dobrinskikh, E.; Gazal, S.; Kannengiesser, C.; Ottaviani, S.; Oka, S.; Tohma, S.; et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N. Engl. J. Med. 2018, 379, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Miossec, P. Interleukin-17 and Lupus: Enough to be a Target? For Which Patients? Lupus 2019, 0, 1–9. [Google Scholar] [CrossRef]
- Crispín, J.C.; Oukka, M.; Bayliss, G.; Cohen, R.A.; Van Beek, C.A.; Stillman, I.E.; Kyttaris, V.C.; Juang, Y.T.; Tsokos, G.C. Expanded Double Negative T Cells in Patients with Systemic Lupus Erythematosus Produce IL-17 and Infiltrate the Kidneys. J. Immunol. 2008, 181, 8761–8766. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Miossec, P. IL-17 in rheumatoid arthritis and precision medicine: From synovitis expression to circulating bioactive levels. Front. Med. 2019, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Takami, A.; Nakata, K.; Onizuka, M.; Kawase, T.; Akiyama, H.; Miyamura, K.; Morishima, Y.; Fukuda, T.; Kodera, Y.; et al. A Genetic Variant in the IL-17 Promoter Is Functionally Associated with Acute Graft-Versus-Host Disease after Unrelated Bone Marrow Transplantation. PLoS ONE 2011, 6, e26229. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ji, X.; Wu, B.; Wang, T.; Han, L.; Yang, J.; Zhu, B.; Ni, C. Polymorphisms in interleukin 17A gene and coal workers’ pneumoconiosis risk in a Chinese population. BMC Pulm. Med. 2015, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Behrens Pinto, G.L.; Carboni RC de, S.; de Souza, F.H.C.; Shinjo, S.K. A prospective cross-sectional study of serum IL-17A in antisynthetase syndrome. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Zou, J.F.; Cheng, Z.S. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-β1 mediated Smad2/3 and ERK1/2 activation. PLoS ONE. 2017, 12, e0183972. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, C.; Qin, F.; He, Z.Y.; Wang, X.; Zhong, X.N. Th17 Cells and IL-17 Promote the Skin and Lung Inflammation and Fibrosis Process in a Bleomycin-Induced Murine Model of Systemic Sclerosis. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 100), 14–22. [Google Scholar]
- Wu, S.; Tang, X.; Wu, L.; Lu, L.J.; Feng, X. Association of anti-Ro52 autoantibodies with interstitial lung disease in connective tissue diseases. Ann. Rheum. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
| Variables | ASSD | HS | p-Value | Anti-Jo1 | Non-Anti-Jo1 | p-Value |
|---|---|---|---|---|---|---|
| (n = 121) | (n = 346) | (n = 52) | (n = 69) | |||
| Age, (years) | 55 (27–83) | 55 (21–80) | 0.66 | 54 (41–73) | 58 (38–75) | 0.29 |
| Sex, female (%) | 82 (67.77) | 263 (76.01) | 0.06 | 35 (67.31) | 47 (79.66) | 0.14 |
| BMI, kg/m2 | 27.93 (15.61–51) | 27.78 (17.09–45.12) | 0.68 | 27.42 (23–34.29) | 27.75 (15.61–34.92) | 0.56 |
| Pulmonary function | ||||||
| FVC, % | 60.5 (32–114) | 56.5 (32–114) | 61 (35–109) | 0.43 | ||
| DLCO, % | 50 (2–110) | 48.5 (6.25–102) | 53 (2–110) | 0.85 | ||
| Arthritis | n = 105 | n = 46 | n = 59 | |||
| Yes (%) | 77 (73.33) | 38 (82.61) | 39 (66.10) | 0.057 | ||
| Mechanic’s hands | n = 46 | n = 59 | ||||
