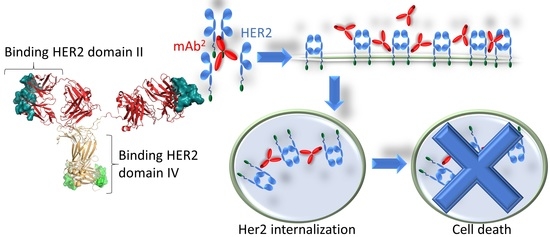
A Tetravalent Biparatopic Antibody Causes Strong HER2 Internalization and Inhibits Cellular Proliferation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression Constructs
2.2. Protein Production
2.3. Labelling of Antibody Preparations
2.4. High Performance Liquid Chromatography—Size Exclusion Chromatography (HPLC-SEC)
2.5. Cell Culture and Cell Staining
2.6. Fluorescence Microscopy
3. Results
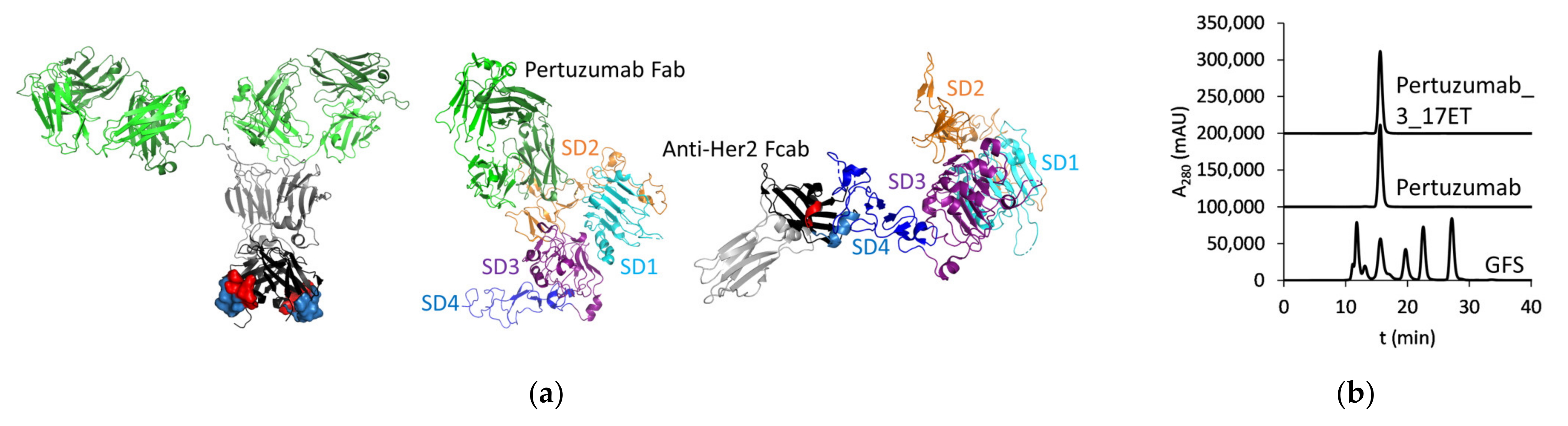
3.1. Expression of the Bispecific Antibody
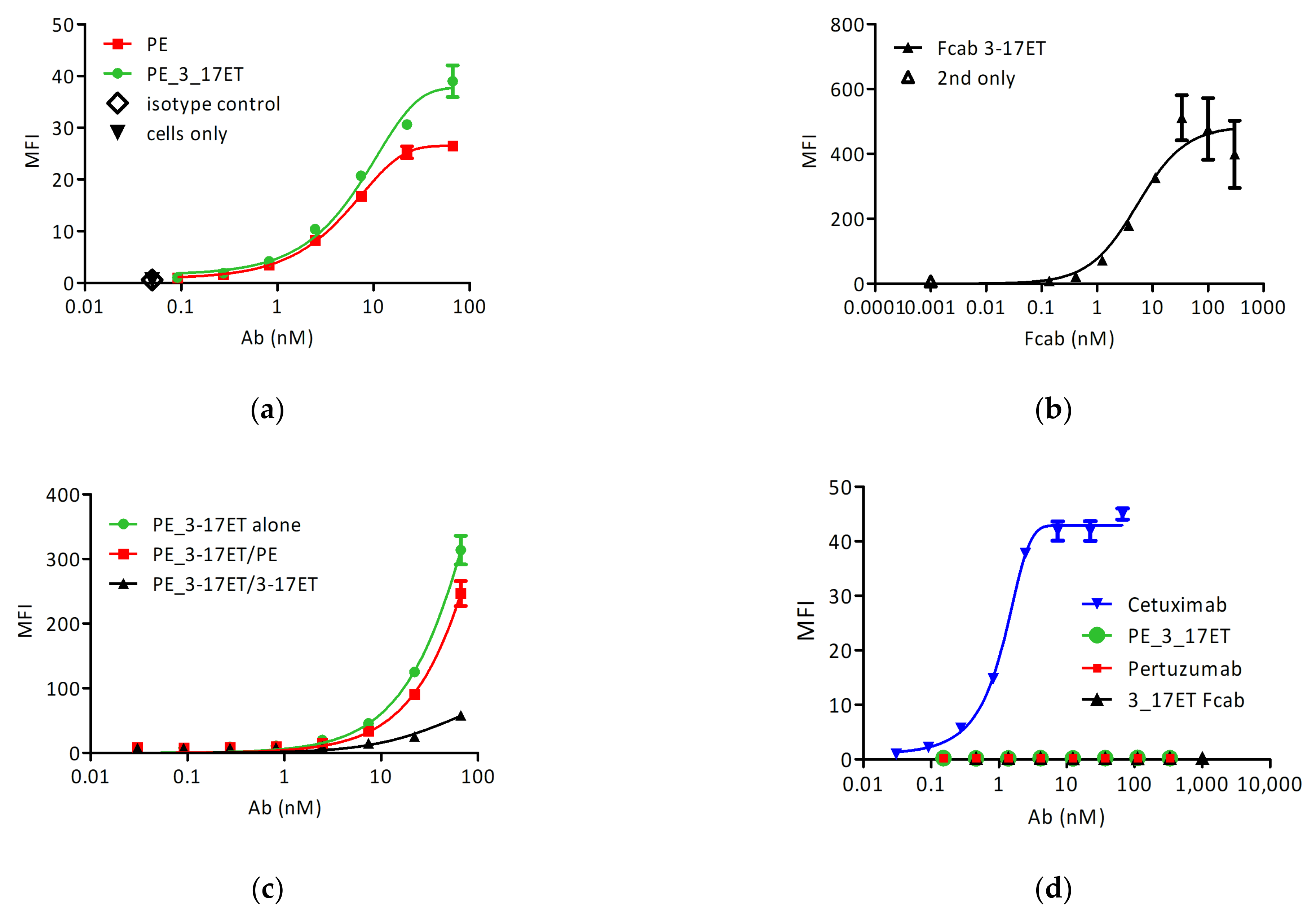
3.2. Cell Surface Binding
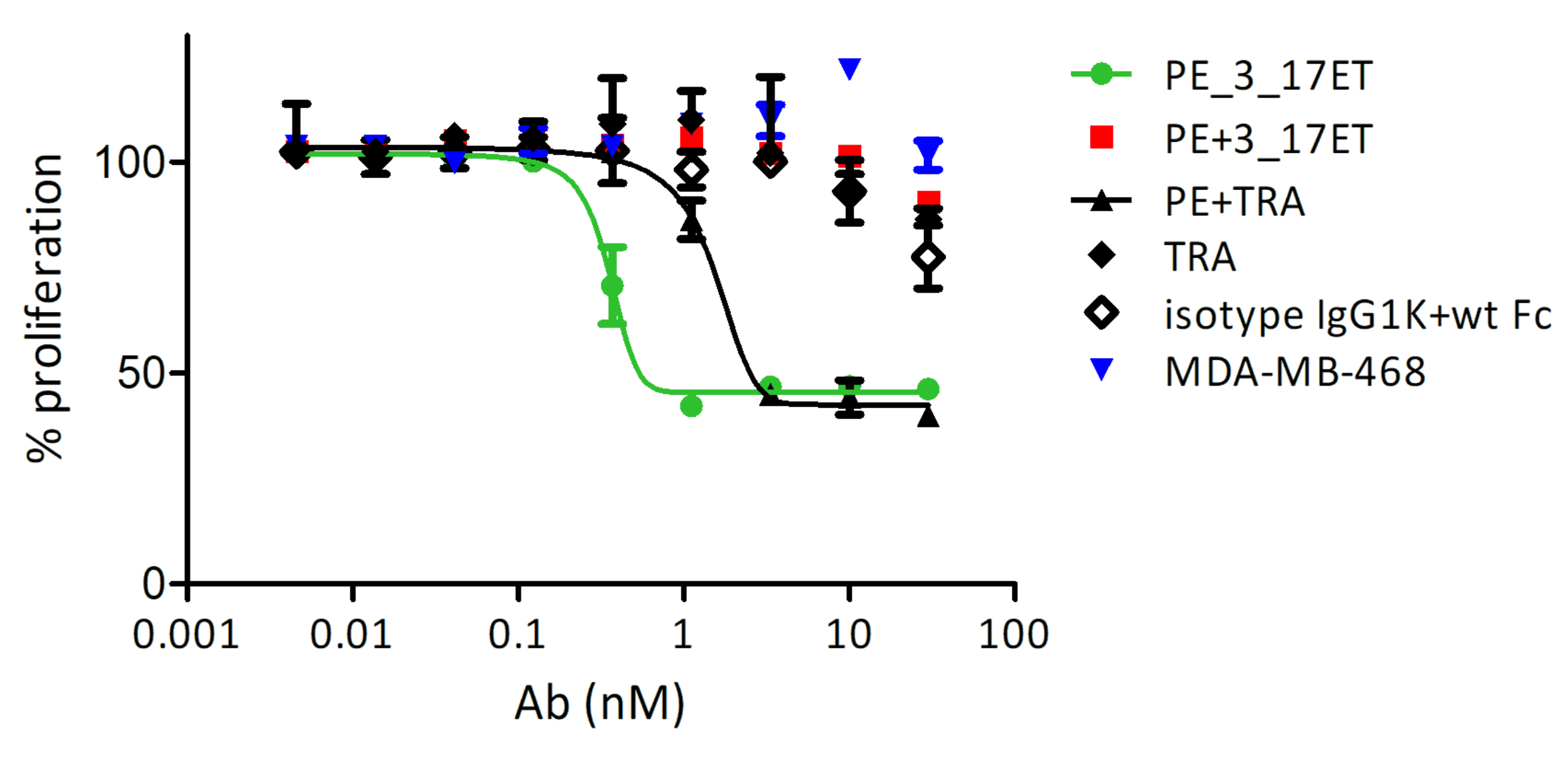
3.3. Effect of the Bispecific Antibody on HER2-Overexpressing Cells
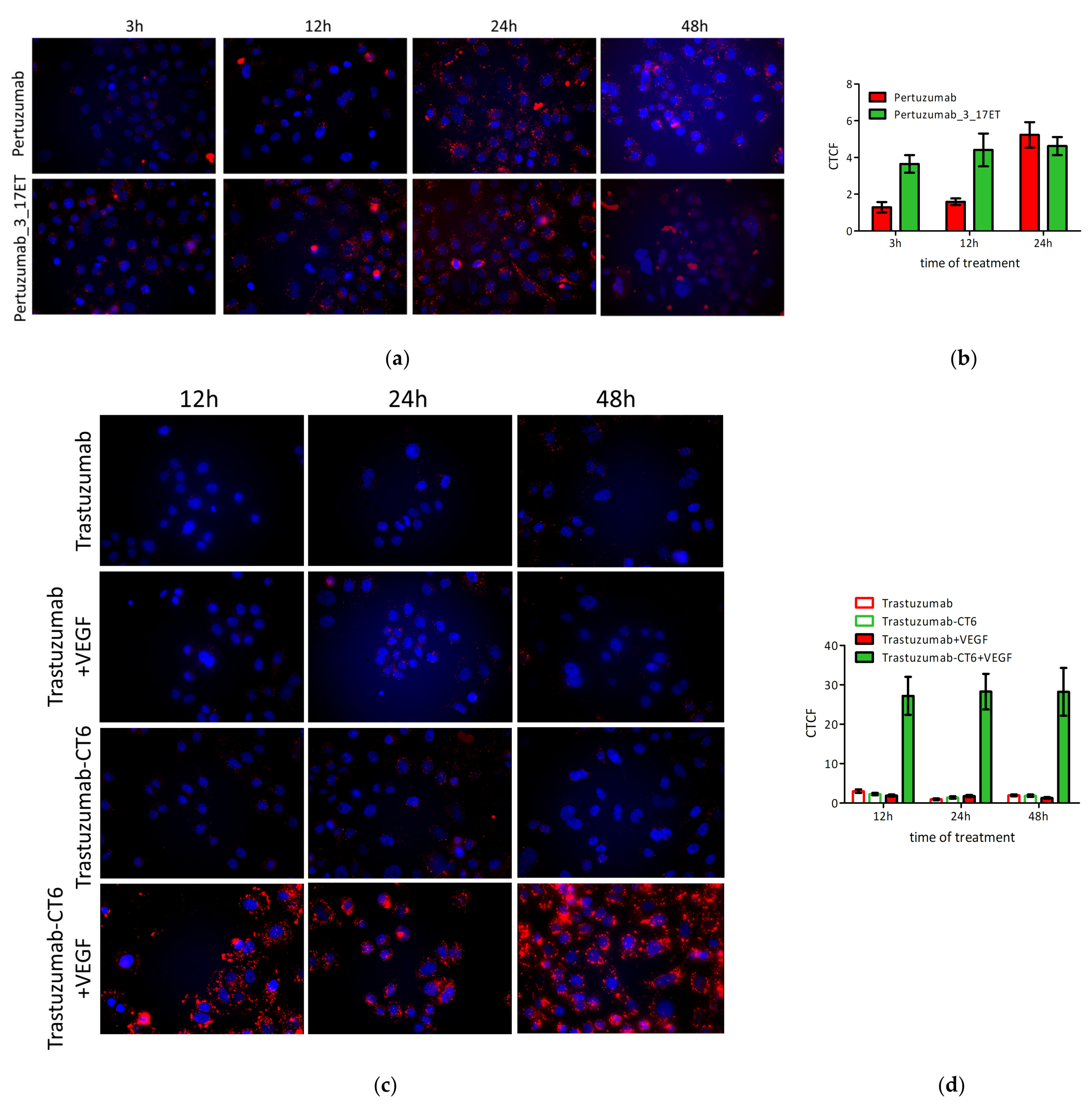
3.4. Bispecific Antibody Depletes HER2 from the Cell Surface
3.5. Induction of Apoptosis As a Reason for Anti-Proliferative Activity of the Bispecific Antibody
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slamon, D.; Clark, G.; Wong, S.; Levin, W.; Ullrich, A.; McGuire, W. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Yagisawa, M.; Sawada, K.; Nakamura, Y.; Fujii, S.; Yuki, S.; Komatsu, Y.; Yoshino, T.; Sakamoto, N.; Taniguchi, H. Prognostic Value and Molecular Landscape of HER2 Low-Expressing Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2020, 20, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.C.; Lau, Y.K. Basic science of HER-2/neu: A review. Semin. Oncol. 