Selective Inhibition of IL-6 Trans-Signaling Has No Beneficial Effect on the Posttraumatic Cytokine Release after Multiple Trauma in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Group Distribution
2.3. Anesthesia and Perioperative Analgesia
2.4. Control
2.5. Intramedullary Femur Pin
2.6. Femoral Fracture
2.7. Bilateral Chest Trauma
2.8. Activity Score
2.9. Final Blood Sampling and Sacrifice
2.10. Organ Removal, Bronchoalveolar Lavage (BAL) and Hepatic Rinsing
2.11. BALF Processing and Isolation of Alveolar Macrophages
2.12. Isolation of Kupffer Cells
2.13. Immunoassay for Cytokine Measurement
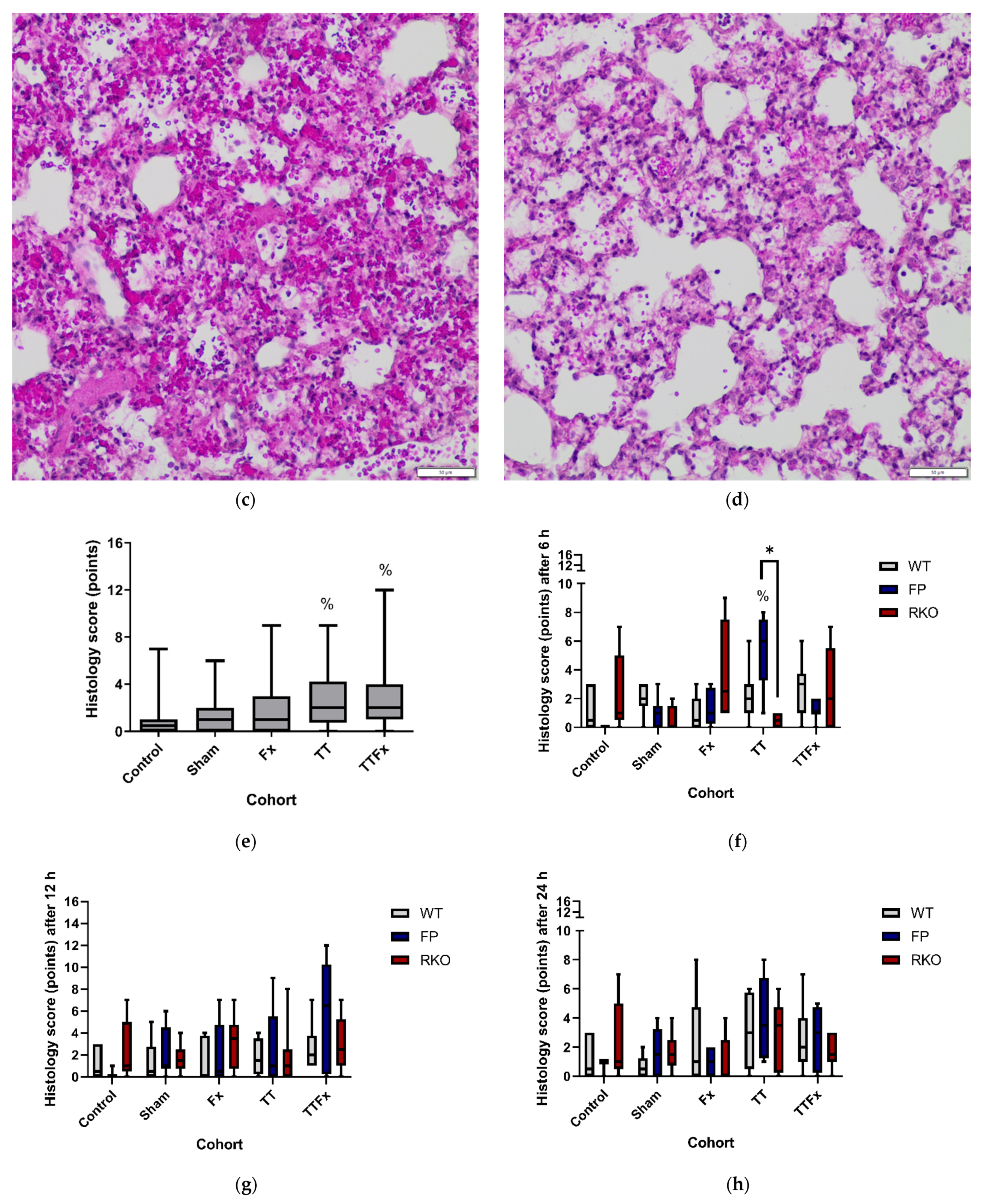
2.14. Histological Examination
2.15. Statistical Analysis
3. Results
3.1. Survival, Activity and Histological Examination
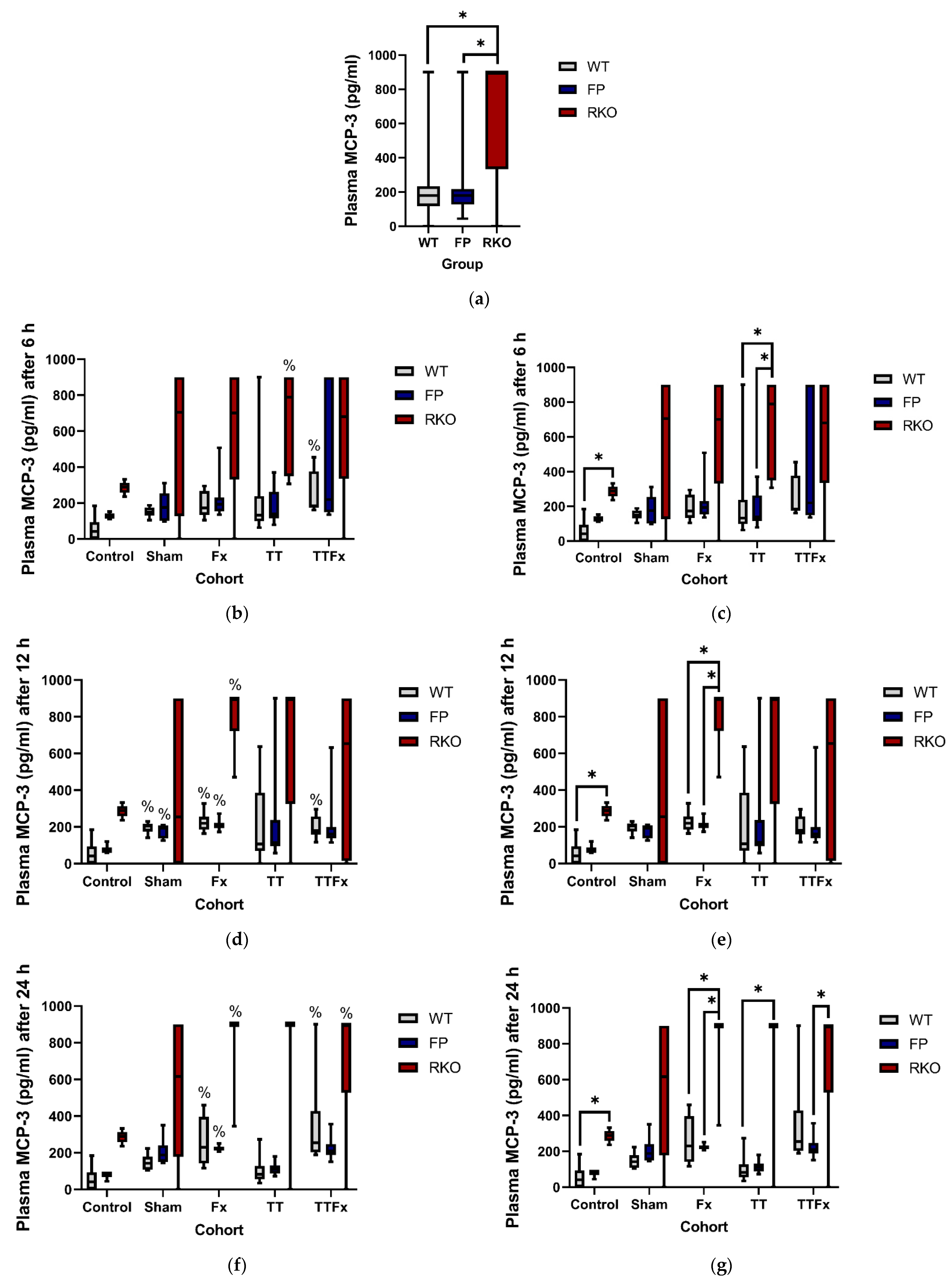
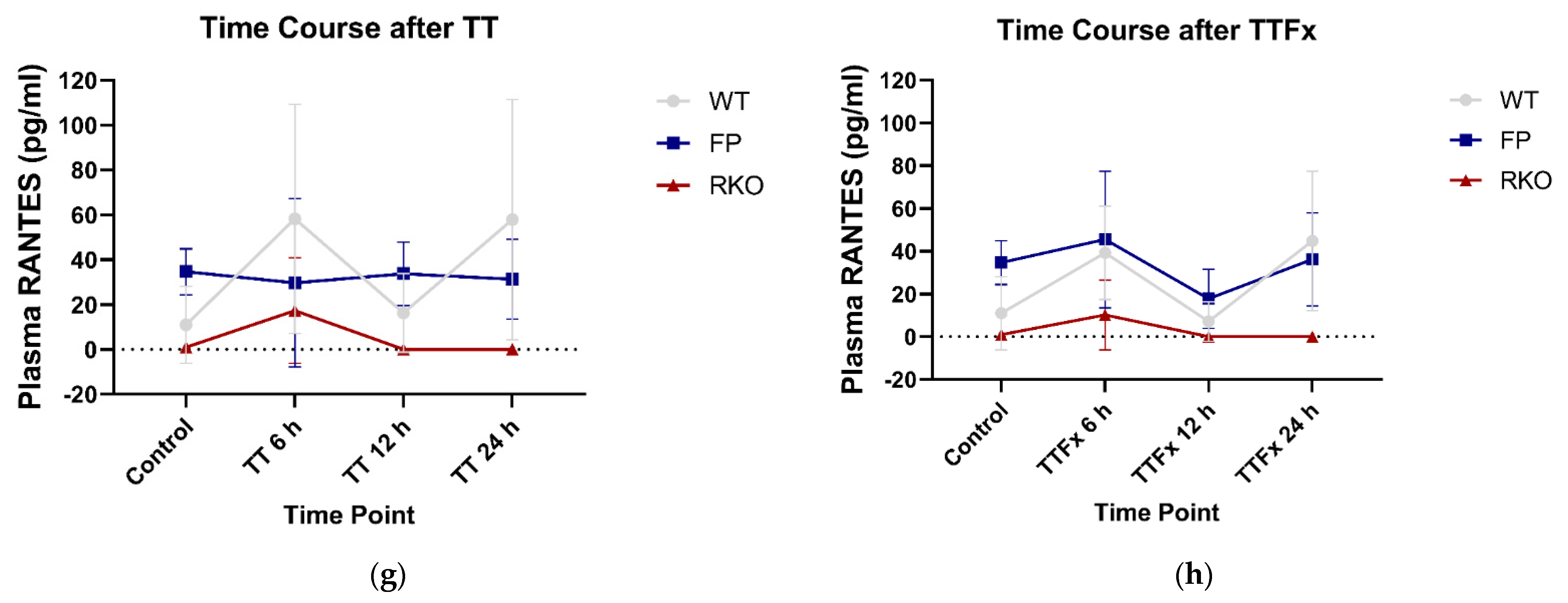
3.2. Cytokines in Blood Plasma
3.2.1. IL-6
3.2.2. MCP-3
3.2.3. RANTES
3.2.4. MCP-1, MIP-1β, GM-CSF
3.3. Cytokines in Kupffer Cell Supernatant
3.3.1. MCP-1
3.3.2. IL-6, MCP-3, RANTES, GM-CSF and MIP-1β
3.4. Cytokines in Alveolar Macrophages Supernatant
3.5. Cytokines in BAL
4. Discussion
Limitations and Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mock, C.; World Health Organization; International Society of Surgery; International Association of Trauma Surgery and Intensive Care. Guidelines for Essential Trauma Care; World Health Organization: Geneva, Switzerland, 2004; p. 3. ISBN 9241546409. [Google Scholar]
- German Society of Trauma. Whitebook Medical Care of the Severely Injured, 2nd ed.; Orthopaedics and Traumatology, Communications and News; German Society of Trauma: Berlin, Germany, 2012; p. 14. [Google Scholar]
- Ruchholtz, S.; Lefering, R.; Paffrath, T.; Oestern, H.J.; Neugebauer, E.; Nast-Kolb, D.; Pape, H.C.; Bouillon, B. Rückgang der traumaletalität: Ergebnisse des traumaregisters der Deutschen gesellschaft für unfallchirurgie. Dtsch. Arztebl. 2008, 105, 225–231. [Google Scholar] [CrossRef]
- Faist, E.; Baue, A.E.; Dittmer, H.; Heberer, G. Multiple organ failure in polytrauma patients. J. Trauma Inj. Infect. Crit. Care 1983, 23, 775–787. [Google Scholar] [CrossRef]