| Yes (%) | 64 (60.95) | 30 (65.22) | 28 (47.46) | 0.07 | ||
| Fever | n = 46 | n = 59 | ||||
| Yes (%) | 60 (57.14) | 30 (65.22) | 30 (50.85) | 0.14 | ||
| Raynaud’s phenomenon | n = 46 | n = 59 | ||||
| Yes (%) | 50 (47.62) | 24 (52.17) | 26 (44.07) | 0.41 | ||
| CPK, U/I | 109 (18–14270) | 242.5 (24–7210) | 67.5 (18–14270) | 0.001 | ||
| Autoantibodies | ||||||
| Anti-Jo1 (%) | 52 (42.98) | 52 (100) | 0 | |||
| Anti-PL12 (%) | 39 (32.23) | 4 (7.69) | 35 (50.72) | |||
| Anti-PL7 (%) | 24 (19.83) | 3 (5.77) | 21 (30.43) | |||
| Anti-EJ (%) | 14 (11.57) | 1 (1.92) | 13 (18.84) | |||
| Anti-OJ (%) | 10 (8.26) | 0 | 10 (14.49) | |||
| Anti-Ro52 (%) | 62 (51.23) | 28 (53.85) | 34 (49.28) | 0.61 | ||
| HRCT | n = 104 | n = 46 | n = 58 | |||
| NSIP (%) | 45 (43.27) | 19 (41.30) | 26 (44.83) | 0.71 | ||
| COP (%) | 38 (36.54) | 19 (41.30) | 19 (32.76) | 0.37 | ||
| UIP (%) | 17 (16.35) | 6 (13.05) | 11 (18.97) | 0.41 | ||
| LIP (%) | 4 (3.84) | 2 (4.35) | 2 (3.44) | 0.81 |
| Model | ASSD | HS | p-Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| n = 120 | F (%) | n = 340 | F (%) | ||||
| rs2275913 | |||||||
| Genotypes | |||||||
| GG | 81 | 67.50 | 243 | 71.47 | 0.41 | 0.83 | 0.53–1.30 |
| GA | 34 | 28.33 | 92 | 27.06 | 0.79 | 1.07 | 0.67–1.69 |
| AA | 5 | 4.17 | 5 | 1.47 | 0.08 | 2.91 | 0.82–10.24 |
| Alleles | |||||||
| G | 196 | 81.67 | 578 | 85 | 0.22 | 0.78 | 0.53–1.16 |
| A | 44 | 18.33 | 102 | 15 | 1.27 | 0.86–1.88 | |
| Dominant | |||||||
| GG | 81 | 67.50 | 243 | 71.47 | 0.41 | 0.83 | 0.53–1.30 |
| GA+AA | 39 | 32.50 | 97 | 28.53 | 1.21 | 0.77–1.89 | |
| Recessive | |||||||
| GG+GA | 115 | 95.83 | 335 | 98.53 | 0.08 | 0.34 | 0.10–1.21 |
| AA | 5 | 4.17 | 5 | 1.47 | 2.91 | 0.82–10.24 | |
| rs8193036 | |||||||
| Genotypes | n = 115 | F (%) | n = 343 | F (%) | |||
| TT | 66 | 57.39 | 199 | 58.02 | 0.91 | 0.97 | 0.64–1.49 |
| TC | 42 | 36.52 | 125 | 36.44 | 0.99 | 1 | 0.65–1.56 |
| CC | 7 | 6.09 | 19 | 5.54 | 0.83 | 1.11 | 0.45–2.70 |
| Alleles | |||||||
| T | 174 | 75.65 | 523 | 76.24 | 0.86 | 0.97 | 0.68–1.37 |
| C | 56 | 24.35 | 163 | 23.76 | 1.03 | 0.73–1.46 | |
| Dominant | |||||||
| TT | 66 | 57.39 | 199 | 58.02 | 0.91 | 0.97 | 0.64–1.49 |
| TC+CC | 49 | 42.61 | 144 | 41.98 | 1.03 | 0.67–1.57 | |
| Recessive | |||||||
| TT+TC | 108 | 93.91 | 324 | 94.46 | 0.83 | 0.90 | 0.37–2.21 |
| CC | 7 | 6.09 | 19 | 5.54 | 1.11 | 0.45–2.70 | |
| Model | Anti-Jo1 | Non-Anti-Jo1 | p-Value | ||
|---|---|---|---|---|---|
| n = 51 | F (%) | n = 69 | F (%) | ||
| rs2275913 | |||||
| Genotypes | |||||
| GG | 39 | 76.47 | 42 | 60.87 | 0.07 |
| GA | 9 | 17.65 | 25 | 36.23 | 0.03 |
| AA | 3 | 5.88 | 2 | 2.90 | 0.42 |
| Alleles | |||||
| G | 87 | 85.29 | 109 | 78.99 | 0.21 |
| A | 15 | 14.71 | 29 | 21.01 | |
| rs8193036 | |||||
| Genotypes | n = 48 | F (%) | n = 67 | F (%) | |
| TT | 27 | 56.25 | 39 | 58.21 | 0.83 |
| TC | 14 | 29.17 | 28 | 41.79 | 0.17 |
| CC | 7 | 14.58 | 0 | 0.00 | <0.001 |
| Alleles | |||||
| T | 68 | 70.83 | 106 | 79.10 | 0.15 |
| C | 28 | 29.17 | 28 | 20.90 | |
