The Prebiotic Kitchen: A Guide to Composing Prebiotic Soup Recipes to Test Origins of Life Hypotheses
Abstract
:1. Introduction
2. General Principles and Challenges for Designing Experimental Prebiotic Soups
3. Prebiotic Sources of Organics and Challenges for Soup Design
3.1. Terrestrial Sources of Organics
3.2. Exogenous Delivery of Organics
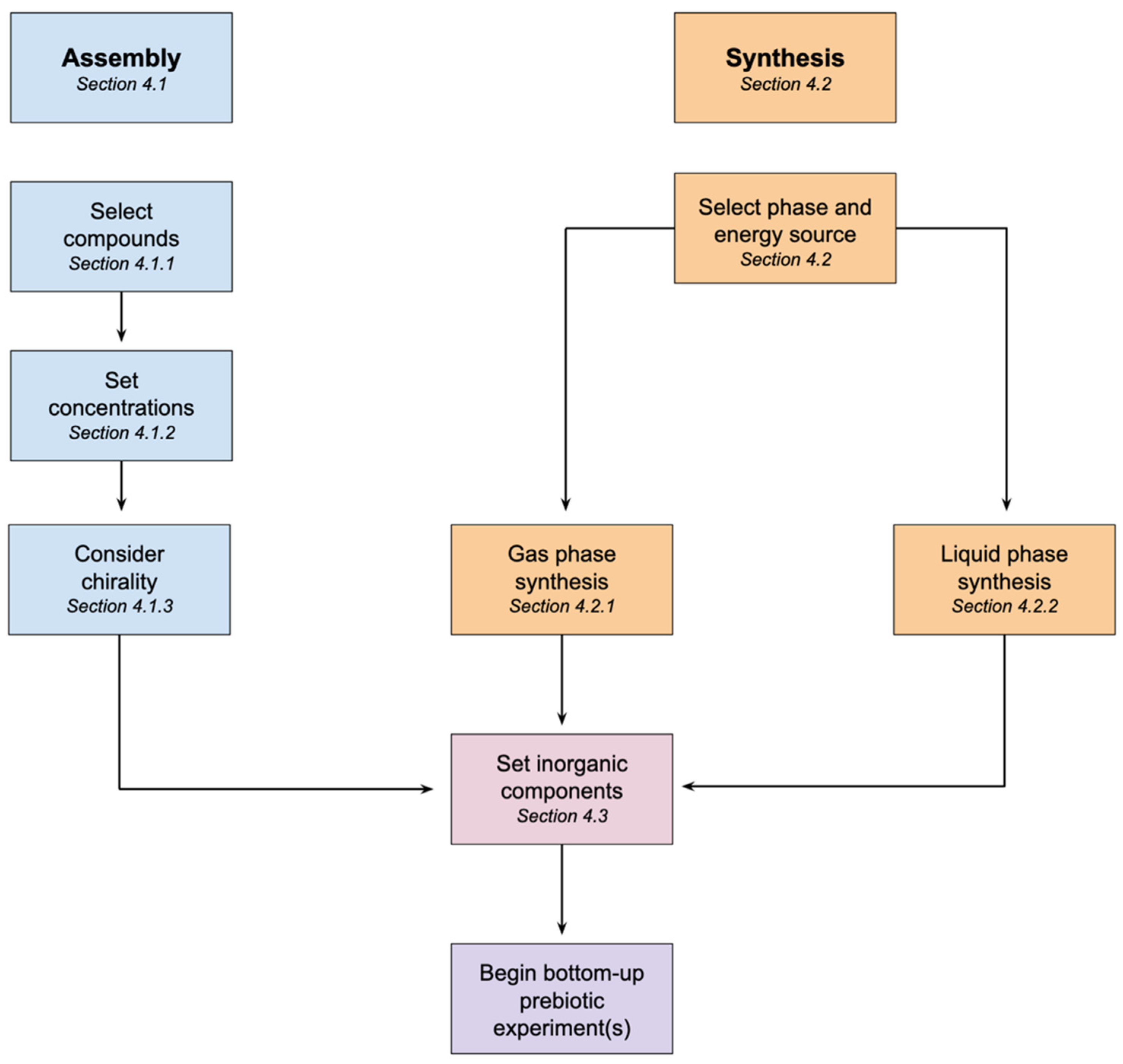
4. How to Make Prebiotic Soup
4.1. Assembled Prebiotic Soup
4.1.1. Selection of Compounds
4.1.2. Setting Concentrations
4.1.3. Chirality
4.2. Synthesizing Prebiotic Soup
4.2.1. Gas Phase Synthesis
4.2.2. Liquid Phase Synthesis
4.3. Inorganic Components
4.4. Storage and Transport
5. A Shared Infrastructure for Complex Prebiotic Chemistry
6. Conclusions: The Future of Messy Prebiotic Chemistry and Its Interplay with Reductionist Approaches
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.L.; Urey, H.C. Organic Compound Synthesis on the Primitive Earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M. Prebiotic materials from on and off the early Earth. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1689–1702. [Google Scholar] [CrossRef] [Green Version]
- Cleaves, H.J. Prebiotic Chemistry: What We Know, What We Don’t. Evol. Educ. Outreach 2012, 5, 342–360. [Google Scholar] [CrossRef] [Green Version]
- McCollom, T.M. Miller-Urey and Beyond: What Have We Learned About Prebiotic Organic Synthesis Reactions in the Past 60 Years? Annu. Rev. Earth Planet. Sci. 2013, 41, 207–229. [Google Scholar] [CrossRef]
- Haldane, J.B.S. The origin of life. Ration. Annu. 1929, 3, 3–10. [Google Scholar]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Morowitz, H.J.; Deamer, D.W.; Smith, T. Biogenesis as an evolutionary process. J. Mol. Evol. 1991, 33, 207–208. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [CrossRef]
- Raggi, L.; Bada, J.L.; Lazcano, A. On the lack of evolutionary continuity between prebiotic peptides and extant enzymes. Phys. Chem. Chem. Phys. 2016, 18, 20028–20032. [Google Scholar] [CrossRef]
- Kauffman, S.A.; Jelenfi, D.P.; Vattay, G.; Dávid, J. Theory of chemical evolution of molecule compositions in the universe, in the Miller–Urey experiment and the mass distribution of interstellar and intergalactic molecules. J. Theor. Biol. 2019, 486, 110097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollrab, E.; Scherer, S.; Aubriet, F.; Carré, V.; Carlomagno, T.; Codutti, L.; Ott, A. Chemical Analysis of a “Miller-Type” Complex Prebiotic Broth. Orig. Life Evol. Biosph. 2015, 46, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Doran, D.; Abul-Haija, Y.M.; Cronin, L.; Cronin, L. Emergence of Function and Selection from Recursively Programmed Polymerisation Reactions in Mineral Environments. Angew. Chem. Int. Ed. 2019, 58, 11253–11256. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Haynes, J.W.; Martin, C.; Petrov, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preiner, M.; Asche, S.; Becker, S.; Betts, H.C.; Boniface, A.; Camprubi, E.; Chandru, K.; Erastova, V.; Garg, S.G.; Khawaja, N.; et al. The Future of Origin of Life Research: Bridging Decades-Old Divisions. Life 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preiner, M.; Igarashi, K.; Muchowska, K.B.; Yu, M.; Varma, S.J.; Kleinermanns, K.; Nobu, M.K.; Kamagata, Y.; Tüysüz, H.; Moran, J.; et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 2020, 4, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Virgo, N. Thresholds in messy chemistries. In Proceedings of the Artificial Life Conference, Cancún, Mexico, 4–8 July 2016. [Google Scholar]
