Chronic Stress and Gonadectomy Affect the Expression of Cx37, Cx40 and Cx43 in the Spinal Cord
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Immunohistochemical Staining
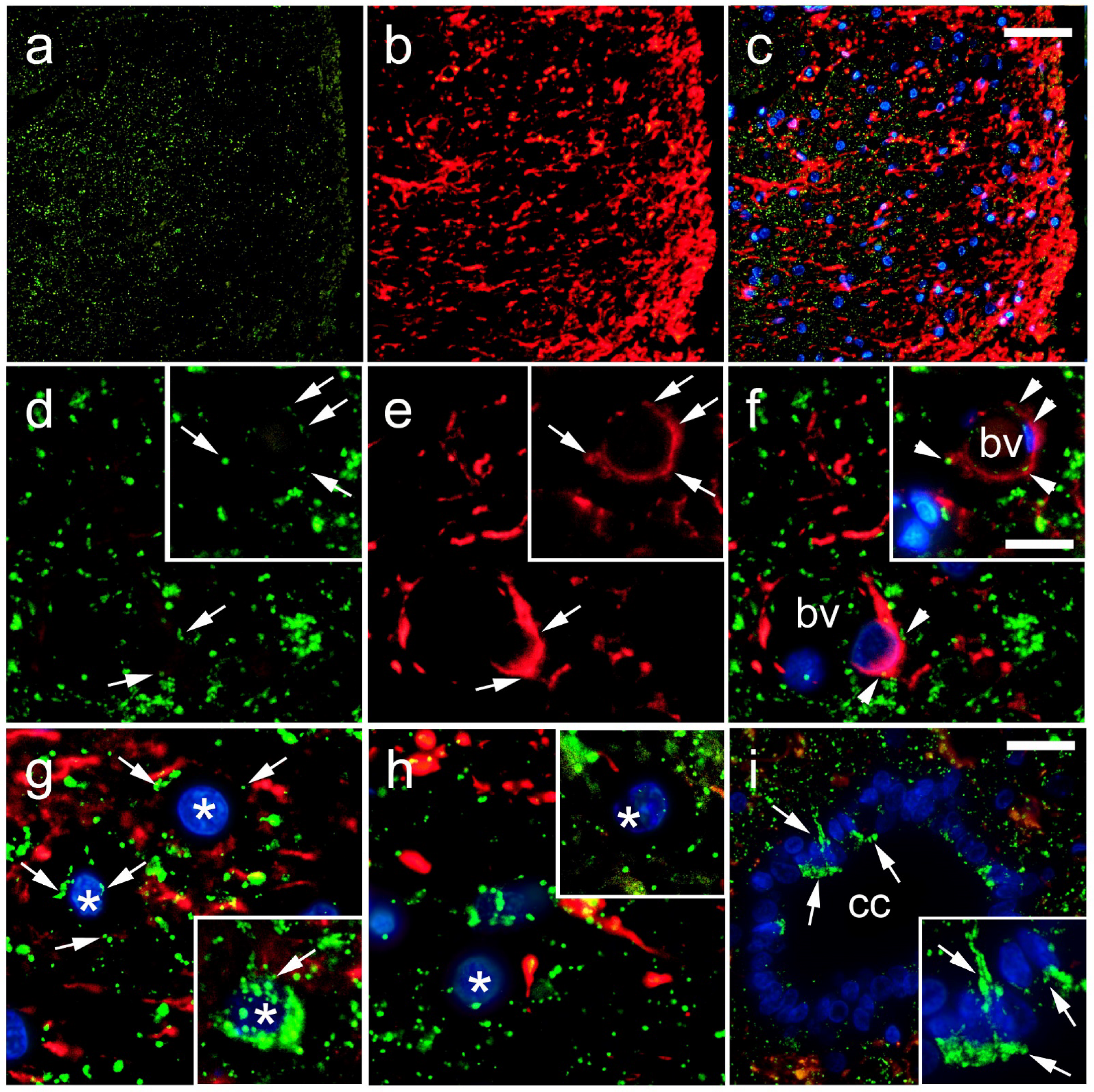
2.3. Data Acquisition and Immunohistochemical Analysis
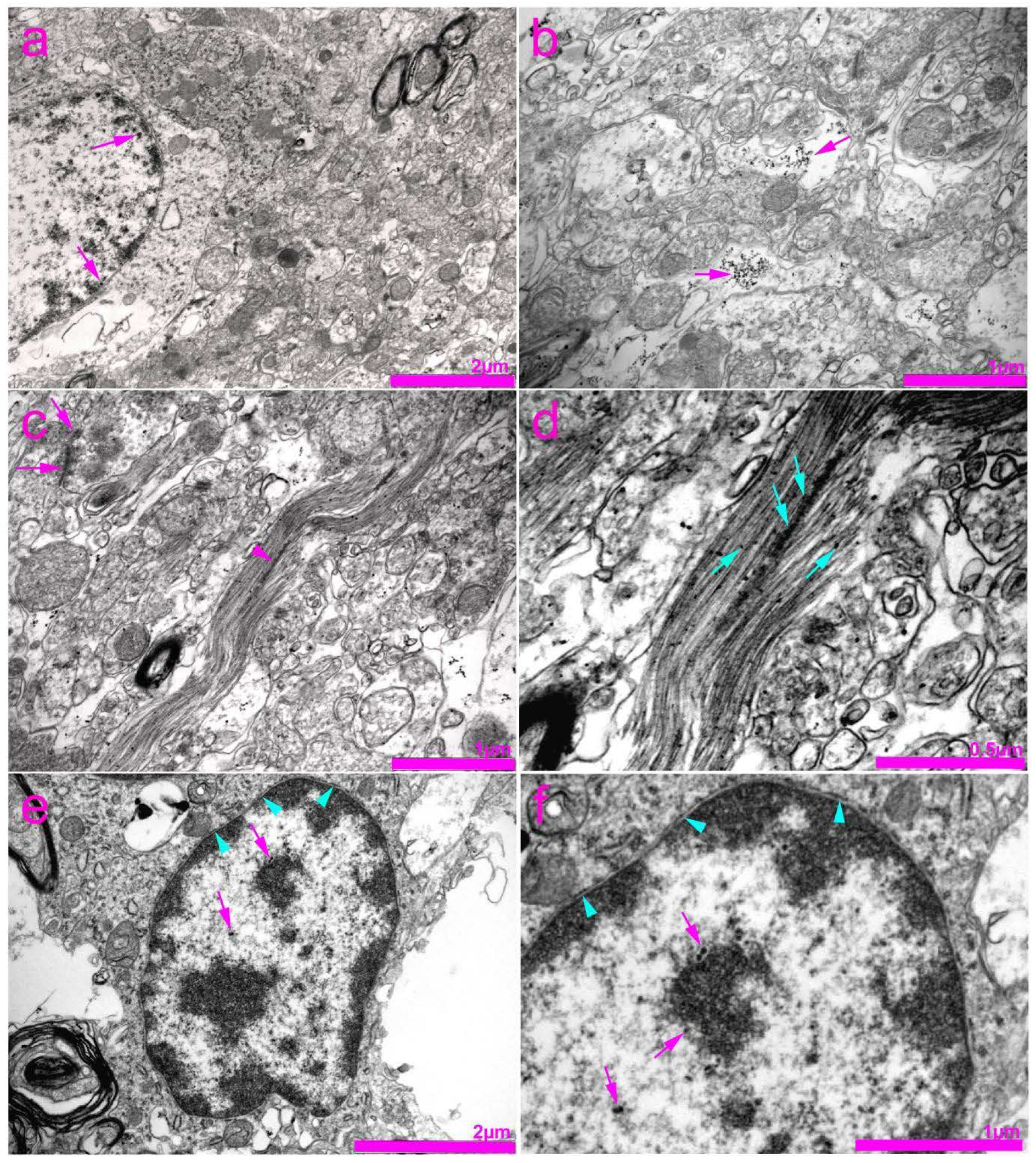
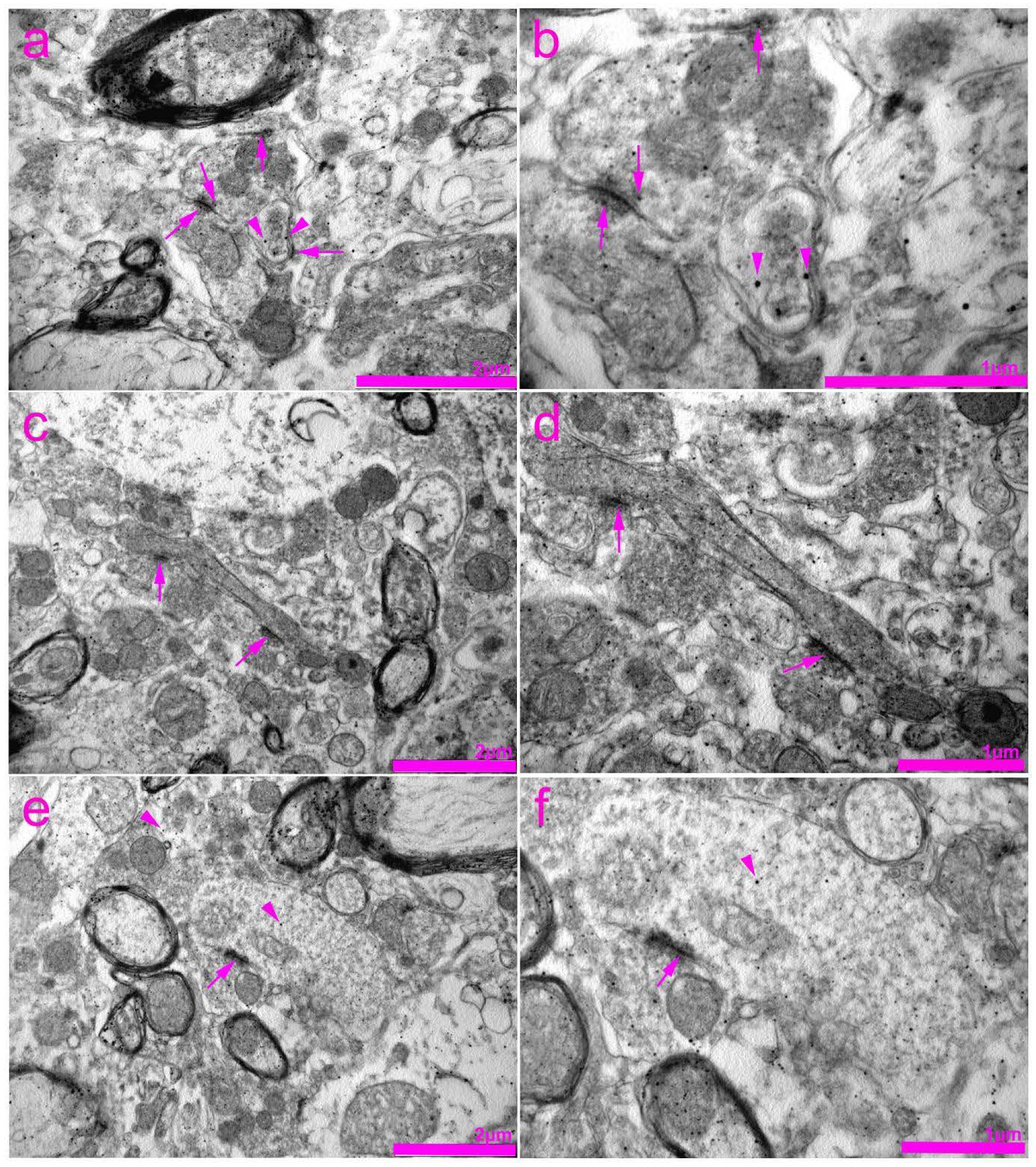
2.4. Tissue Preparation for Transmission Electron Microscopy (TEM)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woda, A.; Picard, P.; Dutheil, F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology 2016, 71, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Greenwood-Van Meerveld, B.; Johnson, A.C. Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin. Front. Syst. Neurosci. 2017, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Bicikova, M.; Sosvorova, L. How hormones influence composition and physiological function of the brain-blood barrier. Physiol. Res. 2015, 64 (Suppl. S2), S259–S264. [Google Scholar] [CrossRef] [PubMed]
- Golovatscka, V.; Ennes, H.; Mayer, E.A.; Bradesi, S. Chronic stress-induced changes in pro-inflammatory cytokines and spinal glia markers in the rat: A time course study. Neuroimmunomodulation 2012, 19, 367–376. [Google Scholar] [CrossRef]
- Lunde, C.E.; Sieberg, C.B. Walking the Tightrope: A Proposed Model of Chronic Pain and Stress. Front. Neurosci. 2020, 14, 270. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Wang, R.; Tawfik, O.; Luyendyk, J.P. Protective and damaging effects of platelets in acute cholestatic liver injury revealed by depletion and inhibition strategies. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 115, 286–294. [Google Scholar] [CrossRef]
- Elzahaf, R.A.; Johnson, M.I.; Tashani, O.A. The epidemiology of chronic pain in Libya: A cross-sectional telephone survey. BMC Public Health 2016, 16, 776. [Google Scholar] [CrossRef]
- Zeng, O.; Li, F.; Li, Y.; Li, L.; Xiao, T.; Chu, C.; Yang, J. Effect of Novel Gasotransmitter hydrogen sulfide on renal fibrosis and connexins expression in diabetic rats. Bioengineered 2016, 7, 314–320. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, B.; Li, J.; Traub, R.J. Opposing Roles of Estradiol and Testosterone on Stress-Induced Visceral Hypersensitivity in Rats. J. Pain Off. J. Am. Pain Soc. 2018, 19, 764–776. [Google Scholar] [CrossRef]
