Serology for Borrelia spp. in Northwest Italy: A Climate-Matched 10-Year Trend
Abstract
:1. Introduction
2. Materials and Methods
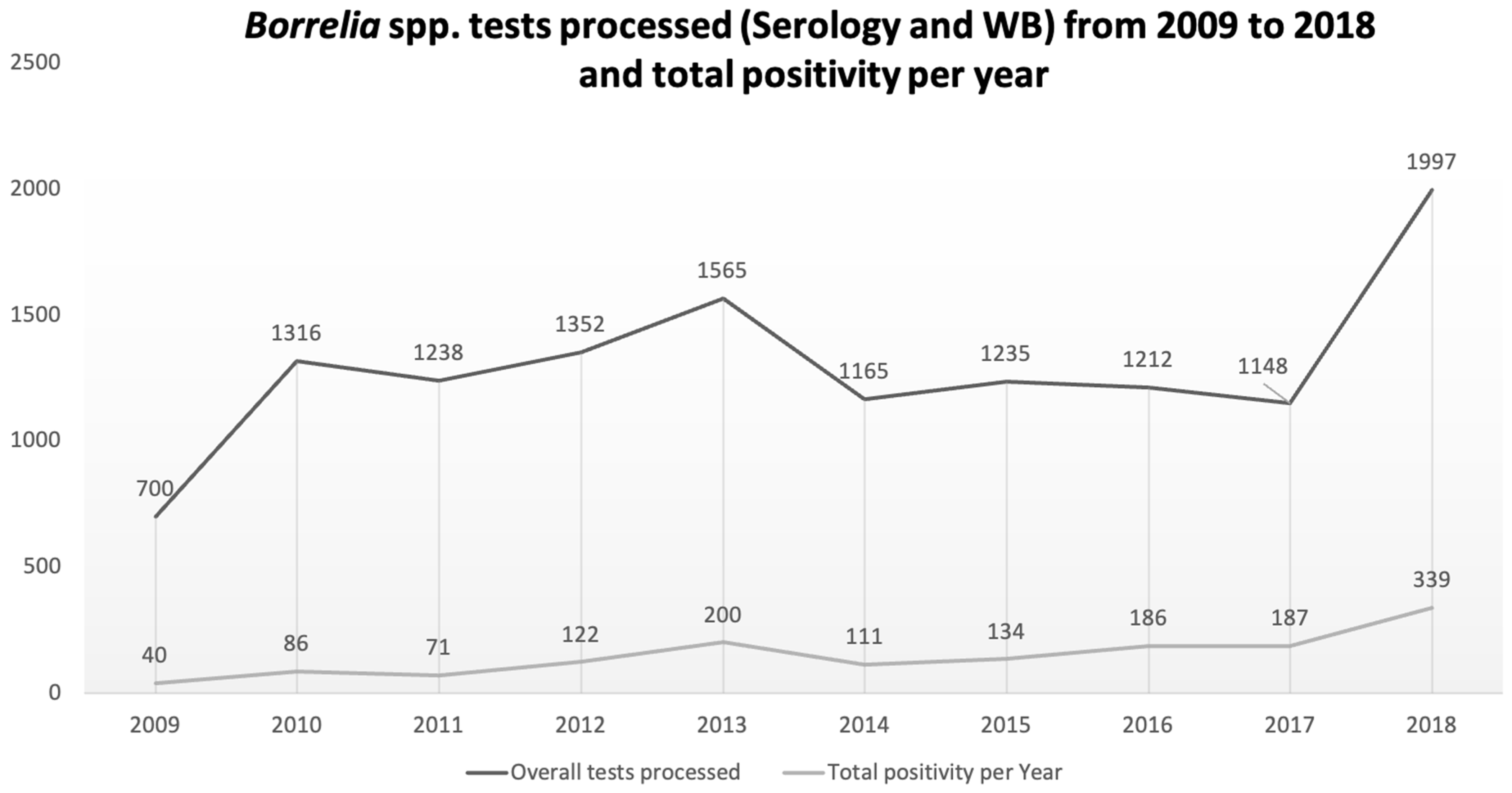
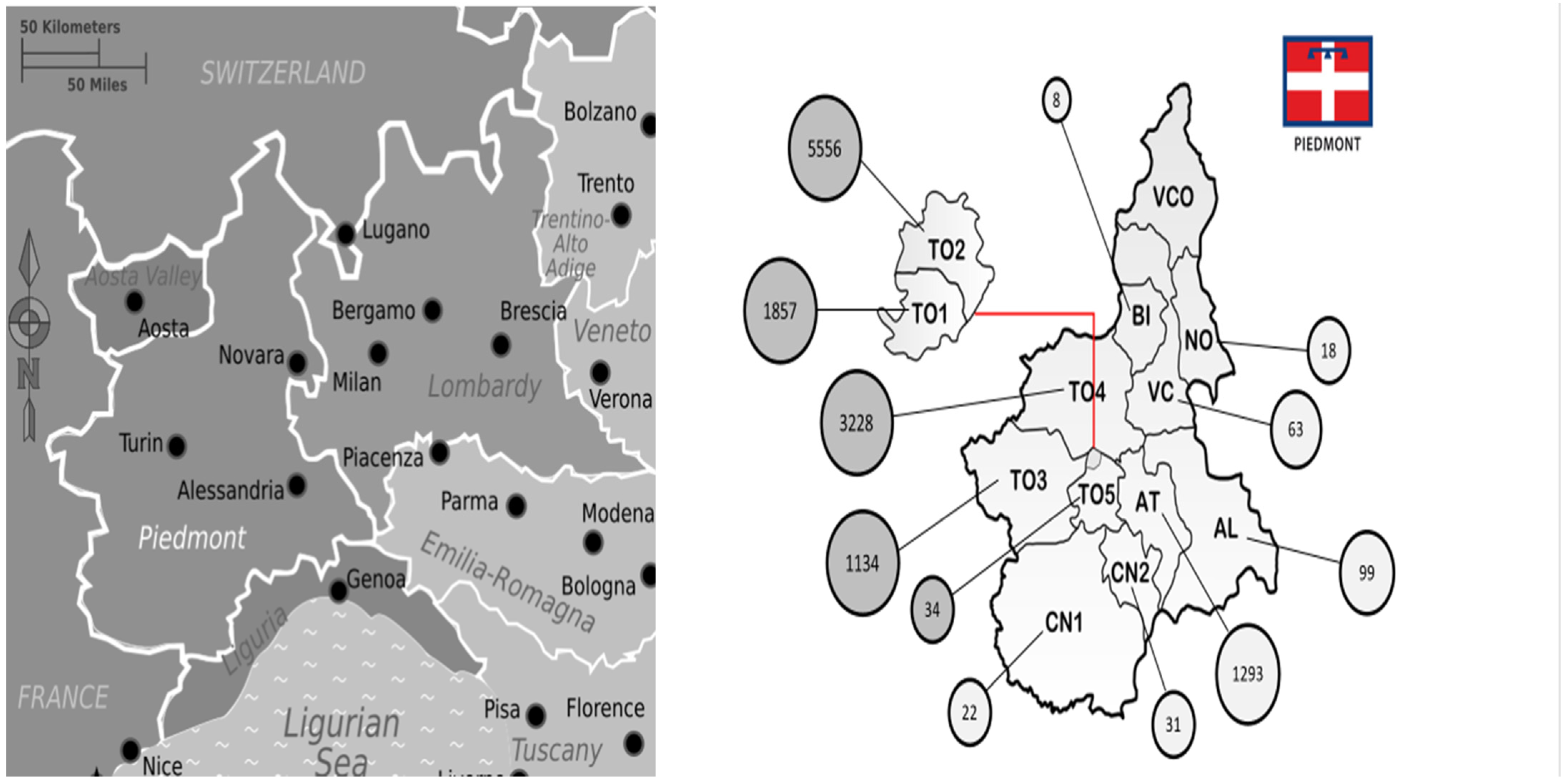
3. Results
4. Discussion
4.1. Epidemiology of TBDs in Europe, Italy and Piedmont
4.2. Our Data in the Picture Frame
4.3. The Impact of Climate Change and Human Behaviour on the Incidence of TBD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The National Aeronautics and Space Administration (NASA). Overview: Weather, Global Warming and Climate Change. Climate Change: Vital Signs of the Planet. Available online: https://climate.nasa.gov/resources/global-warming-vs-climate-change (accessed on 13 October 2021).