- Durham, R.M.; Moran, J.J.; Mazuski, J.E.; Shapiro, M.J.; Baue, A.E.; Flint, L.M. Multiple organ failure in trauma patients. J. Trauma 2003, 55, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, M.; Lefering, R.; Probst, C.; Paffrath, T.; Schneider, M.M.; Maegele, M.; Sakka, S.G.; Bouillon, B.; Wafaisade, A. Epidemiology and risk factors of multiple-organ failure after multiple trauma: An analysis of 31,154 patients from the TraumaRegister DGU. J. Trauma Acute Care Surg. 2014, 76, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Sauaia, A.; Moore, E.E.; Johnson, J.L.; Chin, T.L.; Banerjee, A.; Sperry, J.L.; Maier, R.V.; Burlew, C.C. Temporal trends of postinjury multiple-organ failure: Still resource intensive, morbid, and lethal. J. Trauma Acute Care Surg. 2014, 76, 582–593. [Google Scholar] [CrossRef] [Green Version]
- Sauaia, A.; Moore, F.A.; Moore, E.E. Postinjury Inflammation and Organ Dysfunction. Crit. Care Clin. 2017, 33, 167–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keel, M.; Trentz, O. Pathophysiology of polytrauma. Injury 2005, 36, 691–709. [Google Scholar] [CrossRef]
- Bone, R.C. Immunologic Dissonance: A Continuing Evolution in Our Understanding of the Systemic Inflammatory Response Syndrome (SIRS) and the Multiple Organ Dysfunction Syndrome (MODS). Ann. Intern. Med. 1996, 125, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, P.; Faure, E.; Kipnis, E. Inflammasomes in Tissue Damages and Immune Disorders after Trauma. Front. Immunol. 2018, 9, 1900. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.N.; Bradley, B.A.; Ceredig, R. Sterile post-traumatic immunosuppression. Clin. Transl. Immunol. 2016, 5, e77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Chanthaphavong, R.S.; Pape, H.-C. Current theories on the pathophysiology of multiple organ failure after trauma. Injury 2010, 41, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wang, W.; Yin, L.; Luo, P.; Greven, J.; Horst, K.; Hildebrand, F. Using IL-6 concentrations in the first 24 h following trauma to predict immunological complications and mortality in trauma patients: A meta-analysis. Eur. J. Trauma Emerg. Surg. 2018, 44, 679–687. [Google Scholar] [CrossRef]