- Chaloner, A.; Greenwood-Van Meerveld, B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J. Pain Off. J. Am. Pain Soc. 2013, 14, 270–280. [Google Scholar] [CrossRef]
- Handa, R.J.; Burgess, L.H.; Kerr, J.E.; O’Keefe, J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994, 28, 464–476. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, Y.; He, Z.; Hu, W. Function of Connexins in the Interaction between Glial and Vascular Cells in the Central Nervous System and Related Neurological Diseases. Neural Plast. 2018, 2018, 6323901. [Google Scholar] [CrossRef]
- Castro Dias, M.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef]
- Brocardo, L.; Acosta, L.E.; Piantanida, A.P.; Rela, L. Beneficial and Detrimental Remodeling of Glial Connexin and Pannexin Functions in Rodent Models of Nervous System Diseases. Front. Cell. Neurosci. 2019, 13, 491. [Google Scholar] [CrossRef]
- Harris, A.L.; Locke, D. Connexins: A Guide; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Aasen, T.; Johnstone, S.; Vidal-Brime, L.; Lynn, K.S.; Koval, M. Connexins: Synthesis, Post-Translational Modifications, and Trafficking in Health and Disease. Int. J. Mol. Sci. 2018, 19, 1296. [Google Scholar] [CrossRef]
- Beilharz, T.H.; See, M.M.; Boag, P.R. 3′-UTRs and the Control of Protein Expression in Space and Time. Adv. Exp. Med. Biol. 2019, 1203, 133–148. [Google Scholar] [CrossRef]
- Bautista, W.; Rash, J.E.; Vanderpool, K.G.; Yasumura, T.; Nagy, J.I. Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus. Eur. J. Neurosci. 2014, 39, 757–770. [Google Scholar] [CrossRef]
- Lai, L.L.; See, M.H.; Rampal, S.; Ng, K.S.; Chan, L. Significant factors influencing inadvertent hypothermia in pediatric anesthesia. J. Clin. Monit. Comput. 2019, 33, 1105–1112. [Google Scholar] [CrossRef]
- Vinken, M.; Decrock, E.; Leybaert, L.; Bultynck, G.; Himpens, B.; Vanhaecke, T.; Rogiers, V. Non-channel functions of connexins in cell growth and cell death. Biochim. Biophys. Acta 2012, 1818, 2002–2008. [Google Scholar] [CrossRef]
- Söhl, G.; Maxeiner, S.; Willecke, K. Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 2005, 6, 191–200. [Google Scholar] [CrossRef]
- Decrock, E.; Vinken, M.; Bol, M.; D’Herde, K.; Rogiers, V.; Vandenabeele, P.; Krysko, D.V.; Bultynck, G.; Leybaert, L. Calcium and connexin-based intercellular communication, a deadly catch? Cell Calcium 2011, 50, 310–321. [Google Scholar] [CrossRef]