1999, 26 (Suppl. 12), 51–59. [Google Scholar] [PubMed]
- Hudziak, R.M.; Schlessinger, J.; Ullrich, A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7159–7163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holbro, T.; Beerli, R.R.; Maurer, F.; Koziczak, M.; Barbas, C.F.; Hynes, N.E. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl. Acad. Sci. USA 2003, 100, 8933–8938. [Google Scholar] [CrossRef] [Green Version]
- Nami, B.; Wang, Z. HER2 in breast cancer stemness: A negative feedback loop towards trastuzumab resistance. Cancers 2017, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.Q.; Ying, R.C.; Zhou, C.H.; Zhu, A.K.; Ye, J.; Zhu, W.; Ju, T.F.; Jin, H.C. MMP-9 is increased in the pathogenesis of gastric cancer by the mediation of HER2. Cancer Gene Ther. 2015, 22, 101–107. [Google Scholar] [CrossRef]
- Lollini, P.L.; Nicoletti, G.; Landuzzi, L.; De Giovanni, C.; Rossi, I.; Di Carlo, E.; Musiani, P.; Muller, W.J.; Nanni, P. Down regulation of major histocompatibility complex class I expression in mammary carcinoma of HER-2/neu transgenic mice. Int. J. Cancer 1998, 77, 937–941. [Google Scholar] [CrossRef]
