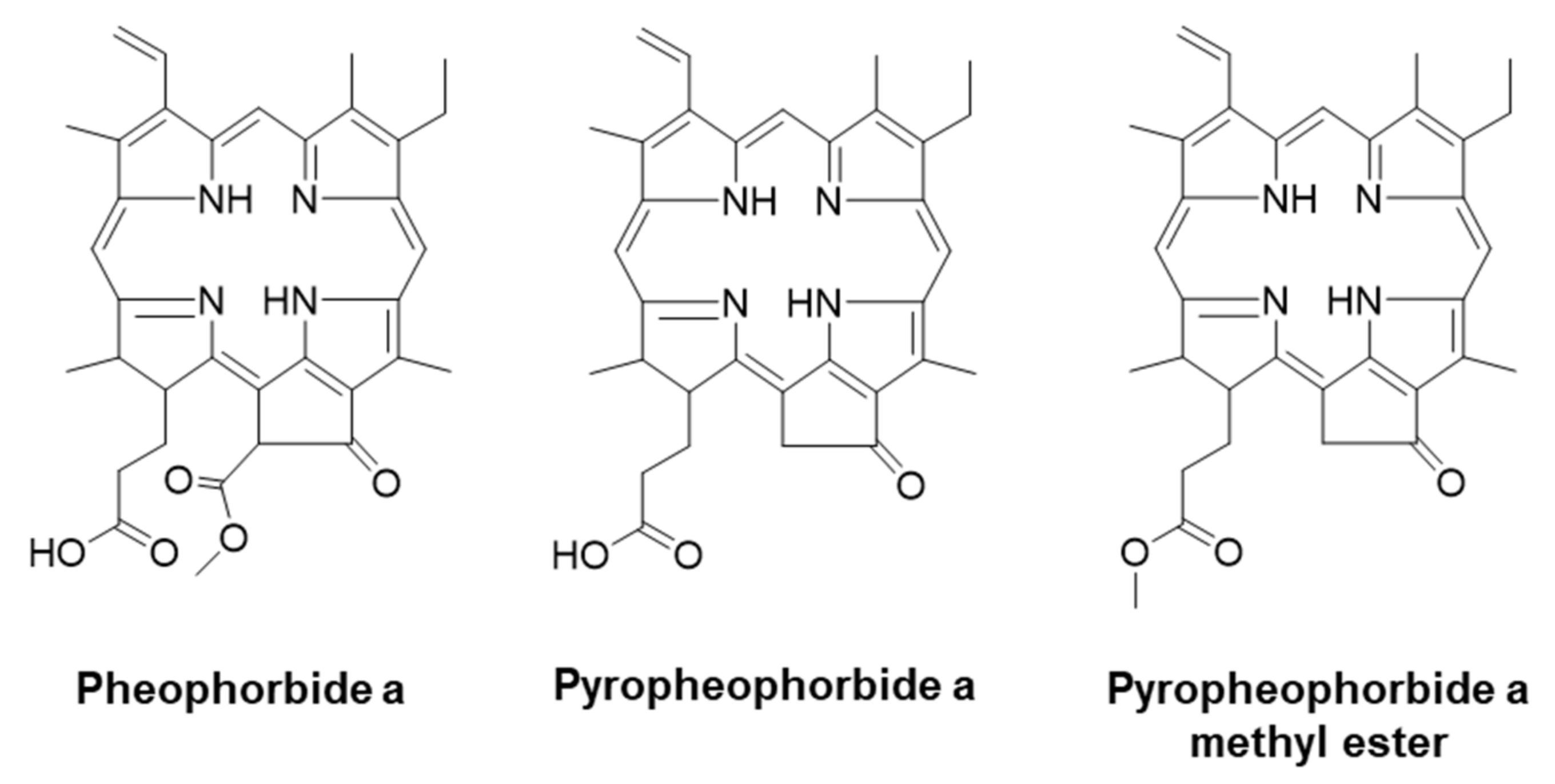
Pheophorbide a Derivatives Exert Antiwrinkle Effects on UVB-Induced Skin Aging in Human Fibroblasts
Abstract
1. Introduction
2. Results
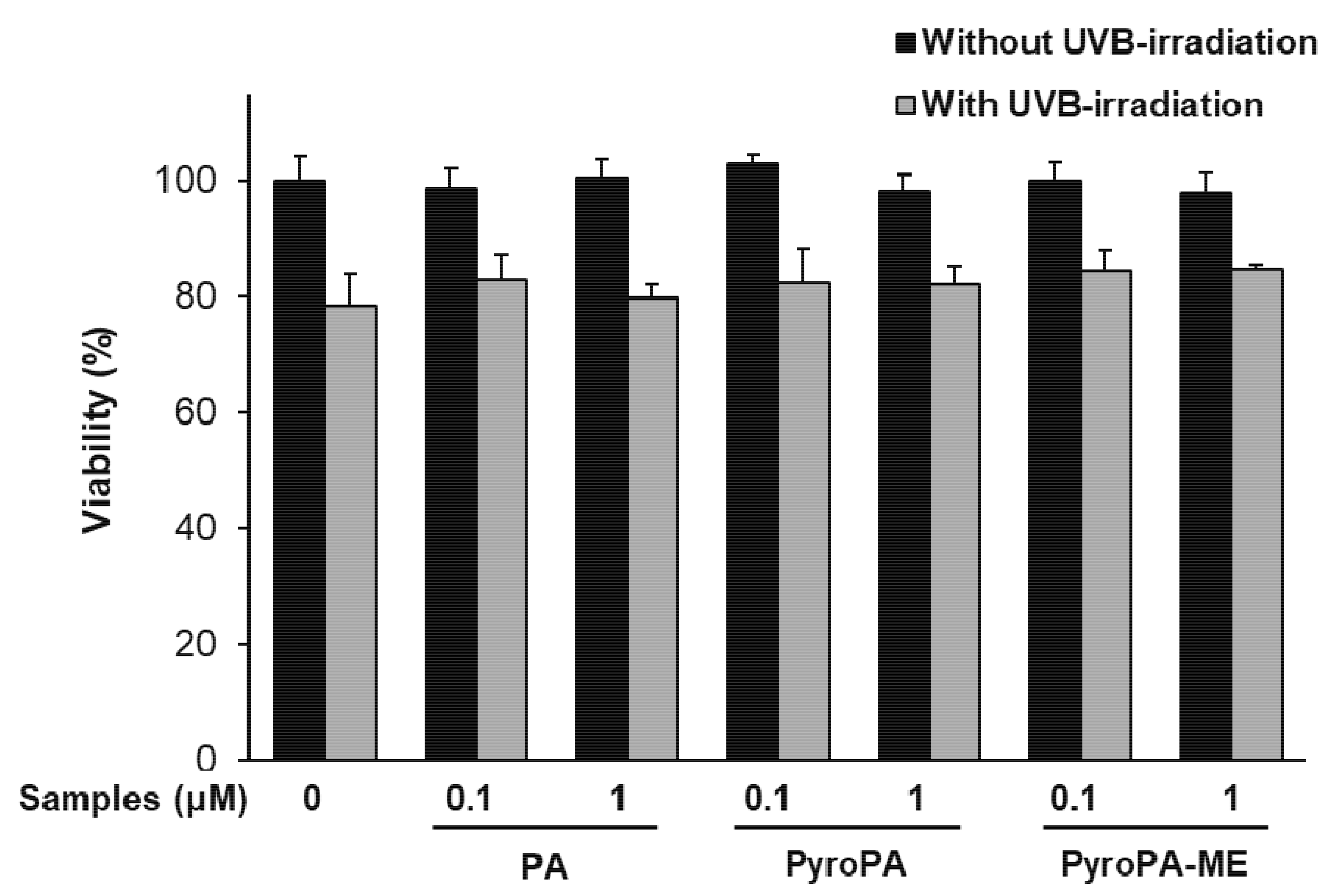
2.1. Effects of Pheophorbides on the Viability of CCD-986sk Cells
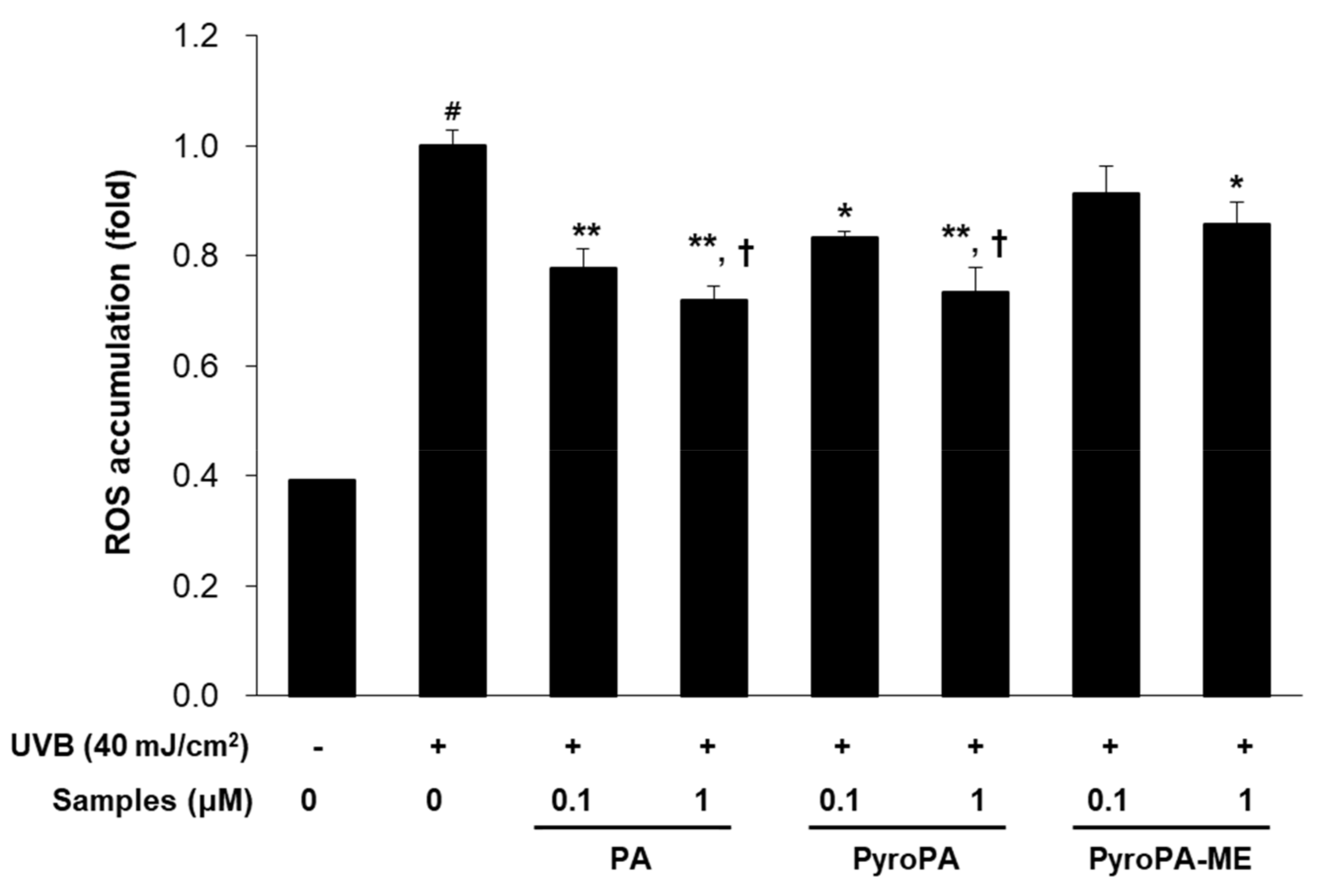
2.2. Effects of Pheophorbides on ROS Accumulation in CCD-986sk Cells
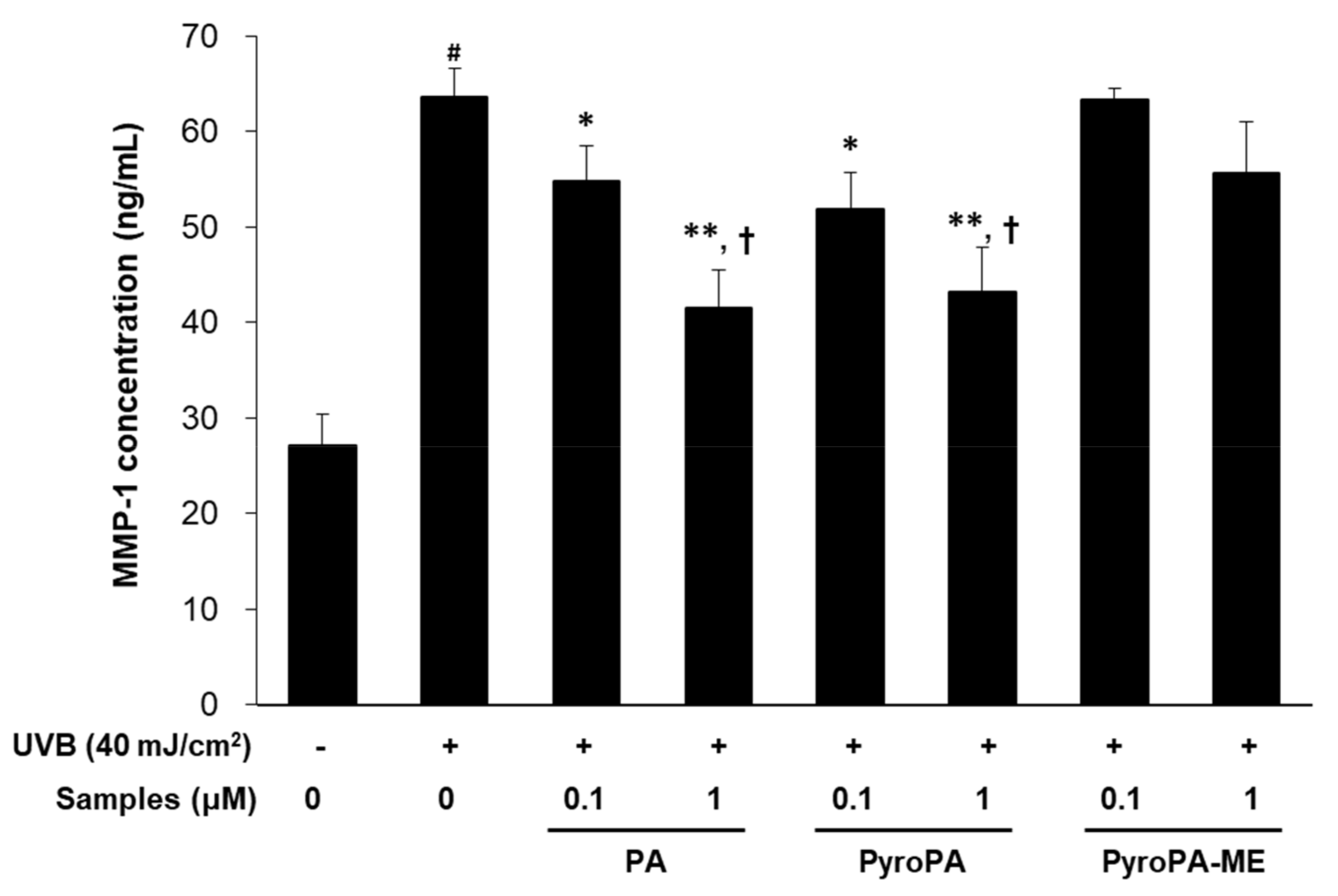
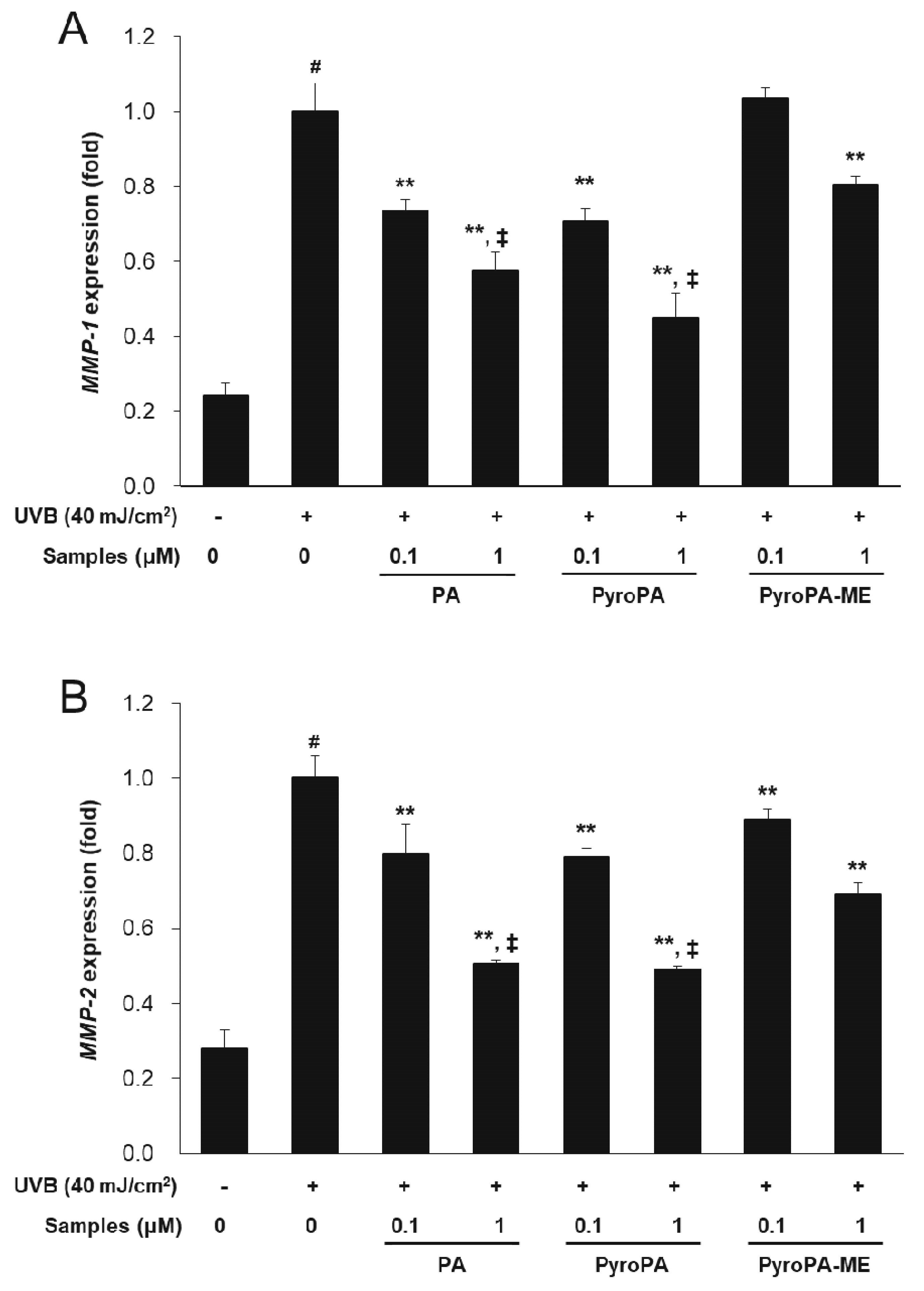
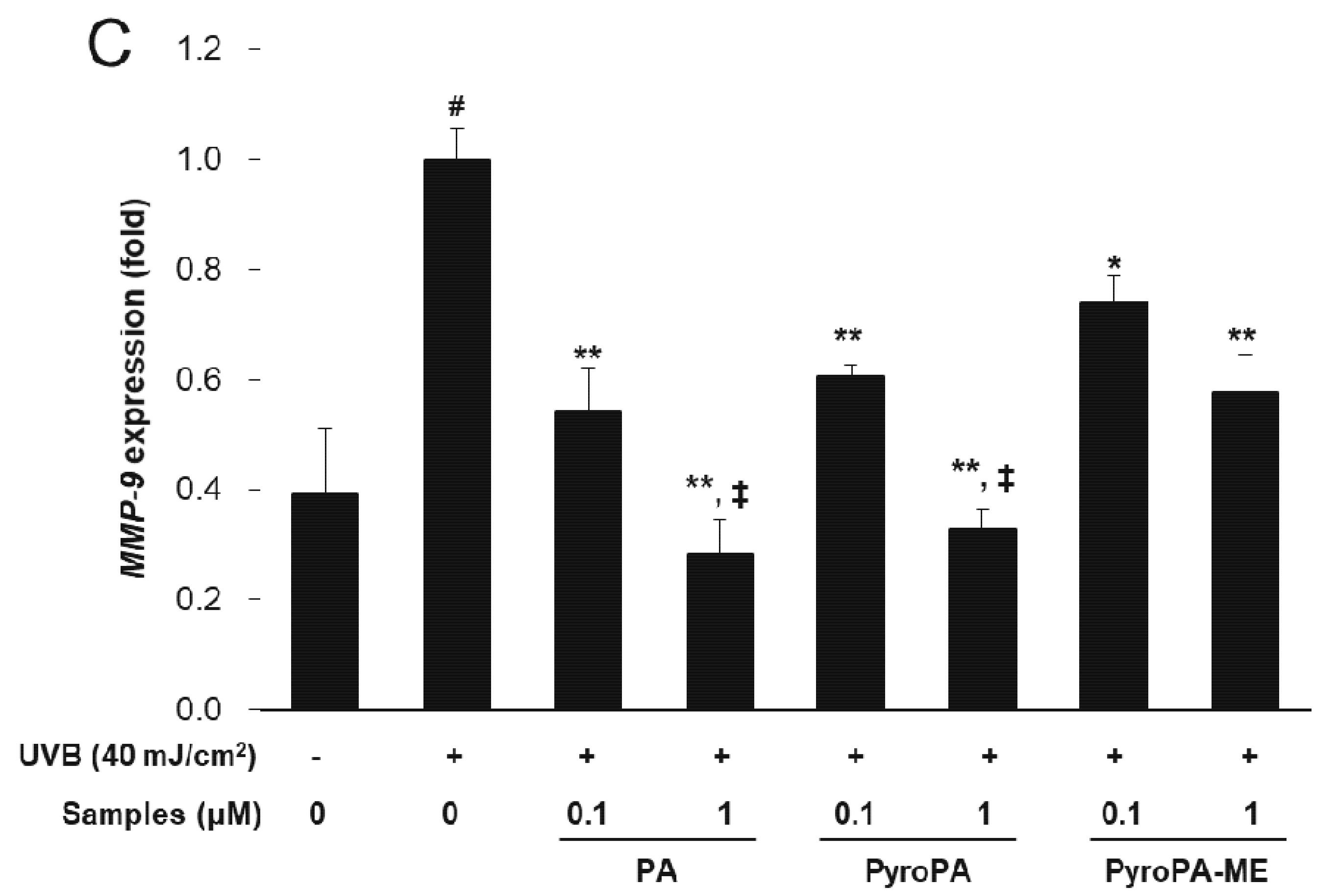
2.3. Effects of Pheophorbides on MMP Expression in CCD-986sk Cells
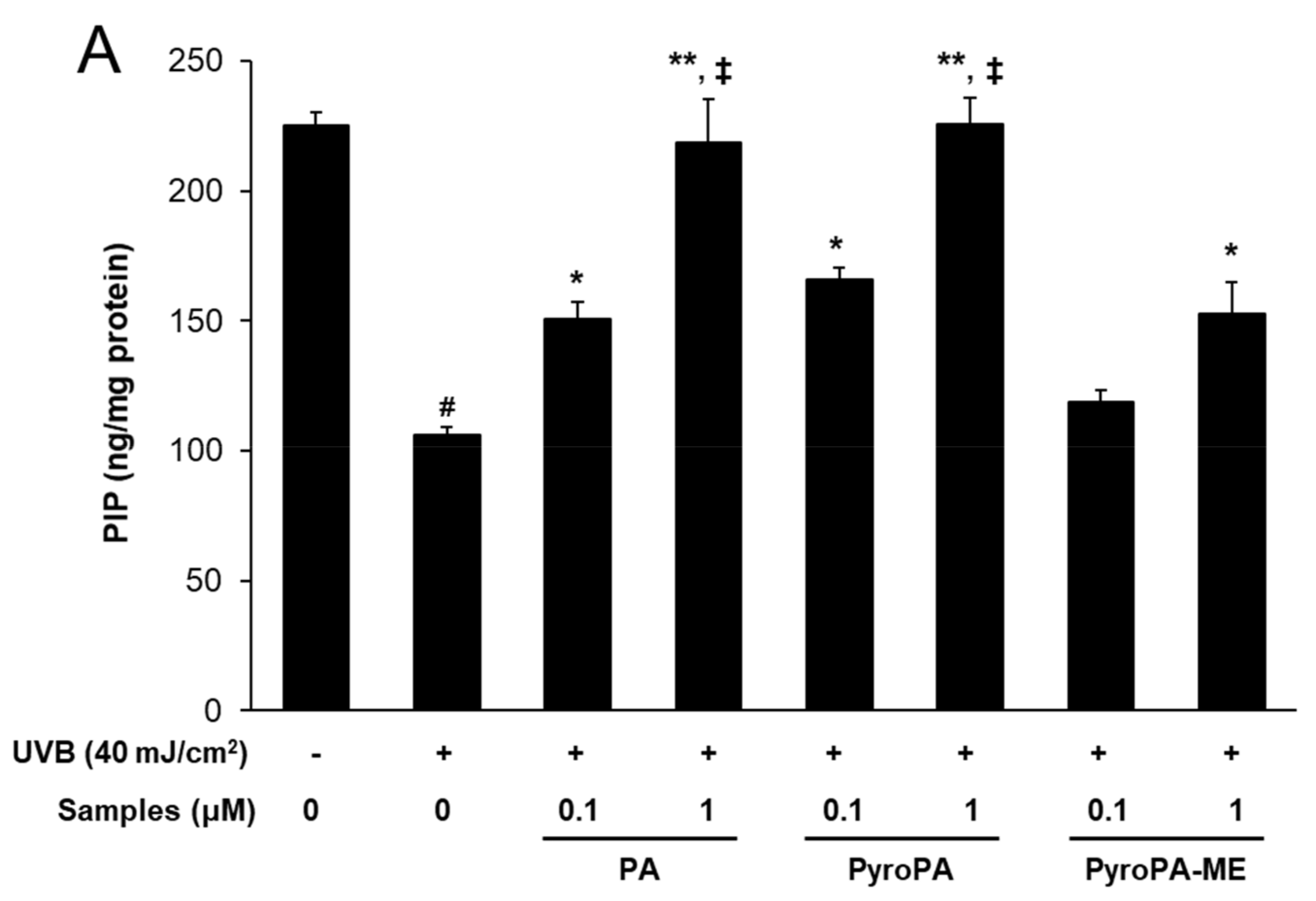
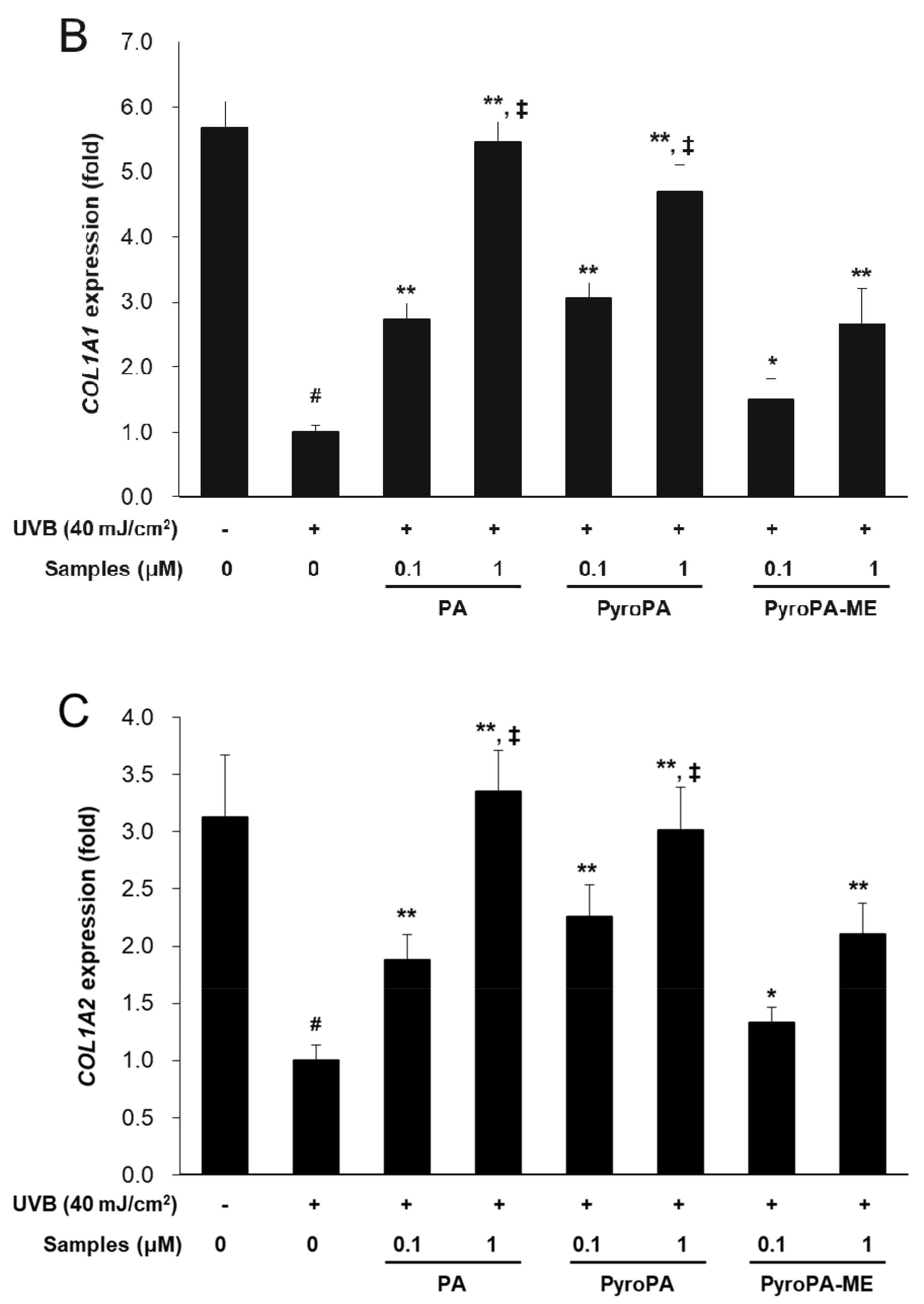
2.4. Effects of Pheophorbides on Collagen Expression in CCD-986sk Cells
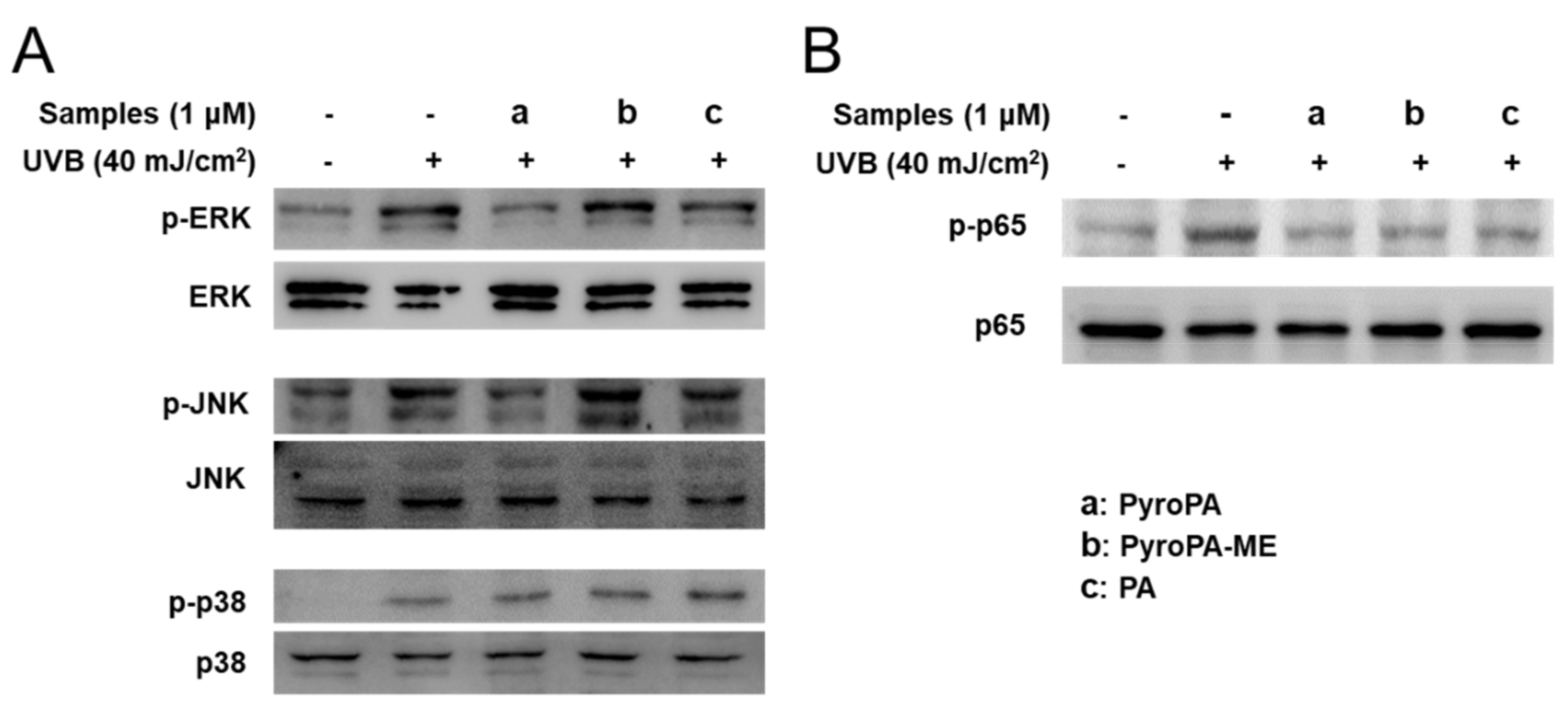
2.5. Effects of Pheophorbides on MAPK and NF-κB Signaling in CCD-986sk Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Cell Viability Assay
4.3. Measurement of ROS Accumulation
4.4. Detection of MMP-1 and Procollagen Concentration
4.5. Real-Time Quantitative RT-PCR (qRT-PCR)
4.6. Immunoblotting