- Rimkute, L.; Kraujalis, T.; Snipas, M.; Palacios-Prado, N.; Jotautis, V.; Skeberdis, V.A.; Bukauskas, F.F. Modulation of Connexin-36 Gap Junction Channels by Intracellular pH and Magnesium Ions. Front. Physiol. 2018, 9, 362. [Google Scholar] [CrossRef]
- Wang, A.; Xu, C. The role of connexin43 in neuropathic pain induced by spinal cord injury. Acta Biochim. Biophys. Sin. 2019, 51, 555–561. [Google Scholar] [CrossRef]
- Morioka, N.; Fujii, S.; Kondo, S.; Zhang, F.F.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Downregulation of spinal astrocytic connexin43 leads to upregulation of interleukin-6 and cyclooxygenase-2 and mechanical hypersensitivity in mice. Glia 2018, 66, 428–444. [Google Scholar] [CrossRef]
- Tonkin, R.S.; Bowles, C.; Perera, C.J.; Keating, B.A.; Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Tran, C.; Don, A.S.; Fath, T.; et al. Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp. Neurol. 2018, 300, 1–12. [Google Scholar] [CrossRef]
- Hang, L.H.; Li, S.N.; Luo, H.; Shu, W.W.; Mao, Z.M.; Chen, Y.F.; Shi, L.L.; Shao, D.H. Connexin 43 Mediates CXCL12 Production from Spinal Dorsal Horn to Maintain Bone Cancer Pain in Rats. Neurochem. Res. 2016, 41, 1200–1208. [Google Scholar] [CrossRef]
- Zupanc, G.K.H. Development of a sexual dimorphism in a central pattern generator driving a rhythmic behavior: The role of glia-mediated potassium buffering in the pacemaker nucleus of the weakly electric fish Apteronotus leptorhynchus. Dev. Neurobiol. 2020, 80, 6–15. [Google Scholar] [CrossRef]
- Wilson, A.C.; Clemente, L.; Liu, T.; Bowen, R.L.; Meethal, S.V.; Atwood, C.S. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim. Biophys. Acta 2008, 1782, 401–407. [Google Scholar] [CrossRef]
- Valdes-Sustaita, B.; Lopez-Rubalcava, C.; Gonzalez-Trujano, M.E.; Garcia-Viguera, C.; Estrada-Camarena, E. Aqueous Extract of Pomegranate Alone or in Combination with Citalopram Produces Antidepressant-Like Effects in an Animal Model of Menopause: Participation of Estrogen Receptors. Int. J. Mol. Sci. 2017, 18, 2643. [Google Scholar] [CrossRef]
- Idris, A.I. Ovariectomy/orchidectomy in rodents. Methods Mol. Biol. 2012, 816, 545–551. [Google Scholar] [CrossRef]