- Colón-Santos, S.; Cooper, G.J.T.; Cronin, L.; Cronin, L. Taming the Combinatorial Explosion of the Formose Reaction via Recursion within Mineral Environments. ChemSystemsChem 2019, 1, e1900014. [Google Scholar] [CrossRef] [Green Version]
- Vincent, L.; Berg, M.; Krismer, M.; Saghafi, S.T.; Cosby, J.; Sankari, T.; Vetsigian, K.; Ii, H.J.C.; Baum, D.A. Chemical Ecosystem Selection on Mineral Surfaces Reveals Long-Term Dynamics Consistent with the Spontaneous Emergence of Mutual Catalysis. Life 2019, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Surman, A.J.; Rodriguez-Garcia, M.; Abul-Haija, Y.M.; Cooper, G.J.T.; Gromski, P.S.; Turk-MacLeod, R.; Mullin, M.; Mathis, C.; Walker, S.I.; Cronin, L. Environmental control programs the emergence of distinct functional ensembles from unconstrained chemical reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 5387–5392. [Google Scholar] [CrossRef] [Green Version]
- Sojo, V.; Ohno, A.; McGlynn, E.S.; Yamada, M.A.Y.; Nakamura, R. Microfluidic Reactors for Carbon Fixation under Ambient-Pressure Alkaline-Hydrothermal-Vent Conditions. Life 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, R.; de Graaf, R.; Rodin, M.S.; Ohno, A.; Lane, N.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R.; Barge, L.M.; Braun, D.; et al. CO2 reduction driven by a pH gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 22873–22879. [Google Scholar] [CrossRef]
- Dworkin, L.P.; Deamer, D.W.; Sandford, S.A.; Allamandola, L.J. Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. USA 2001, 98, 815–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menor-Salván, C.; Ruiz-Bermejo, D.M.; Guzmán, M.I.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases. Chem.-A Eur. J. 2009, 15, 4411–4418. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.; Ruzicka, J.; Stern, J.; Glavin, D.; House, C.H.; Dworkin, J. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, D.A.; Vetsigian, K. An Experimental Framework for Generating Evolvable Chemical Systems in the Laboratory. Orig. Life Evol. Biosph. 2016, 47, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pross, A. What Is Life? How Chemistry Becomes Biology; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Oparin, A. Proiskhozhdenie Zhizny; Izd. Moskovski Rabochii: Moscow, Russia, 1924. [Google Scholar]
- Chyba, C.; Sagan, C. Endogenous Production, Exogenous Delivery and Impact-Shock Synthesis of Organic-Molecules—An Inventory for the Origins of Life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Preiner, M.; Martin, W.F. Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution. Curr. Opin. Microbiol. 2018, 43, 77–83. [Google Scholar] [CrossRef]
- Huber, C.; Waächtershaäuser, G. Activated Acetic Acid by Carbon Fixation on (Fe, Ni)S Under Primordial Conditions. Science 1997, 276, 245–247. [Google Scholar] [CrossRef] [Green Version]