- Mimura, K.; Ando, T.; Poschke, I.; Mougiakakos, D.; Johansson, C.C.; Ichikawa, J.; Okita, R.; Nishimura, M.I.; Handke, D.; Krug, N.; et al. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int. J. Cancer 2011, 128, 390–401. [Google Scholar] [CrossRef]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [Green Version]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.W.; Allison, D.E.; Flagella, K.; Presta, L.; Clarke, J.; Dybdal, N.; McKeever, K.; Sliwkowski, M.X. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol. Immunother. 2006, 55, 717–727. [Google Scholar] [CrossRef]
- Garrett, T.P.J.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 2003, 11, 495–505. [Google Scholar] [CrossRef]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.B.; Henner, D.; Wong, W.L.T.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef] [Green Version]
- Baselga, J.; Albanell, J.; Molina, M.A.; Arribas, J. Mechanism of action of trastuzumab and scientific update. Semin. Oncol. 2001, 28, 4–11. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Guo, D.; Jiang, Z.; Tong, R.; Jiang, P.; Bai, L.; Chen, L.; Zhu, Y.; Guo, C.; Shi, J.; et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur. J. Med. Chem. 2019, 183, 111682. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Phillips, G.D.L.; Fields, C.T.; Li, G.; Dowbenko, D.; Schaefer, G.; Miller, K.; Andre, F.; Burris, H.A.; Albain, K.S.; Harbeck, N.; et al. Dual Targeting of HER2-Positive Cancer with Trastuzumab Emtansine and Pertuzumab: Critical Role for Neuregulin Blockade in Antitumor Response to Combination Therapy. Clin. Cancer Res. 2014, 20, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Ying, H.; Grinnell, C.; Bryant, S.; Miller, R.; Clabbers, A.; Bose, S.; McCarthy, D.; Zhu, R.R.; Santora, L.; et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat. Biotechnol. 2007, 25, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, Y.; Zheng, L.; Zhang, X.; Tong, Q.; Tan, W.; Hu, S.; Li, H.; Chen, Y.; Song, J.; et al. Bispecific Antibody to ErbB2 Overcomes Trastuzumab Resistance through Comprehensive Blockade of ErbB2 Heterodimerization. Cancer Res. 2013, 73, 6471–6483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Li, F.; Liu, H.; Ye, P.; Fan, X.; Yuan, X.; Wu, Z.; Chen, J.; Jin, C.; Shen, B.; et al. Structural and functional characterization of MBS301, an afucosylated bispecific anti-HER2 antibody. mAbs 2018, 10, 