- Balog, M.; Miljanovic, M.; Blazetic, S.; Labak, I.; Ivic, V.; Viljetic, B.; Borbely, A.; Papp, Z.; Blazekovic, R.; Vari, S.G.; et al. Sex-specific chronic stress response at the level of adrenal gland modified sexual hormone and leptin receptors. Croat. Med. J. 2015, 56, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Mlinarevic, D.; Seric, V.; Miljanovic, M.; Blazekovic, R.; Degmecic, I.V.; Blazetic, S.; Orsolic, I.; Vari, S.G.; Heffer, M. Plasma Content of Glucose, C-reactive Protein, Uric Acid and Cholesterol in Male, Female and Ovariectomized Rats upon Acute and Chronic Stress—A Path for Development of Cardiovascular Diseases. Coll. Antropol. 2015, 39, 385–392. [Google Scholar] [PubMed]
- Ivic, V.; Blazetic, S.; Labak, I.; Balog, M.; Vondrak, L.; Blazekovic, R.; Vari, S.G.; Heffer, M. Ovariectomy and chronic stress lead toward leptin resistance in the satiety centers and insulin resistance in the hippocampus of Sprague-Dawley rats. Croat. Med. J. 2016, 57, 194–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balog, M.; Ivić, V.; Scitovski, R.; Labak, I.; Szűcs, K.F.; Gaspar, R.; Vári, S.G.; Heffer, M. A mathematical model reveals sex-specific changes in glucose and insulin tolerance during rat puberty and maturation. Croat. Med. J. 2020, 61, 107–118. [Google Scholar] [CrossRef]
- Agnic, I.; Filipovic, N.; Vukojevic, K.; Saraga-Babic, M.; Grkovic, I. Isoflurane post-conditioning influences myocardial infarct healing in rats. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2018, 93, 354–363. [Google Scholar] [CrossRef]
- Filipovic, N.; Vukojevic, K.; Bocina, I.; Saraga, M.; Durdov, M.G.; Kablar, B.; Saraga-Babic, M. Immunohistochemical and electronmicroscopic features of mesenchymal-to-epithelial transition in human developing, postnatal and nephrotic podocytes. Histochem. Cell Biol. 2017, 147, 481–495. [Google Scholar] [CrossRef]
- Vitlov Uljevic, M.; Starcevic, K.; Masek, T.; Bocina, I.; Restovic, I.; Kevic, N.; Racetin, A.; Kretzschmar, G.; Grobe, M.; Vukojevic, K.; et al. Dietary DHA/EPA supplementation ameliorates diabetic nephropathy by protecting from distal tubular cell damage. Cell Tissue Res. 2019, 378, 301–317. [Google Scholar] [CrossRef]
- Kosovic, I.; Filipovic, N.; Benzon, B.; Vukojevic, K.; Saraga, M.; Glavina Durdov, M.; Bocina, I.; Saraga-Babic, M. Spatio-temporal patterning of different connexins in developing and postnatal human kidneys and in nephrotic syndrome of the Finnish type (CNF). Sci. Rep. 2020, 10, 8756. [Google Scholar] [CrossRef]