- Bonfio, C.; Valer, L.; Scintilla, S.; Shah, S.N.; Evans, D.; Jin, L.; Szostak, J.W.; Sasselov, D.D.; Sutherland, J.D.; Mansy, S.S. UV-light-driven prebiotic synthesis of iron–sulfur clusters. Nat. Chem. 2017, 9, 1229–1234. [Google Scholar] [CrossRef]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef]
- Mißbach, H.; Schmidt, B.C.; Duda, J.-P.; Lünsdorf, N.K.; Goetz, W.; Thiel, V. Assessing the diversity of lipids formed via Fischer-Tropsch-type reactions. Org. Geochem. 2018, 119, 110–121. [Google Scholar] [CrossRef]
- Konn, C.; Charlou, J.; Donval, J.; Holm, N.; Dehairs, F.; Bouillon, S. Hydrocarbons and oxidized organic compounds in hydrothermal fluids from Rainbow and Lost City ultramafic-hosted vents. Chem. Geol. 2009, 258, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Sleep, N.H.; Meibom, A.; Fridriksson, T.; Coleman, R.G.; Bird, D.K. H2-rich fluids from serpentinization: Geochemical and biotic implications. Proc. Natl. Acad. Sci. USA 2004, 101, 12818–12823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.J.; Muchowska, K.B.; Chatelain, P.; Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2018, 2, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. The Origin of Life. New Biol. 1954, 16, 12–27. [Google Scholar]
- Mehta, C.; Perez, A.; Thompson, G.; Pasek, M.A. Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation. Life 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. J. Mol. Evol. 1983, 19, 376–382. [Google Scholar] [CrossRef]
- Miller, S.L.; Schlesinger, G. Carbon and energy yields in prebiotic syntheses using atmospheres containing CH4, CO and CO2. Orig. Life Evol. Biosph. 1984, 14, 83–90. [Google Scholar] [CrossRef]
- Tian, F.; Toon, O.B.; Pavlov, A.A.; De Sterck, H. A Hydrogen-Rich Early Earth Atmosphere. Science 2005, 308, 1014–1017. [Google Scholar] [CrossRef] [Green Version]
- Zahnle, K.; Schaefer, L.; Fegley, B.; Mckay, C.P.; Chen, I.A.; Walde, P.; Sutherland, J.D.; Ehrenfreund, P.; Cami, J. Earth’s Earliest Atmospheres. Cold Spring Harb. Perspect. Biol. 2010, 2, a004895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folsome, C.E.; Brittain, A.; Smith, A.; Chang, S. Hydrazines and carbohydrazides produced from oxidized carbon in Earth’s primitive environment. Nature 1981, 294, 64–65. [Google Scholar] [CrossRef]
- Cleaves, H.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities and the Origins of Life. Cold Spring Harb. Perspect Biol. 2010, 2, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Cleaves, J.; Michalkova Scott, A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R.M. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 24, 5502–5525. [Google Scholar] [CrossRef]
- Zahnle, K.; Arndt, N.; Cockell, C.; Halliday, A.; Nisbet, E.; Selsis, F.; Sleep, N.H. Emergence of a Habitable Planet. Space Sci. Rev. 2007, 129, 35–78. [Google Scholar] [CrossRef]
- Rapf, R.J.; Vaida, V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem. Chem. Phys. 2016, 18, 20067–20084. [Google Scholar] [CrossRef]
- Sagan, C.; Mullen, G. Earth and Mars: Evolution of Atmospheres and Surface Temperatures. Science 1972, 177, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Sagan, C.; Chyba, C. The Early Faint Sun Paradox: Organic Shielding of Ultraviolet-Labile Greenhouse Gases. Science 1997, 276, 1217–1221. [Google Scholar] [CrossRef]