| Model | Anti-Jo1 | HS | p-Value | p-Value Adj-Bon | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| n = 51 | F (%) | n = 340 | F (%) | |||||
| rs2275913 | ||||||||
| Genotypes | ||||||||
| GG | 39 | 76.47 | 243 | 71.47 | 0.46 | 1.30 | 0.65–2.58 | |
| GA | 9 | 17.65 | 92 | 27.06 | 0.15 | 0.58 | 0.27–1.23 | |
| AA | 3 | 5.88 | 5 | 1.47 | 0.07 | 4.18 | 0.97–18.09 | |
| Alleles | ||||||||
| G | 87 | 85.29 | 578 | 85.00 | 0.94 | 1.02 | 0.57–1.84 | |
| A | 15 | 14.71 | 102 | 15.00 | 0.98 | 0.54–1.76 | ||
| Dominant | ||||||||
| GG | 39 | 76.47 | 243 | 71.47 | 0.46 | 1.30 | 0.65–2.58 | |
| GA+AA | 12 | 23.53 | 97 | 28.53 | 0.77 | 0.39–1.53 | ||
| Recessive | ||||||||
| GG+GA | 48 | 94.12 | 335 | 98.53 | 0.07 | 0.24 | 0.06–1.03 | |
| AA | 3 | 5.88 | 5 | 1.47 | 4.18 | 0.97–18.09 | ||
| rs8193036 | ||||||||
| Genotypes | n = 48 | F (%) | n = 343 | F (%) | ||||
| TT | 27 | 56.25 | 199 | 58.02 | 0.82 | 0.93 | 0.51–1.71 | |
| TC | 14 | 29.17 | 125 | 36.44 | 0.32 | 0.72 | 0.37–1.39 | |
| CC | 7 | 14.58 | 19 | 5.54 | 0.018 | 0.036 | 2.91 | 1.15–7.35 |
| Alleles | ||||||||
| T | 68 | 70.83 | 523 | 76.24 | 0.25 | 0.76 | 0.47–1.22 | |
| C | 28 | 29.17 | 163 | 23.76 | 1.32 | 0.82–2.12 | ||
| Dominant | ||||||||
| TT | 27 | 56.25 | 199 | 58.02 | 0.82 | 0.93 | 0.51–1.71 | |
| TC+CC | 21 | 43.75 | 144 | 41.98 | 1.07 | 0.58–1.98 | ||
| Recessive | ||||||||
| TT+TC | 41 | 85.42 | 324 | 94.46 | 0.018 | 0.036 | 0.16 | 0.06–0.46 |
| CC | 7 | 14.58 | 19 | 5.54 | 2.91 | 1.15–7.35 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce-Gallegos, M.A.; González-Pérez, M.I.; Mejía, M.; Nava-Quiroz, K.J.; Pérez-Rubio, G.; Buendía-Roldán, I.; Ramos-Martínez, E.; Rojas-Serrano, J.; Falfán-Valencia, R. Single Nucleotide Polymorphism in the IL17A Gene Is Associated with Interstitial Lung Disease Positive to Anti-Jo1 Antisynthetase Autoantibodies. Life 2021, 11, 174. https://doi.org/10.3390/life11020174
Ponce-Gallegos MA, González-Pérez MI, Mejía M, Nava-Quiroz KJ, Pérez-Rubio G, Buendía-Roldán I, Ramos-Martínez E, Rojas-Serrano J, Falfán-Valencia R. Single Nucleotide Polymorphism in the IL17A Gene Is Associated with Interstitial Lung Disease Positive to Anti-Jo1 Antisynthetase Autoantibodies. Life. 2021; 11(2):174. https://doi.org/10.3390/life11020174
Chicago/Turabian StylePonce-Gallegos, Marco Antonio, Montserrat I. González-Pérez, Mayra Mejía, Karol J. Nava-Quiroz, Gloria Pérez-Rubio, Ivette Buendía-Roldán, Espiridión Ramos-Martínez, Jorge Rojas-Serrano, and Ramcés Falfán-Valencia. 2021. "Single Nucleotide Polymorphism in the IL17A Gene Is Associated with Interstitial Lung Disease Positive to Anti-Jo1 Antisynthetase Autoantibodies" Life 11, no. 2: 174. https://doi.org/10.3390/life11020174
APA StylePonce-Gallegos, M. A., González-Pérez, M. I., Mejía, M., Nava-Quiroz, K. J., Pérez-Rubio, G., Buendía-Roldán, I., Ramos-Martínez, E., Rojas-Serrano, J., & Falfán-Valencia, R. (2021). Single Nucleotide Polymorphism in the IL17A Gene Is Associated with Interstitial Lung Disease Positive to Anti-Jo1 Antisynthetase Autoantibodies. Life, 11(2), 174. https://doi.org/10.3390/life11020174