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 report of The Lancet Countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- IPCC, 2021: Summary for Policymakers. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 16 September 2021).
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.-Y.; Bi, P.; Cazelles, B.; Zhou, S.; Huang, S.-Q.; Yang, J.; Pei, Y.; Wu, X.-X.; Fu, S.-H.; Tong, S.-L.; et al. How environmental conditions impact mosquito ecology and Japanese encephalitis: An eco-epidemiological approach. Environ. Int. 2015, 79, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Bajer, A.; Paziewska-Harris, A.; Baumann-Popczyk, A.; Siński, E. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv. Med. Sci. 2012, 57, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Heyman, P.; Cochez, C.; Hofhuis, A.; Van Der Giessen, J.; Sprong, H.; Porter, S.R.; Losson, B.; Saegerman, C.; Donoso-Mantke, O.; Niedrig, M.; et al. A clear and present danger: Tick-borne diseases in Europe. Expert Rev. Anti-Infect. Ther. 2010, 8, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Romi, R. Arthropod-borne diseases in Italy: From a neglected matter to an emerging health problem. Ann. Dell’istituto Super. Sanità 2010, 46, 436–443. [Google Scholar] [CrossRef]
- ECDC Crimean-Congo Haemorrhagic Fever Annual Epidemiological Report 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/crimean-congo-haemorrhagic-fever-annual-epidemiological-report-2019 (accessed on 13 October 2021).
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer Nature: Cham, Switzerland, 2017; pp. 279–291. [Google Scholar]
- Spengler, J.R.; Bente, D.A. Crimean-Congo Hemorrhagic Fever in Spain—New Arrival or Silent Resident? N. Engl. J. Med. 2017, 377, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Viljugrein, H.; Hofshagen, M.; Brun-Hansen, H.; Kristoffersen, A.B.; Nygård, K.; Brun, E.; Ottesen, P.; Sævik, B.K.; Ytrehus, B. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasites Vectors 2011, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randolph, S.; Green, R.; Hoodless, A.; Peacey, M. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 2002, 32, 979–989. [Google Scholar] [CrossRef]
- Eestrada-Pena, A.; Eayllon, N.; La Fuente, J.E. Impact of Climate Trends on Tick-Borne Pathogen Transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uiterwijk, M.; Ibáñez-Justicia, A.; van de Vossenberg, B.; Jacobs, F.; Overgaauw, P.; Nijsse, R.; Dabekaussen, C.; Stroo, A.; Sprong, H. Imported Hyalomma ticks in the Netherlands 2018–2020. Parasites Vectors 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Süss, J. Tick-borne encephalitis in Europe and beyond—the epidemiological situation as of 2007. Eurosurveillance 2008, 13, 18916. [Google Scholar] [CrossRef] [PubMed]
- Alfano, N.; Tagliapietra, V.; Rosso, F.; Ziegler, U.; Arnoldi, D.; Rizzoli, A. Tick-borne encephalitis foci in northeast Italy revealed by combined virus detection in ticks, serosurvey on goats and human cases. Emerg. Microbes Infect. 2020, 9, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, A.; Rodari, P.; Mauroner, L.; Zanella, F.; Moro, L.; Bertoli, G.; Da Re, F.; Russo, F.; Napoletano, G.; Silva, R. Emergence of Lyme borreliosis in the province of Verona, Northern Italy: Five-years of sentinel surveillance. Ticks Tick-Borne Dis. 2021, 12, 101628. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Farkas, R.; Jaenson, T.G.T.; Koenen, F.; Madder, M.; Pascucci, I.; Salman, M.; Tarrés-Call, J.; Jongejan, F. Association of environmental traits with the geographic ranges of ticks (Acari: Ixodidae) of medical and veterinary importance in the western Palearctic. A digital data set. Exp. Appl. Acarol. 2013, 59, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Peña, A.E.; Sánchez, N.; Estrada-Sánchez, A. An Assessment of the Distribution and Spread of the Tick Hyalomma marginatum in the Western Palearctic under Different Climate Scenarios. Vector-Borne Zoonotic Dis. 2012, 12, 758–768. [Google Scholar] [CrossRef]
- Mysterud, A.; Easterday, W.R.; Stigum, V.M.; Aas, A.B.; Meisingset, E.L.; Viljugrein, H. Contrasting Emergence of Lyme Disease across Ecosystems. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- I.Stat, la Banca Dati Completa per gli Esperti. Available online: http://dati.istat.it/ (accessed on 13 November 2021).