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar] [PubMed]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Friedman, O. Changes associated with the aging face. Facial Plast. Surg. Clin. N. Am. 2005, 13, 371–380. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- de Gruijl, F.R. Photocarcinogenesis: UVA vs. UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar]
- Tamura, B.M.; Cuce, L.C.; Rodrigues, C.J. Allergic reaction to botulinum toxin: Positive intradermal test. Dermatol. Surg. 2008, 34, 1117–1119. [Google Scholar] [CrossRef]
- Jin, Y.; Jin, X.; Chen, Y.; Zhu, J. Acupuncture and constraint-induced movement therapy for a patient with chronic stroke: One-year follow-up case report. Medicine 2017, 96, e8737. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar]
- Barrantes, E.; Guinea, M. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003, 72, 843–850. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.R.; Choi, H.S.; Moon, H.I.; Chung, J.H.; Lee, D.G.; Woo, E.R. The effect of curculigoside on the expression of matrix metalloproteinase-1 in cultured human skin fibroblasts. Arch. Pharm. Res. 2009, 32, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wu, Y.; Wei, H.C.; Xu, Y.Y.; Jia, L.L.; Chen, J.; Yang, X.S.; Dong, G.H.; Gao, X.H.; Chen, H.D. Protective effects of green tea extracts on photoaging and photommunosuppression. Skin Res. Technol. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hortensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef]
- Ratnoglik, S.L.; Aoki, C.; Sudarmono, P.; Komoto, M.; Deng, L.; Shoji, I.; Fuchino, H.; Kawahara, N.; Hotta, H. Antiviral activity of extracts from Morinda citrifolia leaves and chlorophyll catabolites, pheophorbide a and pyropheophorbide a, against hepatitis C virus. Microbiol. Immunol. 2014, 58, 188–194. [Google Scholar] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and chlorophyll-derived molecules from the diatom cylindrotheca closterium with anti-inflammatory activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.J.; Han, J.S. Pheophorbide A from Gelidium amansii improves postprandial hyperglycemia in diabetic mice through alpha-glucosidase inhibition. Phytother. Res. 2019, 33, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, H.S.; Kang, J.