- Filipovic, N.; Bocina, I.; Restovic, I.; Grobe, M.; Kretzschmar, G.; Kevic, N.; Masek, T.; Vitlov Uljevic, M.; Juric, M.; Vukojevic, K.; et al. Ultrastructural characterization of vitamin D receptors and metabolizing enzymes in the lipid droplets of the fatty liver in rat. Acta Histochem. 2020, 122, 151502. [Google Scholar] [CrossRef]
- Khosla, K.; Naus, C.C.; Sin, W.C. Cx43 in Neural Progenitors Promotes Glioma Invasion in a 3D Culture System. Int. J. Mol. Sci. 2020, 21, 5216. [Google Scholar] [CrossRef]
- Chang, Q.; Gonzalez, M.; Pinter, M.J.; Balice-Gordon, R.J. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 10813–10828. [Google Scholar] [CrossRef]
- Lin, S.H.; Lu, C.Y.; Muhammad, R.; Chou, W.Y.; Lin, F.C.; Wu, P.C.; Lin, C.R.; Yang, L.C. Induction of connexin 37 expression in a rat model of neuropathic pain. Brain Res. Mol. Brain Res. 2002, 99, 134–140. [Google Scholar] [CrossRef]
- Lavrov, I.; Fox, L.; Shen, J.; Han, Y.; Cheng, J. Gap Junctions Contribute to the Regulation of Walking-Like Activity in the Adult Mudpuppy (Necturus Maculatus). PLoS ONE 2016, 11, e0152650. [Google Scholar] [CrossRef]
- Krutovskikh, V.A.; Troyanovsky, S.M.; Piccoli, C.; Tsuda, H.; Asamoto, M.; Yamasaki, H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene 2000, 19, 505–513. [Google Scholar] [CrossRef]
- Kanczuga-Koda, L.; Sulkowski, S.; Koda, M.; Sulkowska, M. Alterations in connexin26 expression during colorectal carcinogenesis. Oncology 2005, 68, 217–222. [Google Scholar] [CrossRef]
- Rodriguez-Jimenez, F.J.; Alastrue-Agudo, A.; Stojkovic, M.; Erceg, S.; Moreno-Manzano, V. Connexin 50 Expression in Ependymal Stem Progenitor Cells after Spinal Cord Injury Activation. Int. J. Mol. Sci. 2015, 16, 26608–26618. [Google Scholar] [CrossRef]
- Dang, X.; Doble, B.W.; Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell. Biochem. 2003, 242, 35–38. [Google Scholar] [CrossRef]
- Ke, Q.; Li, L.; Cai, B.; Liu, C.; Yang, Y.; Gao, Y.; Huang, W.; Yuan, X.; Wang, T.; Zhang, Q.; et al. Connexin 43 is involved in the generation of human-induced pluripotent stem cells. Hum. Mol. Genet. 2013, 22, 2221–2233. [Google Scholar] [CrossRef]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef]
- Mennecier, G.; Derangeon, M.; Coronas, V.; Herve, J.C.; Mesnil, M. Aberrant expression and localization of connexin43 and connexin30 in a rat glioma cell line. Mol. Carcinog. 2008, 47, 391–401. [Google Scholar] [CrossRef]