- Simonov, A.; Pestunova, O.P.; Matvienko, L.G.; Parmon, V.N. The nature of autocatalysis in the Butlerov reaction. Kinet. Catal. 2007, 48, 245–254. [Google Scholar] [CrossRef]
- Ii, H.J.C. The prebiotic geochemistry of formaldehyde. Precambrian Res. 2008, 164, 111–118. [Google Scholar] [CrossRef]
- Omran, A.; Menor-Salvan, C.; Springsteen, G.; Pasek, M. The Messy Alkaline Formose Reaction and Its Link to Metabolism. Life 2020, 10, 125. [Google Scholar] [CrossRef]
- Chyba, C.F.; Thomas, P.J.; Brookshaw, L.; Sagan, C. Cometary Delivery of Organic Molecules to the Early Earth. Science 1990, 249, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.F. The violent environment of the origin of life: Progress and uncertainties. Geochim. Cosmochim. Acta 1993, 57, 3351–3358. [Google Scholar] [CrossRef]
- Zellner, N.E.B. Cataclysm No More: New Views on the Timing and Delivery of Lunar Impactors. Orig. Life Evol. Biosph. 2017, 47, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The Cold Origin of Life: A. Implications Based on The Hydrolytic Stabilities Of Hydrogen Cyanide And Formamide. Orig. Life Evol. Biosph. 2002, 32, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Hammer, P.G.; Yi, R.; Yoda, I.; Cleaves, H.J.; Callahan, M.P. Radiolysis of solid-state nitrogen heterocycles provides clues to their abundance in the early solar system. Int. J. Astrobiol. 2018, 18, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Schrader, D.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [Green Version]
- Wolman, Y.; Haverland, W.J.; Miller, S.L. Nonprotein Amino Acids from Spark Discharges and Their Comparison with the Murchison Meteorite Amino Acids. Proc. Natl. Acad. Sci. USA 1972, 69, 809–811. [Google Scholar] [CrossRef] [Green Version]
- Sephton, M.A.; Gilmour, I. Chapter 11—Compound Specific Isotope Analysis of the Organic Constituents in the Murchison Meteorite. In Handbook of Stable Isotope Analytical Techniques; de Groot, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 229–236. ISBN 978-0-444-51114-0. [Google Scholar]
- Adam, Z.R.; Fahrenbach, A.C.; Jacobson, S.M.; Kacar, B.; Zubarev, D.Y. Radiolysis generates a complex organosynthetic chemical network. Sci. Rep. 2021, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.E.; House, C.H.; Smith, K.E.; Roberts, M.R.; Callahan, M.P. Nitrogen heterocycles form peptide nucleic acid precursors in complex prebiotic mixtures. Sci. Rep. 2019, 9, 9281. [Google Scholar] [CrossRef]
- Kissin, Y. Hydrocarbon components in carbonaceous meteorites. Geochim. Cosmochim. Acta 2003, 67, 1723–1735. [Google Scholar] [CrossRef]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sariana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879. [Google Scholar] [CrossRef] [PubMed]
- Stoks, P.G.; Schwartz, A.W. Nitrogen-heterocyclic compounds in meteorites: Significance and mechanisms of formation. Geochim. Cosmochim. Acta 1981, 45, 563–569. [Google Scholar] [CrossRef]
- Yamashita, Y.; Naraoka, H. Two homologous series of alkylpyridines in the Murchison meteorite. Geochem. J. 2014, 48, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.; Cronin, J. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 1995, 59, 1003–1015. [Google Scholar] [CrossRef]