- Hildebrand, F.; Hubbard, W.J.; Choudhry, M.A.; Frink, M.; Pape, H.C.; Kunkel, S.L.; Chaudry, I.H. Kupffer cells and their mediators: The culprits in producing distant organ damage after trauma-hemorrhage. Am. J. Pathol. 2006, 169, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Perl, M.; Gebhard, F.; Braumüller, S.; Tauchmann, B.; Brückner, U.B.; Kinzl, L.; Knöferl, M.W. The pulmonary and hepatic immune microenvironment and its contribution to the early systemic inflammation following blunt chest trauma. Crit. Care Med. 2006, 34, 1152–1159. [Google Scholar] [CrossRef]
- Gauldie, J.; Richards, C.; Harnish, D.; Lansdorp, P.; Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7251–7255. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Jones, S.A. Directing Transition from Innate to Acquired Immunity: Defining a Role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct Effects of IL-6 Classic and Trans-Signaling in Bone Fracture Healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, K.; Prystaz, K.; Vikman, A.; Haffner-Luntzer, M.; Bergdolt, S.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; Ignatius, A. Pharmacological inhibition of IL-6 trans-signaling improves compromised fracture healing after severe trauma. Naunyn. Schmiedebergs. Arch. Pharmacol. 2018, 391, 523–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheller, J.; Rose-John, S. Interleukin-6 and its receptor: From bench to bedside. Med. Microbiol. Immunol. 2006, 195, 173–183. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: Role in inflammation and cancer. J. Leukoc. Biol. 2006, 80, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Garbers, C.; Rose-John, S. Dissecting interleukin-6 classic- and trans-signaling in inflammation and cancer. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1725, pp. 127–140. [Google Scholar]
- Rose-John, S.; Heinrich, P.C. Soluble receptors for cytokines and growth factors: Generation and biological function. Biochem. J. 1994, 300, 281–290. [Google Scholar] [CrossRef]
- Rose-John, S. The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin. Pharmacol. Ther. 2017, 102, 591–598. [Google Scholar] [CrossRef]