- Morel, S.; Burnier, L.; Kwak, B.R. Connexins participate in the initiation and progression of atherosclerosis. Semin. Immunopathol. 2009, 31, 49–61. [Google Scholar] [CrossRef]
- Personius, K.E.; Chang, Q.; Mentis, G.Z.; O’Donovan, M.J.; Balice-Gordon, R.J. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc. Natl. Acad. Sci. USA 2007, 104, 11808–11813. [Google Scholar] [CrossRef]
- Makino, A.; Platoshyn, O.; Suarez, J.; Yuan, J.X.; Dillmann, W.H. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am. J. Physiol. Cell Physiol. 2008, 295, C221–C230. [Google Scholar] [CrossRef]
- Lapato, A.S.; Tiwari-Woodruff, S.K. Connexins and pannexins: At the junction of neuro-glial homeostasis & disease. J. Neurosci. Res. 2018, 96, 31–44. [Google Scholar] [CrossRef]
- Tonkin, R.S.; Mao, Y.; O’Carroll, S.J.; Nicholson, L.F.; Green, C.R.; Gorrie, C.A.; Moalem-Taylor, G. Gap junction proteins and their role in spinal cord injury. Front. Mol. Neurosci. 2014, 7, 102. [Google Scholar] [CrossRef]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef]
- Stauffer, B.L.; Sobus, R.D.; Sucharov, C.C. Sex differences in cardiomyocyte connexin43 expression. J. Cardiovasc. Pharmacol. 2011, 58, 32–39. [Google Scholar] [CrossRef]
- De Bock, M.; Leybaert, L.; Giaume, C. Connexin Channels at the Glio-Vascular Interface: Gatekeepers of the Brain. Neurochem. Res. 2017, 42, 2519–2536. [Google Scholar] [CrossRef]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Maggioli, E.; McArthur, S.; Mauro, C.; Kieswich, J.; Kusters, D.H.; Reutelingsperger, C.P.; Yaqoob, M.; Solito, E. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav. Immun. 2016, 51, 212–222. [Google Scholar] [CrossRef]
- Bake, S.; Sohrabji, F. 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology 2004, 145, 5471–5475. [Google Scholar] [CrossRef] [PubMed]
- Atallah, A.; Mhaouty-Kodja, S.; Grange-Messent, V. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J. Cereb. Blood Flow Metab. 2017, 37, 3161–3175. [Google Scholar] [CrossRef] [PubMed]
- Duque Ede, A.; Munhoz, C.D. The Pro-inflammatory Effects of Glucocorticoids in the Brain. Front. Endocrinol. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, C.D.; Lepsch, L.B.; Kawamoto, E.M.; Malta, M.B.; Lima Lde, S.; Avellar, M.C.; Sapolsky, R.M.; Scavone, C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 3813–3820. [Google Scholar] [CrossRef]