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Lee, C.H.; Lee, I.K.; Yun, B.S.; Jeong, T.S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic beta-cell function and suppressing hepatic lipid accumulation in db/db mice. J. Agric. Food Chem. 2015, 63, 7198–7210. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Yoon, H.E.; Moon, S.Y.; Kim, Y.C.; Yoon, J.H. Intratumoral photodynamic therapy with newly synthesized pheophorbide a in murine oral cancer. Oncol. Res. 2017, 25, 295–304. [Google Scholar] [CrossRef]
- Miranda, N.; Volpato, H.; da Silva Rodrigues, J.H.; Caetano, W.; Ueda-Nakamura, T.; de Oliveira Silva, S.; Nakamura, C.V. The photodynamic action of pheophorbide a induces cell death through oxidative stress in Leishmania amazonensis. J. Photochem. Photobiol. B 2017, 174, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Yoon, H.E.; Kwon, S.M.; Lee, J.; Min, S.K.; Kim, Y.C.; Ahn, S.G.; Yoon, J.H. Synthesized Pheophorbide a-mediated photodynamic therapy induced apoptosis and autophagy in human oral squamous carcinoma cells. J. Oral Pathol. Med. 2013, 42, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Ko, S.H.; Lee, W.Y. Silkworm-pheophorbide alpha mediated photodynamic therapy against B16F10 pigmented melanoma. J. Photochem. Photobiol. B 2004, 74, 1–6. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.M.; Kim, S.N.; Amna, T.; Lee, S.; Shin, W.S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. J Microbiol Biotechnol 2014, 24, 346–353. [Google Scholar] [PubMed]
- Chen, D.; Lu, S.; Yang, G.; Pan, X.; Fan, S.; Xie, X.; Chen, Q.; Li, F.; Li, Z.; Wu, S.; et al. The seafood Musculus senhousei shows anti-influenza A virus activity by targeting virion envelope lipids. Biochem. Pharmacol. 2020, 177, 113982. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, Y.; Zhang, C.C.; Gao, J.; Tang, Y.; Wang, Q. In vitro and in vivo studies of a chlorin-based carbon nanocarrier with photodynamic therapy features. Photochem. Photobiol. Sci. 2018, 17, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Guelluy, P.H.; Fontaine-Aupart, M.P.; Grammenos, A.; Lecart, S.; Piette, J.; Hoebeke, M. Optimizing photodynamic therapy by liposomal formulation of the photosensitizer