- Peters, M.; Jacobs, S.; Ehlers, M.; Vollmer, P.; Müllberg, J.; Wolf, E.; Brem, G.; Meyer Zum Büschenfelde, K.H.; Rose-John, S. The function of the soluble interleukin 6 (IL-6) receptor in vivo: Sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J. Exp. Med. 1996, 183, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Mülberg, J.; Schooltink, H.; Stoyan, T.; Günther, M.; Graeve, L.; Buse, G.; Mackiewicz, A.; Heinrich, P.C.; Rose-John, S. The soluble interleukin-6 receptor is generated by shedding. Eur. J. Immunol. 1993, 23, 473–480. [Google Scholar] [CrossRef]
- Lust, J.A.; Donovan, K.A.; Kline, M.P.; Greipp, P.R.; Kyle, R.A.; Maihle, N.J. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine 1992, 4, 96–100. [Google Scholar] [CrossRef]
- Waetzig, G.H.; Rose-John, S. Hitting a complex target: An update on interleukin-6 trans-signalling. Expert Opin. Ther. Targets 2012, 16, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From basic biology to selective blockade of proinflammatory activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Jostock, T.; Müllberg, J.; Özbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Tenhumberg, S.; Waetzig, G.H.; Chalaris, A.; Rabe, B.; Seegert, D.; Scheller, J.; Rose-John, S.; Grötzinger, J. Structure-guided optimization of the interleukin-6 trans-signaling antagonist sgp130. J. Biol. Chem. 2008, 283, 27200–27207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, J.; Waetzig, G.H.; Chalaris, A.; Reinheimer, T.M.; Wege, H.; Rose-John, S.; Garbers, C. Different soluble forms of the interleukin-6 family signal transducer gp130 fine-tune the blockade of interleukin-6 trans-signaling. J. Biol. Chem. 2016, 291, 16186–16196. [Google Scholar] [CrossRef] [Green Version]
- Barkhausen, T.; Tschernig, T.; Rosenstiel, P.; Van Griensven, M.; Vonberg, R.P.; Dorsch, M.; Mueller-Heine, A.; Chalaris, A.; Scheller, J.; Rose-John, S.; et al. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 2011, 39, 1407–1413. [Google Scholar] [CrossRef]
- Barkhausen, T.; Hildebrand, F.; Krettek, C.; van Griensven, M. DHEA-dependent and organ-specific regulation of TNF-α mRNA expression in a murine polymicrobial sepsis and trauma model. Crit. Care 2009, 13, R114. [Google Scholar] [CrossRef] [Green Version]
- Fitschen-Oestern, S.; Lippross, S.; Klueter, T.; Weuster, M.; Varoga, D.; Tohidnezhad, M.; Pufe, T.; Rose-John, S.; Andruszkow, H.; Hildebrand, F.; et al. Correction to: A new multiple trauma model of the mouse. BMC Musculoskelet. Disord. 2019, 20, 72. [Google Scholar] [CrossRef]