- Fabbiani, G.; Reali, C.; Valentin-Kahan, A.; Rehermann, M.I.; Fagetti, J.; Falco, M.V.; Russo, R.E. Connexin Signaling Is Involved in the Reactivation of a Latent Stem Cell Niche after Spinal Cord Injury. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 2246–2258. [Google Scholar] [CrossRef]
- Russo, R.E.; Reali, C.; Radmilovich, M.; Fernandez, A.; Trujillo-Cenoz, O. Connexin 43 delimits functional domains of neurogenic precursors in the spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 3298–3309. [Google Scholar] [CrossRef]
- Rodriguez-Jimenez, F.J.; Alastrue, A.; Stojkovic, M.; Erceg, S.; Moreno-Manzano, V. Connexin 50 modulates Sox2 expression in spinal-cord-derived ependymal stem/progenitor cells. Cell Tissue Res. 2016, 365, 295–307. [Google Scholar] [CrossRef]
- Todd, A.J.; Wang, F. Central Nervous System Pain Pathways. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Krotov, V.; Tokhtamysh, A.; Kopach, O.; Dromaretsky, A.; Sheremet, Y.; Belan, P.; Voitenko, N. Functional Characterization of Lamina X Neurons in ex-Vivo Spinal Cord Preparation. Front. Cell. Neurosci. 2017, 11, 342. [Google Scholar] [CrossRef]
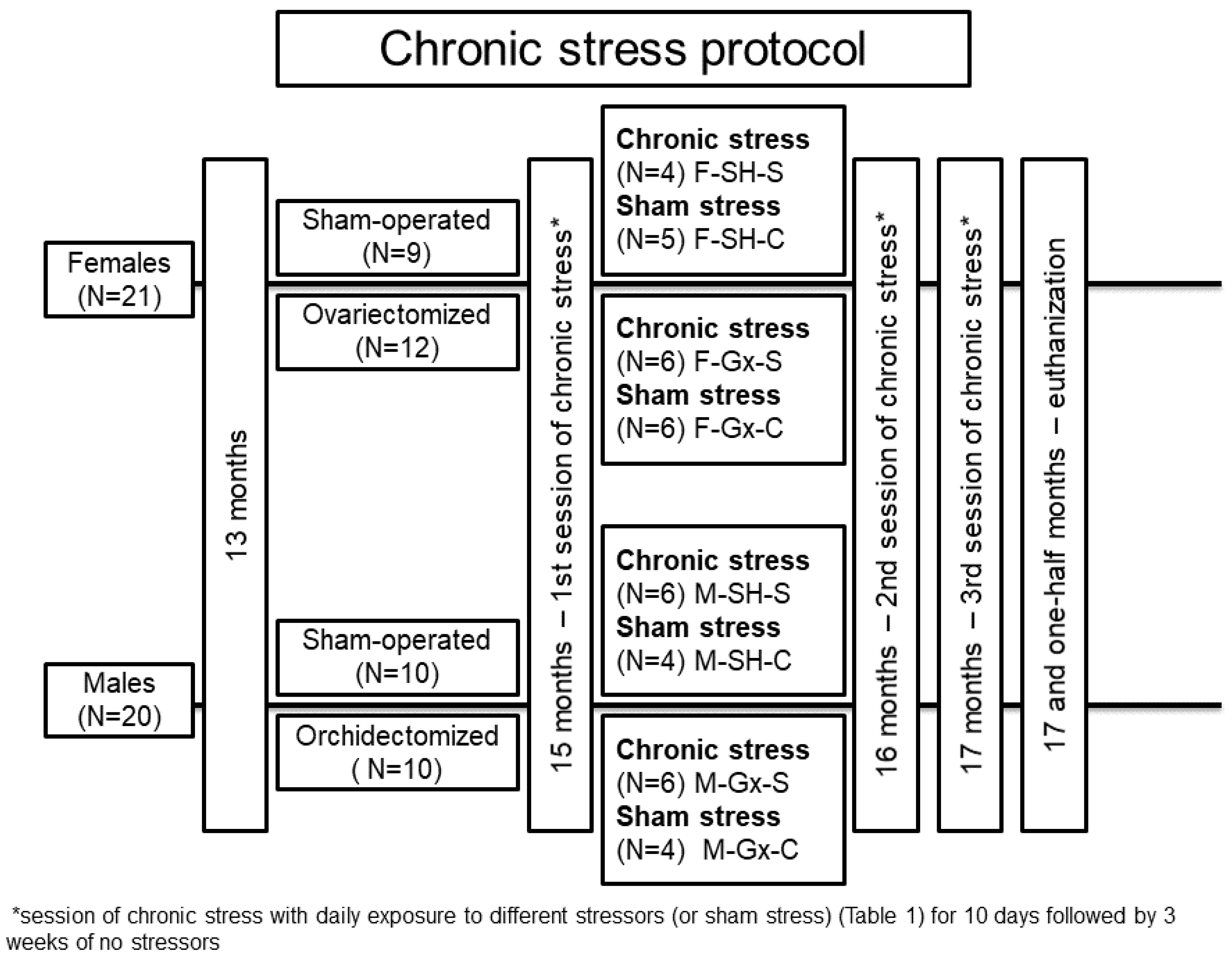
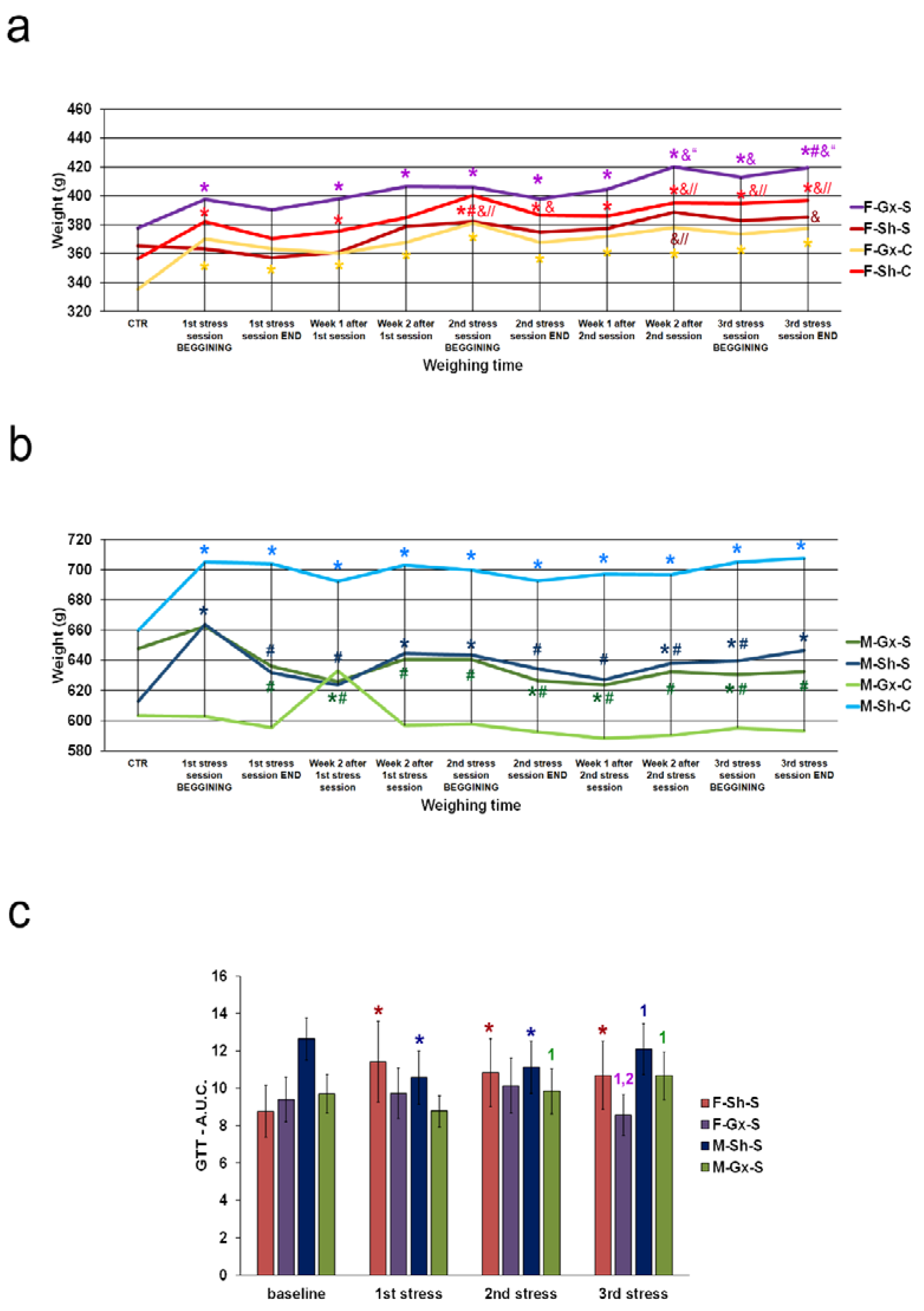
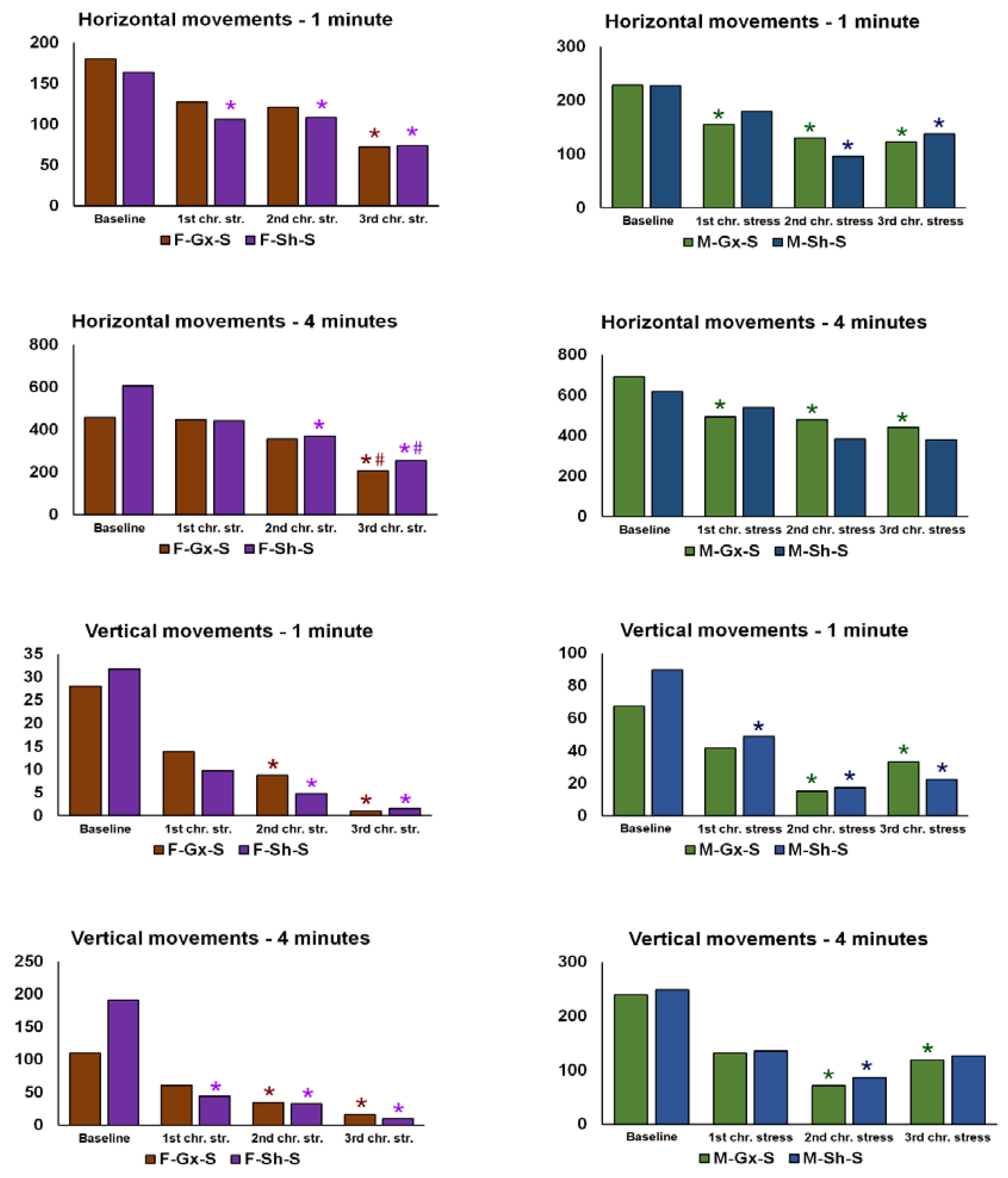

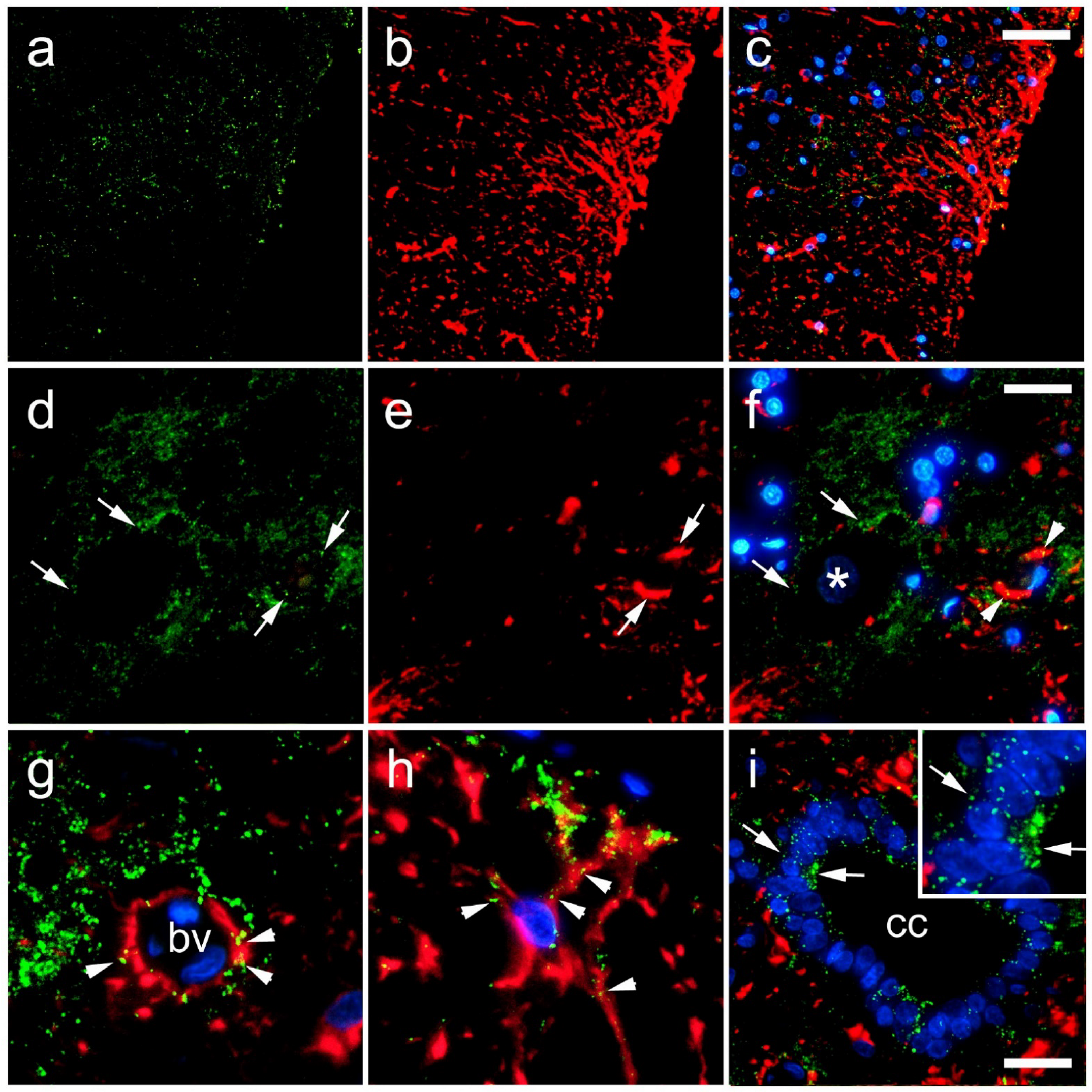
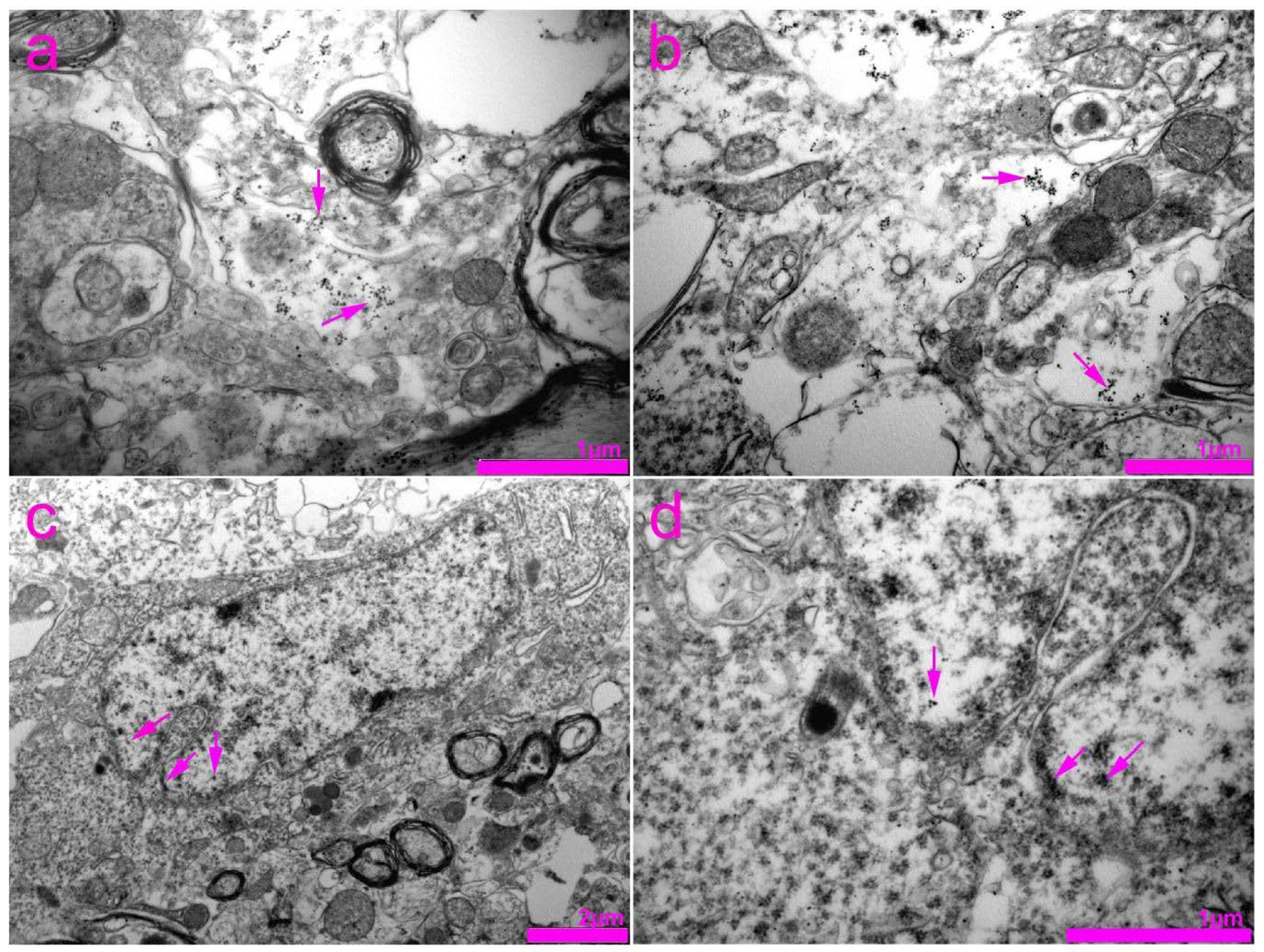
| 1st session of chronic stress protocol | |
| Day | Stressor |
| 1 | Food deprivation (12 h) |
| 2 | GTT ‡ |
| 3 | Cold restraint (+4 °C, 60 min) + food deprivation |
| 4 | GTT |
| 5 | Light overnight (09:00 pm–09:00 am) |
| 6 | Cage rotation (40 min) |
| 7 | Swim test |
| 8 | Noise overnight (09:00 pm–09:00 am) |
| 9 | Cold restraint (+4 °C, 60 min) + food deprivation |
| 10 | GTT |
| 2nd and 3rd sessions of chronic stress protocol | |
| Day | Stressor |
| 1 | Cage rotation (40 min) |
| 2 | Swim test |
| 3 | Cold restraint (+4 °C, 60 min) + food deprivation |
| 4 | Noise overnight (09:00 pm–09:00 am) |
| 5 | Light overnight (09:00 pm–09:00 am) |
| 6 | Cage rotation (40 min) |
| 7 | Swim test |
| 8 | Noise overnight (09:00 pm–09:00 am) |
| 9 | Cold restraint (+4 °C, 60 min) + food deprivation |
| 10 | GTT |
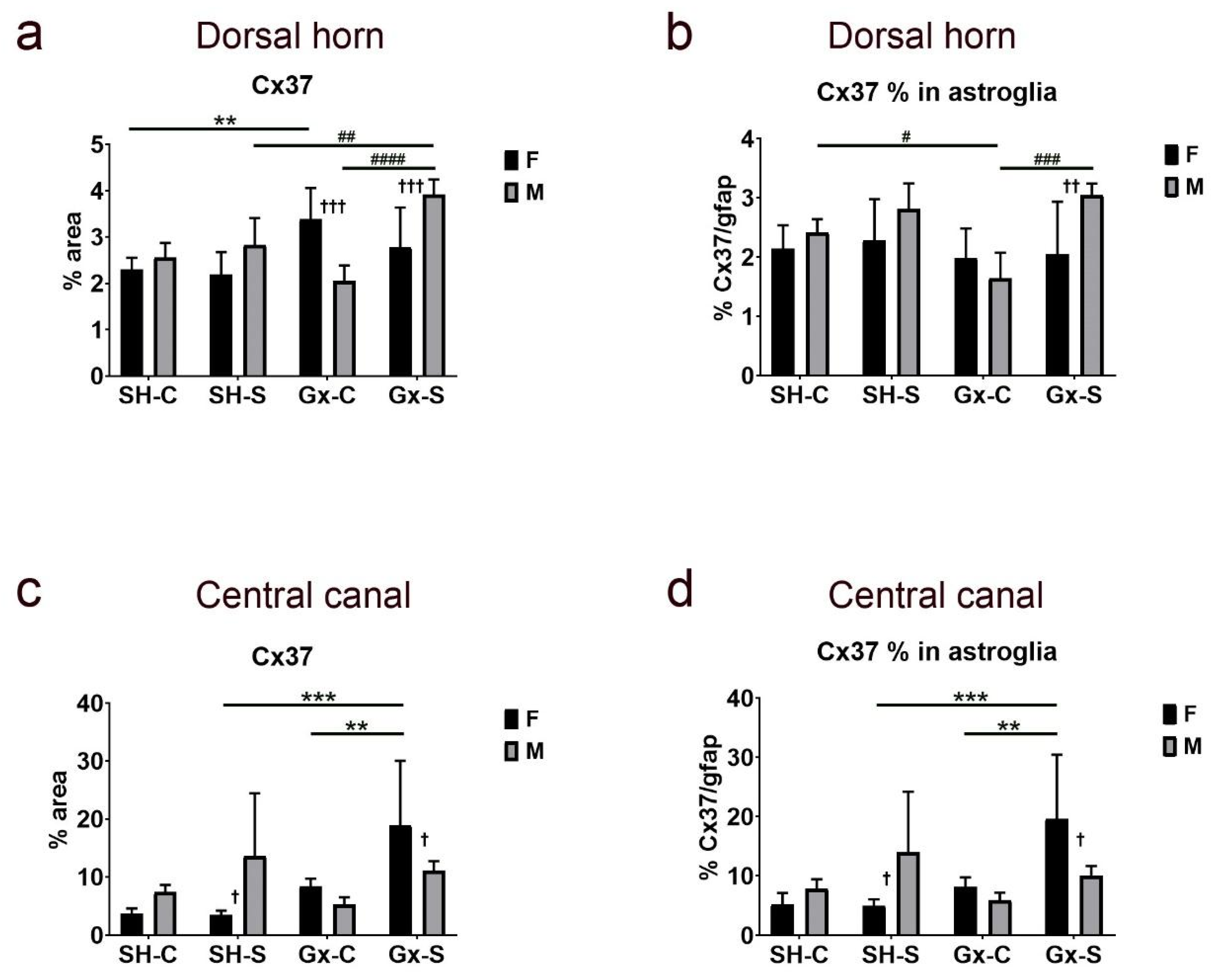
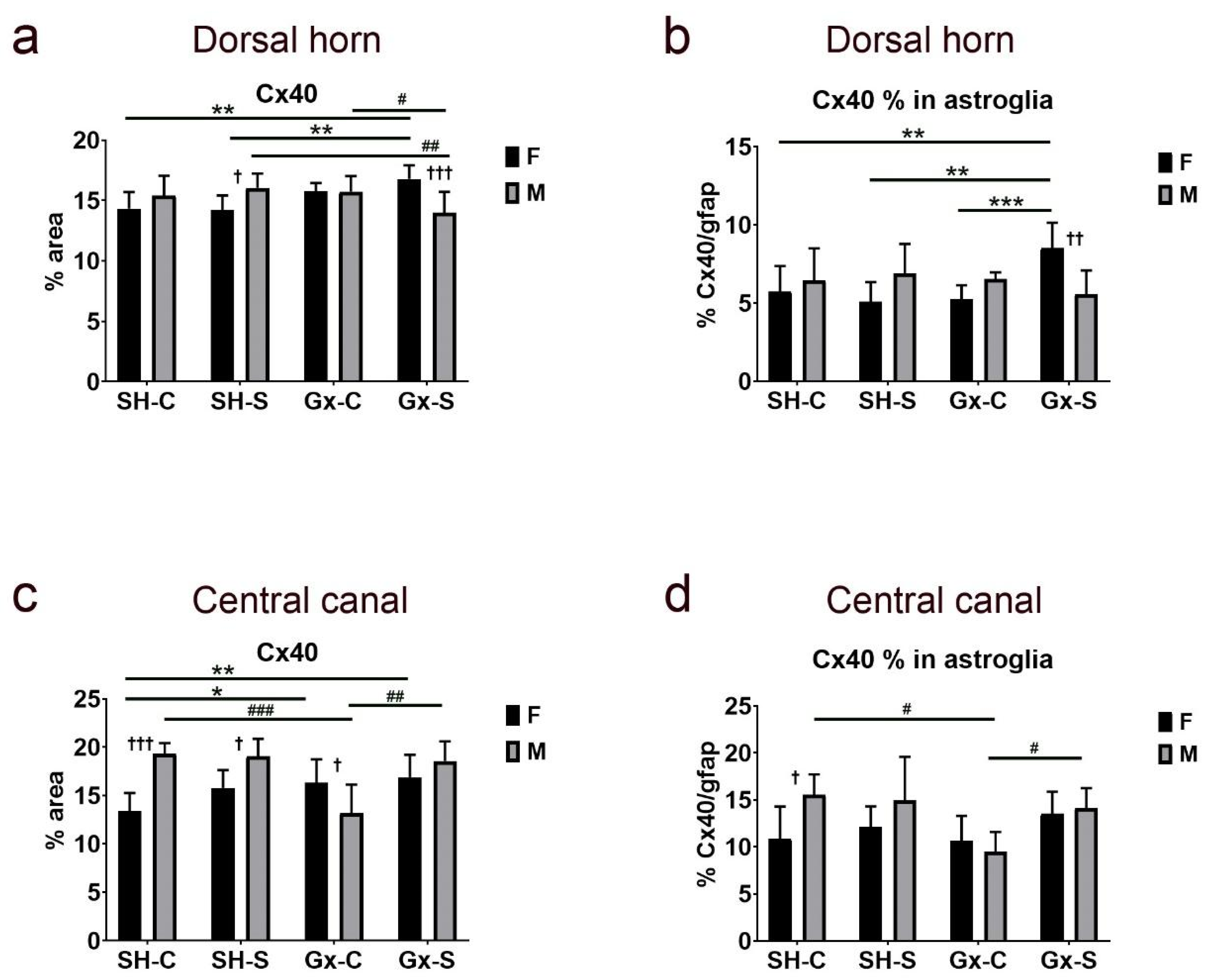
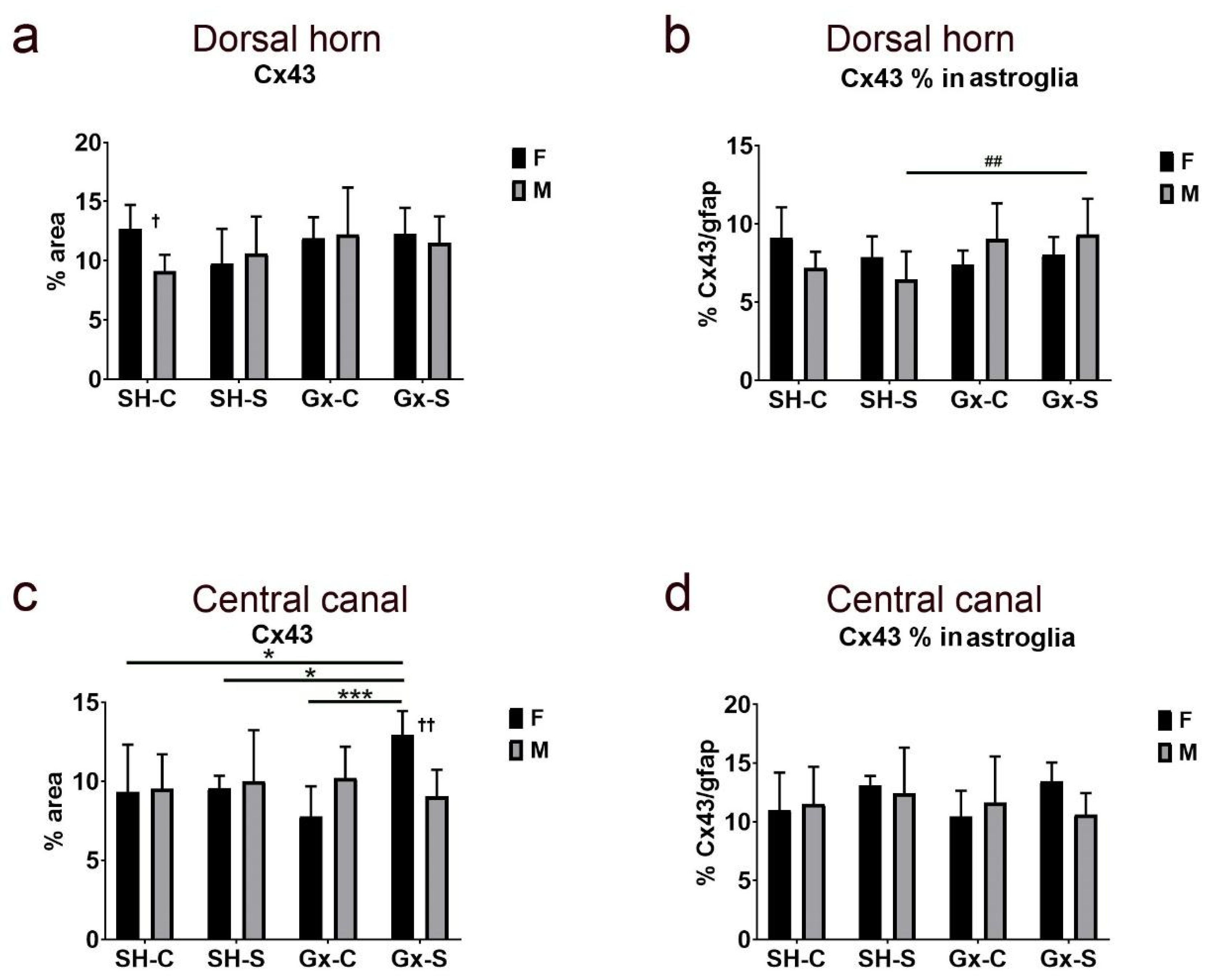
| Cx37 | Cx37/gfap | Cx40 | Cx40/gfap | Cx43 | Cx43/gfap | ||
|---|---|---|---|---|---|---|---|