pyropheophorbide-a methyl ester: In vitro and ex vivo comparative biophysical investigations in a colon carcinoma cell line. Photochem. Photobiol. Sci. 2010, 9, 1252–1256. [Google Scholar] [CrossRef]
- Al-Omari, S.; Ali, A. Photodynamic activity of pyropheophorbide methyl ester and pyropheophorbide a in dimethylformamide solution. Gen. Physiol. Biophys. 2009, 28, 70–77. [Google Scholar] [CrossRef][Green Version]
- Gilchrest, B.A. Photoaging. J. Invest. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suarez-Perez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; Gonzalez, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, L.; Zhang, X.; Li, L.; Moon, S.; Roh, M.R.; Jin, Z. RUNX3 expression is associated with sensitivity to pheophorbide a-based photodynamic therapy in keloids. Lasers Med. Sci. 2015, 30, 67–75. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen-Durr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Bui-Xuan, N.H.; Tang, P.M.; Wong, C.K.; Fung, K.P. Photo-activated pheophorbide-a, an active component of Scutellaria barbata, enhances apoptosis via the suppression of ERK-mediated autophagy in the estrogen receptor-negative human breast adenocarcinoma cells MDA-MB-231. J. Ethnopharmacol. 2010, 131, 95–103. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef]
- Lee, Y.R.; Noh, E.M.; Han, J.H.; Kim, J.M.; Hwang, J.K.; Hwang, B.M.; Chung, E.Y.; Kim, B.S.; Lee, S.H.; Lee, S.J.; et al. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-kappaB pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Asamitsu, K.; Uranishi, H.; Iddamalgoda, A.; Ito, K.; Kojima, H.; Okamoto, T. Protecting skin photoaging by NF-kappaB inhibitor. Curr. Drug Metab. 2010, 11, 431–435. [Google Scholar] [PubMed]
- Heinrich, M.; Bork, P.M.; Schmitz, M.L.; Rimpler, H.; Frei, B.; Sticher, O. Pheophorbide A from Solanum diflorum interferes with NF-κB activation. Planta Med. 2001, 67, 156–157. [Google Scholar] [CrossRef] [PubMed]
| Gene (NCBI Reference Sequence) | Forward Primer | Reverse Primer |
|---|---|---|
| ACTIN (NM_001101) | GGCACCACACCTTCTACAAT | GCCTGGATAGCAACGTACAT |
| COL1A1 (NM_000088) | TGGCCTCGGAGGAAACTTT | GCTTCCCCATCATCTCCATTC |
| COL1A2 (NM_000089) | CGGTGGTGGTTATGACTTTGGT | GAAGGGTCTCAATCTGGTTGTTG |