- Pizzarello, S.; Feng, X.; Epstein, S.; Cronin, J. Isotopic analyses of nitrogenous compounds from the Murchison meteorite: Ammonia, amines, amino acids, and polar hydrocarbons. Geochim. Cosmochim. Acta 1994, 58, 5579–5587. [Google Scholar] [CrossRef]
- Cronin, J.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y. Molecular and isotopic analyses of Tagish Lake alkyl dicarboxylic acids. Meteorit. Planet. Sci. 2002, 37, 687–696. [Google Scholar] [CrossRef]
- Lerner, N.R.; Cooper, G.W. Iminodicarboxylic acids in the Murchison meteorite: Evidence of Strecker reactions. Geochim. Cosmochim. Acta 2005, 69, 2901–2906. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Alexandre, M.R.; Lee, T.; Rose-Petruck, C.; Fuller, M.; Pizzarello, S. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carboneous meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar] [CrossRef]
- Cooper, G.W.; Onwo, W.M.; Cronin, J.R. Alkyl phosphonic acids and sulfonic acids in the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4109–4115. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Miller, S.L. Production of Some Organic Compounds under Possible Primitive Earth Conditions. J. Am. Chem. Soc. 1955, 77, 2351–2361. [Google Scholar] [CrossRef]
- Parker, E.T.; Cleaves, H.; Dworkin, J.; Glavin, D.; Callahan, M.; Aubrey, A.; Lazcano, A.; Bada, J.L. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. USA 2011, 108, 5526–5531. [Google Scholar] [CrossRef] [Green Version]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, P.; Ivanek, O.; Shestivska, V.; Civiš, S. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef] [Green Version]
- Yuen, G.U.; Lawless, J.G.; Edelson, E.H. Quantification of monocarboxylic acids from a spark discharge synthesis. J. Mol. Evol. 1981, 17, 43–47. [Google Scholar] [CrossRef]
- Li, X.-S.; Zhu, A.-M.; Wang, K.-J.; Xu, Y.; Song, Z.-M. Methane conversion to C2 hydrocarbons and hydrogen in atmospheric non-thermal plasmas generated by different electric discharge techniques. Catal. Today 2004, 98, 617–624. [Google Scholar] [CrossRef]
- Lathe, R. Fast tidal cycling and the origin of life. Icarus 2004, 168, 18–22. [Google Scholar] [CrossRef]
- Deamer, D.W. The first living systems: A bioenergetic perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 239–261. [Google Scholar] [PubMed]
- Miyakawa, S.; Cleaves, H.; Miller, S.L. The Cold Origin of Life: B. Implications Based on Pyrimidines and Purines Produced From Frozen Ammonium Cyanide Solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Ziock, H. Eutectic Phase in Water-Ice: A Self-Assembled Environment Conducive to Metal-Catalyzed Non-Enzymatic RNA Polymerization. Chem. Biodivers. 2008, 5, 1521–1539. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M.; Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Natl. Acad. Sci. USA 2000, 97, 11864–11868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzarello, S.; Huang, Y.; Alexandre, M.D.R. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. USA 2008, 105, 3700–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect. Biol. 2010, 2, a002147. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.W.; Lee, J.T.; Chang, L.W. Enantiomeric impurities in chiral catalysts, auxiliaries and synthons used in enantioselective synthesis. Tetrahedron Asymmetry 1998, 9, 2043–2064. [Google Scholar] [CrossRef]