| Source of Variation | p Value | ||||||
| Dorsal horn | Stress | 0.0496 | 0.0054 | 0.9189 | 0.2921 | 0.6081 | 0.6437 |
| Gonadectomy | 0.0028 | 0.1785 | 0.1829 | 0.3833 | 0.0901 | 0.1481 | |
| Sex | 0.3053 | 0.0352 | 0.9571 | 0.6437 | 0.3419 | 0.8565 | |
| Stress ∗ gonadectomy | 0.1258 | 0.1726 | 0.4519 | 0.191 | 0.7184 | 0.1896 | |
| Stress ∗ sex | 0.0002 | 0.0238 | 0.2346 | 0.1029 | 0.3124 | 0.9293 | |
| Gonadectomy ∗ sex | 0.1208 | 0.8167 | 0.0016 | 0.036 | 0.4869 | 0.0065 | |
| Stress ∗ gonadectomy ∗ sex | 0.0048 | 0.1147 | 0.0382 | 0.0082 | 0.1019 | 0.6697 | |
| Central canal | Stress | 0.0102 | 0.0103 | 0.0066 | 0.0424 | 0.1149 | 0.179 |
| Gonadectomy | 0.0647 | 0.1486 | 0.3542 | 0.1584 | 0.6144 | 0.6022 | |
| Sex | 0.7309 | 0.9857 | 0.0087 | 0.0809 | 0.7923 | 0.6254 | |
| Stress ∗ gonadectomy | 0.2145 | 0.2293 | 0.1796 | 0.087 | 0.2723 | 0.7958 | |
| Stress ∗ sex | 0.8294 | 0.8997 | 0.4357 | 0.9413 | 0.049 | 0.1564 | |
| Gonadectomy ∗ sex | 0.0048 | 0.006 | 0.0006 | 0.0454 | 0.4974 | 0.6823 | |
| Stress ∗ gonadectomy ∗ sex | 0.1827 | 0.0958 | 0.0117 | 0.3553 | 0.034 | 0.439 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurić, M.; Balog, M.; Ivić, V.; Benzon, B.; Racetin, A.; Bočina, I.; Kević, N.; Konjevoda, S.; Szűcs, K.F.; Gáspár, R.; et al. Chronic Stress and Gonadectomy Affect the Expression of Cx37, Cx40 and Cx43 in the Spinal Cord. Life 2021, 11, 1330. https://doi.org/10.3390/life11121330
Jurić M, Balog M, Ivić V, Benzon B, Racetin A, Bočina I, Kević N, Konjevoda S, Szűcs KF, Gáspár R, et al. Chronic Stress and Gonadectomy Affect the Expression of Cx37, Cx40 and Cx43 in the Spinal Cord. Life. 2021; 11(12):1330. https://doi.org/10.3390/life11121330
Chicago/Turabian StyleJurić, Marija, Marta Balog, Vedrana Ivić, Benjamin Benzon, Anita Racetin, Ivana Bočina, Nives Kević, Suzana Konjevoda, Kálmán F. Szűcs, Róbert Gáspár, and et al. 2021. "Chronic Stress and Gonadectomy Affect the Expression of Cx37, Cx40 and Cx43 in the Spinal Cord" Life 11, no. 12: 1330. https://doi.org/10.3390/life11121330
APA StyleJurić, M., Balog, M., Ivić, V., Benzon, B., Racetin, A., Bočina, I., Kević, N., Konjevoda, S., Szűcs, K. F., Gáspár, R., Heffer, M., Vukojević, K., Vari, S. G., & Filipović, N. (2021). Chronic Stress and Gonadectomy Affect the Expression of Cx37, Cx40 and Cx43 in the Spinal Cord. Life, 11(12), 1330. https://doi.org/10.3390/life11121330