| MMP-1 (NM_001145938) | AACACATCTGACCTACAGGATTGAAA | CTTGGTGAATGTCAGAGGTGTGA |
| MMP-2 (NM_001127891) | ACTGGAGCAAAAACAAGAAGACATAC | TCCATTTTCTTCTTCACCTCATTG |
| MMP-9 (NM_004994) | GCGCTGGGCTTAGATCATTC | GTGCCGGATGCCATTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Park, H.-Y.; Jeong, T.-S. Pheophorbide a Derivatives Exert Antiwrinkle Effects on UVB-Induced Skin Aging in Human Fibroblasts. Life 2021, 11, 147. https://doi.org/10.3390/life11020147
Lee H, Park H-Y, Jeong T-S. Pheophorbide a Derivatives Exert Antiwrinkle Effects on UVB-Induced Skin Aging in Human Fibroblasts. Life. 2021; 11(2):147. https://doi.org/10.3390/life11020147
Chicago/Turabian StyleLee, Hwa, Ho-Yong Park, and Tae-Sook Jeong. 2021. "Pheophorbide a Derivatives Exert Antiwrinkle Effects on UVB-Induced Skin Aging in Human Fibroblasts" Life 11, no. 2: 147. https://doi.org/10.3390/life11020147
APA StyleLee, H., Park, H.-Y., & Jeong, T.-S. (2021). Pheophorbide a Derivatives Exert Antiwrinkle Effects on UVB-Induced Skin Aging in Human Fibroblasts. Life, 11(2), 147. https://doi.org/10.3390/life11020147