- Bonnarens, F.; Einhorn, T.A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 1984, 2, 97–101. [Google Scholar] [CrossRef]
- Neunaber, C.; Oestern, S.; Andruszkow, H.; Zeckey, C.; Mommsen, P.; Kutter, D.; Stöfen, M.; Krettek, C.; Hildebrand, F. Cytokine productive capacity of alveolar macrophages and Kupffer cells after femoral fracture and blunt chest trauma in a murine trauma model. Immunol. Lett. 2013, 152, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Barkhausen, T.; Probst, C.; Hildebrand, F.; Pape, H.C.; Krettek, C.; van Griensven, M. Insulin therapy induces changes in the inflammatory response in a murine 2-hit model. Injury 2009, 40, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Matsutani, T.; Choudhry, M.A.; Schwacha, M.G.; Rue, L.W.; Bland, K.I.; Chaudry, I.H. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine 2004, 26, 223–230. [Google Scholar] [CrossRef]
- Wichmann, M.W.; Ayala, A.; Chaudry, I.H. Male sex steroids are responsible for depressing macrophage immune function after trauma-hemorrhage. Am. J. Physiol. Cell Physiol. 1997, 273, C1335–C1340. [Google Scholar] [CrossRef]
- Mommsen, P.; Barkhausen, T.; Frink, M.; Zeckey, C.; Probst, C.; Krettek, C.; Hildebrand, F. Productive capacity of alveolar macrophages and pulmonary organ damage after femoral fracture and hemorrhage in IL-6 knockout mice. Cytokine 2011, 53, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Flohé, S.; Lendemans, S.; Selbach, C.; Waydhas, C.; Ackermann, M.; Schade, F.U.; Kreuzfelder, E. Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit. Care Med. 2003, 31, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Nishina, K.; Mikawa, K.; Takao, Y.; Shiga, M.; Maekawa, N.; Obara, H. Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology 1998, 88, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Bardenheuer, M.; Obertacke, U.; Waydhas, C.; Nast-Kolb, D. Epidemiologie des schwerverletzten—Eine prospektive erfassung der praklinischen und klinischen versorgung. Unfallchirurg 2000, 103, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Gunst, M.; Ghaemmaghami, V.; Gruszecki, A.; Urban, J.; Frankel, H.; Shafi, S. Changing Epidemiology of Trauma Deaths Leads to a