- Parker, E.T.; Cleaves, J.H.; Burton, A.; Glavin, D.P.; Dworkin, J.P.; Zhou, M.; Bada, J.L.; Fernández, F.M. Conducting Miller-Urey Experiments. J. Vis. Exp. 2014, 83, e51039. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.J.T.; Surman, A.J.; McIver, J.; Colón-Santos, S.M.; Gromski, P.S.; Buchwald, S.; Marina, I.S.; Cronin, L. Miller–Urey Spark-Discharge Experiments in the Deuterium World. Angew. Chem. Int. Ed. 2017, 56, 8079–8082. [Google Scholar] [CrossRef] [Green Version]
- Stribling, R.; Miller, S.L. Energy yields for hydrogen cyanide and formaldehyde syntheses: The hcn and amino acid concentrations in the primitive ocean. Orig. Life Evol. Biosph. 1987, 17, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Voet, A.B.; Schwartz, A.W. Uracil synthesisvia HCN oligomerization. Orig. Life Evol. Biosph. 1982, 12, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.; Andersen, T.; Flamm, C.; Hanczyc, M.M.; Merkle, D.; Stadler, P.F. Navigating the Chemical Space of HCN Polymerization and Hydrolysis: Guiding Graph Grammars by Mass Spectrometry Data. Entropy 2013, 15, 4066–4083. [Google Scholar] [CrossRef] [Green Version]
- Bada, J.L.; Chalmers, J.H.; Cleaves, H.J. Is formamide a geochemically plausible prebiotic solvent? Phys. Chem. Chem. Phys. 2016, 18, 20085–20090. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, F.; Saitta, A.M. Formamide reaction network in gas phase and solution via a unified theoretical approach: Toward a reconciliation of different prebiotic scenarios. Proc. Natl. Acad. Sci. USA 2015, 112, 15030–15035. [Google Scholar] [CrossRef] [Green Version]
- Ferus, M.; Nesvorný, D.; Sponer, J.; Kubelík, P.; Michalčíková, R.; Shestivska, V.; Šponer, J.E.; Civiš, S. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 2014, 112, 657–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; Di Mauro, E. From the one-carbon amide formamide to RNA all the steps are prebiotically possible. Biochimie 2012, 94, 1451–1456. [Google Scholar] [CrossRef]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.Y.; Krasavin, E.; Di Mauro, E. Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, E2746–E2755. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.W.; De Graaf, R.M. The prebiotic synthesis of carbohydrates: A reassessment. J. Mol. Evol. 1993, 36, 101–106. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Saladino, R.; Delfino, I.; García-Ruiz, J.M.; Di Mauro, E. Prebiotic Organic Chemistry of Formamide and the Origin of Life in Planetary Conditions: What We Know and What Is the Future. Int. J. Mol. Sci. 2021, 22, 917. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Costanzo, G.; Di Mauro, E. About a Formamide-Based Origin of Informational Polymers: Syntheses of Nucleobases and Favourable Thermodynamic Niches for Early Polymers. Orig. Life Evol. Biosph. 2006, 36, 523–531. [Google Scholar] [CrossRef]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate Minerals Stabilize Ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef] [Green Version]