- ARPA: Historical Meteorology Database of the Regional Agency for Environment Protection (ARPA Piemonte). Available online: https://www.arpa.piemonte.it (accessed on 12 October 2021).
- SEREMI, Regional Notification System. Available online: https://www.seremi.it/pubblicazioni (accessed on 12 October 2021).
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Ѐntomol. 2021, 66, 373–388. [Google Scholar] [CrossRef]
- Colwell, D.D.; Torres, F.D.; Otranto, D. Vector-borne parasitic zoonoses: Emerging scenarios and new perspectives. Veter Parasitol. 2011, 182, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Santino, I.; Sessa, R.; Del Piano, M. Lyme Borreliosis Infection in Europe. Eur. J. Inflamm. 2006, 4, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick-Borne Dis. 2020, 11, 101489. [Google Scholar] [CrossRef]
- Acquaotta, F.; Fratianni, S.; Garzena, D. Temperature changes in the North-Western Italian Alps from 1961 to 2010. Theor. Appl. Clim. 2015, 122, 619–634. [Google Scholar] [CrossRef]
- Altpeter, E.; Zimmermann, H.; Oberreich, J.; Péter, O.; Dvořák, C.; Swiss Sentinel Surveillance Network. Tick related diseases in Switzerland, 2008 to 2011. Swiss Med. Wkly. 2013, 143. [Google Scholar] [CrossRef]
- Zanet, S.; Ferroglio, E.; Battisti, E.; Tizzani, P. Ecological niche modeling of Babesia sp. infection in wildlife experimentally evaluated in questing Ixodes ricinus. Geospat. Health 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Giannelli, A.; Latrofa, M.S.; Cascio, A.; Cazzin, S.; Ravagnan, S.; Montarsi, F.; Zanzani, S.A.; Manfredi, M.T.; et al. Ticks infesting humans in Italy and associated pathogens. Parasites Vectors 2014, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Battisti, E.; Zanet, S.; Boraso, F.; Minniti, D.; Giacometti, M.; Duscher, G.G.; Ferroglio, E. Survey on tick-borne pathogens in ticks removed from humans in Northwestern Italy. Veter- Parasitol. Reg. Stud. Rep. 2019, 18, 100352. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Battisti, E.; Pepe, P.; Ciuca, L.; Colombo, L.; Trisciuoglio, A.; Ferroglio, E.; Cringoli, G.; Rinaldi, L.; Maurelli, M.P. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: A country-wide molecular survey. BMC Vet. Res. 2020, 16, 46. [Google Scholar] [CrossRef] [Green Version]
- Audino, T.; Pautasso, A.; Bellavia, V.; Carta, V.; Ferrari, A.; Verna, F.; Grattarola, C.; Iulini, B.; Pintore, M.D.; Bardelli, M.; et al. Ticks infesting humans and associated pathogens: A cross-sectional study in a 3-year period (2017–2019) in northwest Italy. Parasites Vectors 2021, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Giglio, G.; Ramassa, E.; Nobili, F.; Rossi, L.; Tomassone, L. Dermacentor marginatus and Dermacentor reticulatus, and Their Infection by SFG Rickettsiae and Francisella-Like Endosymbionts, in Mountain and Periurban Habitats of Northwestern Italy. Vet. Sci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Ravagnan, S.; Tomassone, L.; Montarsi, F.; Krawczyk, A.I.; Mastrorilli, E.; Sprong, H.; Milani, A.; Rossi, L.; Capelli, G. First detection of Borrelia miyamotoi in Ixodes ricinus ticks from northern Italy. Parasites Vectors 2018, 11, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Carnevali, L.; Pedrotti, L.; Riga, F.; Toso, S. Banca Dati Ungulati. Status, distribuzione, consistenza, gestione e prelievo venatorio delle popolazioni di ungulati in Italia: Rapporto 2001–2005. Biol. Cons. Fauna. 2009, 117, 1–168. [Google Scholar]