Bimodal Distribution. Baylor Univ. Med. Cent. Proc. 2010, 23, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Garbers, C.; Thaiss, W.; Jones, G.W.; Waetzig, G.H.; Lorenzen, I.; Guilhot, F.; Lissilaa, R.; Ferlin, W.G.; Grötzinger, J.; Jones, S.A.; et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011, 286, 42959–42970. [Google Scholar] [CrossRef] [Green Version]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Wintges, K.; Beil, F.T.; Albers, J.; Jeschke, A.; Schweizer, M.; Claass, B.; Tiegs, G.; Amling, M.; Schinke, T. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J. Bone Miner. Res. 2013, 28, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Frink, M.; Pape, H.C.; Van Griensven, M.; Krettek, C.; Chaudry, I.H.; Hildebrand, F. Influence of sex and age on mods and cytokines after multiple injuries. Shock 2007, 27, 151–156. [Google Scholar] [CrossRef]
- Crockett, E.T.; Spielman, W.; Dowlatshahi, S.; He, J. Sex differences in inflammatory cytokine production in hepatic ischemia-reperfusion. J. Inflamm. 2006, 3, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, M.W.; Zellweger, R.; DeMaso, C.M.; Ayala, A.; Chaudry, I.H. Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock and resuscitation. Cytokine 1996, 8, 853–863. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homeier, J.-M.; Bundkirchen, K.; Winkelmann, M.; Graulich, T.; Relja, B.; Neunaber, C.; Macke, C. Selective Inhibition of IL-6 Trans-Signaling Has No Beneficial Effect on the Posttraumatic Cytokine Release after Multiple Trauma in Mice. Life 2021, 11, 1252. https://doi.org/10.3390/life11111252
Homeier J-M, Bundkirchen K, Winkelmann M, Graulich T, Relja B, Neunaber C, Macke C. Selective Inhibition of IL-6 Trans-Signaling Has No Beneficial Effect on the Posttraumatic Cytokine Release after Multiple Trauma in Mice. Life. 2021; 11(11):1252. https://doi.org/10.3390/life11111252
Chicago/Turabian StyleHomeier, Jil-Madeline, Katrin Bundkirchen, Marcel Winkelmann, Tilman Graulich, Borna Relja, Claudia Neunaber, and Christian Macke. 2021. "Selective Inhibition of IL-6 Trans-Signaling Has No Beneficial Effect on the Posttraumatic Cytokine Release after Multiple Trauma in Mice" Life 11, no. 11: 1252. https://doi.org/10.3390/life11111252