- Damer, B. A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond. Life 2016, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Maurer, S. The Impact of Salts on Single Chain Amphiphile Membranes and Implications for the Location of the Origin of Life. Life 2017, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Barge, L.M.; Steinbock, O. Microfluidic Production of Pyrophosphate Catalyzed by Mineral Membranes with Steep pH Gradients. Chem.-A Eur. J. 2019, 25, 4732–4739. [Google Scholar] [CrossRef] [PubMed]
- Anizelli, P.R.; Baú, J.P.; Nabeshima, H.S.; Costa, M.; de Santana, H.; Zaia, D.A. An experimental and theoretical vibrational study of interaction of adenine and thymine with artificial seawaters: A prebiotic chemistry experiment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 126, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Barajas, S.A.; Baú, J.P.T.; Colín-García, M.; Negrón-Mendoza, A.; Heredia-Barbero, A.; Pi-Puig, T.; Zaia, D.A.M. Salinity Effects on the Adsorption of Nucleic Acid Compounds on Na-Montmorillonite: A Prebiotic Chemistry Experiment. Orig. Life Evol. Biosph. 2018, 48, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F. Earth ’ s Early Atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sillanpää, M.; Gjessing, E.T.; Vogt, R.D. Water quality in the Tibetan Plateau: Major ions and trace elements in the headwaters of four major Asian rivers. Sci. Total Environ. 2009, 407, 6242–6254. [Google Scholar] [CrossRef]
- Pasek, M.A. Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 853–858. [Google Scholar] [CrossRef] [Green Version]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an Ancient Metabolism without Phosphate. Cell 2017, 168, 1126–1134.e9. [Google Scholar] [CrossRef] [Green Version]
- Halevy, I.; Bachan, A. The geologic history of seawater pH. Science 2017, 355, 1069–1071. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.M. Considering planetary environments in origin of life studies. Nat. Commun. 2018, 9, 5170. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.M.; Flores, E.; Baum, M.M.; VanderVelde, D.G.; Russell, M.J. Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc. Natl. Acad. Sci. USA 2019, 116, 4828–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.S.; Karson, J.A.; Blackman, D.K.; Früh-Green, G.L.; Butterfield, D.; Lilley, M.D.; Olson, E.J.; Schrenk, M.; Roe, K.K.; Lebon, G.T.; et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature 2001, 412, 145–149. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Genet. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Arnold, W.A. Inorganic chemical composition of natural waters. In Water Chemistry; Oxford University Press: New York, NY, USA, 2011; pp. 41–75. [Google Scholar]
- Cleaves, H.J. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Seyler, L.; Kujawinski, E.B.; Azua-Bustos, A.; Lee, M.D.; Marlow, J.; Perl, S.M.; Ii, H.J.C. Metabolomics as an Emerging Tool in the Search for Astrobiologically Relevant Biomarkers. Astrobiology 2020, 20, 1251–1261. [Google Scholar] [CrossRef]