- Bellato, A.; Pintore, M.D.; Catelan, D.; Pautasso, A.; Torina, A.; Rizzo, F.; Mandola, M.L.; Mannelli, A.; Casalone, C.; Tomassone, L. Risk of tick-borne zoonoses in urban green areas: A case study from Turin, northwestern Italy. Urban For. Urban Green. 2021, 64, 127297. [Google Scholar] [CrossRef]
- Pintore, M.D.; Ceballos, L.A.; Iulini, B.; Tomassone, L.; Pautasso, A.; Corbellini, D.; Rizzo, F.; Mandola, M.L.; Bardelli, M.; Peletto, S.; et al. Detection of Invasive Borrelia burgdorferi Strains in North-Eastern Piedmont, Italy. Zoonoses Public Health 2015, 62, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Giglio, G.; Ramassa, E.; Nobili, F.; Rossi, L.; Tomassone, L. Low Risk Perception about Ticks and Tick-Borne Diseases in an Area Recently Invaded by Ticks in Northwestern Italy. Vet. Sci. 2021, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Millet, I.; Ragionieri, M.; Tomassone, L.; Trentin, C.; Mannelli, A. Assessment of the Exposure of People to Questing Ticks Carrying Agents of Zoonoses in Aosta Valley, Italy. Vet. Sci. 2019, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguero-Rosenfeld, M.E.; Wormser, G.P. Lyme disease: Diagnostic issues and controversies. Expert Rev. Mol. Diagn. 2014, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rutz, H.; Hogan, B.; Hook, S.; Hinckley, A.; Feldman, K. Impacts of misclassification on Lyme disease surveillance. Zoonoses Public Health 2018, 66, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Jaulhac, B.; Mediannikov, O.; Arzouni, J.-P.; Raoult, D. Values of diagnostic tests for the various species of spirochetes. Med. Mal. Infect. 2019, 49, 102–111. [Google Scholar] [CrossRef] [PubMed]


| 2016 | 2017 | 2018 | |
|---|---|---|---|
| Borrelia spp. WB, IgM and IgG both positive (Current report) | 36 | 39 | 61 |
| SEREMI surveillance confirmed Lyme diseases | 12 | 6 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroffolini, G.; Segala, F.V.; Lupia, T.; Faraoni, S.; Rossi, L.; Tomassone, L.; Zanet, S.; De Rosa, F.G.; Di Perri, G.; Calcagno, A. Serology for Borrelia spp. in Northwest Italy: A Climate-Matched 10-Year Trend. Life 2021, 11, 1310. https://doi.org/10.3390/life11121310
Stroffolini G, Segala FV, Lupia T, Faraoni S, Rossi L, Tomassone L, Zanet S, De Rosa FG, Di Perri G, Calcagno A. Serology for Borrelia spp. in Northwest Italy: A Climate-Matched 10-Year Trend. Life. 2021; 11(12):1310. https://doi.org/10.3390/life11121310
Chicago/Turabian StyleStroffolini, Giacomo, Francesco Vladimiro Segala, Tommaso Lupia, Silvia Faraoni, Luca Rossi, Laura Tomassone, Stefania Zanet, Francesco Giuseppe De Rosa, Giovanni Di Perri, and Andrea Calcagno. 2021. "Serology for Borrelia spp. in Northwest Italy: A Climate-Matched 10-Year Trend" Life 11, no. 12: 1310. https://doi.org/10.3390/life11121310
APA StyleStroffolini, G., Segala, F. V., Lupia, T., Faraoni, S., Rossi, L., Tomassone, L., Zanet, S., De Rosa, F. G., Di Perri, G., & Calcagno, A. (2021). Serology for Borrelia spp. in Northwest Italy: A Climate-Matched 10-Year Trend. Life, 11(12), 1310. https://doi.org/10.3390/life11121310