- Wołos, A.; Roszak, R.; Żądło-Dobrowolska, A.; Beker, W.; Mikulak-Klucznik, B.; Spólnik, G.; Dygas, M.; Szymkuć, S.; Grzybowski, B.A. Synthetic connectivity, emergence, and self-regeneration in the network of prebiotic chemistry. Science 2020, 369. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical Method for Analysis of Ultrahigh-Resolution Broadband Mass Spectra of Natural Organic Matter, the Van Krevelen Diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- Powner, M.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salván, C.M.; Bouza, M.; Fialho, D.M.; Burcar, B.T.; Fernández, F.M.; Hud, N.V. Prebiotic Origin of Pre-RNA Building Blocks in a Urea “Warm Little Pond” Scenario. ChemBioChem 2020, 21, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Krishnamurthy, R. The role of sugar-backbone heterogeneity and chimeras in the simultaneous emergence of RNA and DNA. Nat. Chem. 2019, 11, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Meringer, M.; Cleaves, H.J. Computational exploration of the chemical structure space of possible reverse tricarboxylic acid cycle constituents. Sci. Rep. 2017, 7, 17540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef]
- Keller, M.A.; Kampjut, D.; Harrison, S.A.; Ralser, M. Sulfate radicals enable a non-enzymatic Krebs cycle precursor. Nat. Ecol. Evol. 2017, 1, 0083. [Google Scholar] [CrossRef] [Green Version]
- Muchowska, K.; Varma, S.J.; Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nat. Cell Biol. 2019, 569, 104–107. [Google Scholar] [CrossRef]
- Stubbs, R.T.; Yadav, M.; Krishnamurthy, R.; Springsteen, G. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of α-ketoacids. Nat. Chem. 2020, 12, 1016–1022. [Google Scholar] [CrossRef]
- Ludlow, R.F.; Otto, S. Systems chemistry. Chem. Soc. Rev. 2007, 37, 101–108. [Google Scholar] [CrossRef]
- Peyralans, J.J.; Otto, S. Recent highlights in systems chemistry. Curr. Opin. Chem. Biol. 2009, 13, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2013, 114, 285–366. [Google Scholar] [CrossRef]
- Ashkenasy, G.; Hermans, T.M.; Otto, S.; Taylor, A.F. Systems chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Crick, F. The origin of the genetic code. J. Mol. Biol. 1968, 38, 367–379. [Google Scholar] [CrossRef]
| Consideration | Assembled Mixture | Synthesized Mixture | |
|---|---|---|---|
| Atmospheric Synthesis | Liquid Synthesis | ||
| Complexity of Products | Low | High | High |
| Procedure Difficulty | Low | High | Low |
| Control of Chirality | High | Low | Low |
| Control of Composition | High | Low | Low |
| Analytical Tractability | High | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, L.; Colón-Santos, S.; Cleaves, H.J., II; Baum, D.A.; Maurer, S.E. The Prebiotic Kitchen: A Guide to Composing Prebiotic Soup Recipes to Test Origins of Life Hypotheses. Life 2021, 11, 1221. https://doi.org/10.3390/life11111221
Vincent L, Colón-Santos S, Cleaves HJ II, Baum DA, Maurer SE. The Prebiotic Kitchen: A Guide to Composing Prebiotic Soup Recipes to Test Origins of Life Hypotheses. Life. 2021; 11(11):1221. https://doi.org/10.3390/life11111221
Chicago/Turabian StyleVincent, Lena, Stephanie Colón-Santos, H. James Cleaves, II, David A. Baum, and Sarah E. Maurer. 2021. "The Prebiotic Kitchen: A Guide to Composing Prebiotic Soup Recipes to Test Origins of Life Hypotheses" Life 11, no. 11: 1221. https://doi.org/10.3390/life11111221
APA StyleVincent, L., Colón-Santos, S., Cleaves, H. J., II, Baum, D. A., & Maurer, S. E. (2021). The Prebiotic Kitchen: A Guide to Composing Prebiotic Soup Recipes to Test Origins of Life Hypotheses. Life, 11(11), 1221. https://doi.org/10.3390/life11111221