864–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, F.; Schwill, M.; Stüber, J.C.; Pfundstein, S.; Nagy-Davidescu, G.; Rodríguez, J.M.M.; Seehusen, F.; Richter, C.P.; Honegger, A.; Hartmann, K.P.; et al. Engineering an anti-HER2 biparatopic antibody with a multimodal mechanism of action. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Jost, C.; Schilling, J.; Tamaskovic, R.; Schwill, M.; Honegger, A.; Plückthun, A. Structural basis for eliciting a cytotoxic effect in HER2-overexpressing cancer cells via binding to the extracellular domain of HER2. Structure 2013, 21, 1979–1991. [Google Scholar] [CrossRef] [Green Version]
- Wozniak-Knopp, G.; Bartl, S.; Bauer, A.; Mostageer, M.; Woisetschläger, M.; Antes, B.; Ettl, K.; Kainer, M.; Weberhofer, G.; Wiederkum, S.; et al. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng. Des. Sel. 2010, 23, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Lobner, E.; Humm, A.-S.; Göritzer, K.; Mlynek, G.; Puchinger, M.G.; Hasenhindl, C.; Rüker, F.; Traxlmayr, M.W.; Djinović-Carugo, K.; Obinger, C. Fcab-HER2 Interaction: A Ménage à Trois. Lessons from X-Ray and Solution Studies. Structure 2017, 25, 878–889.e5. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, M.; Pravin, J.; Rodrigues, L.; Uhlenbroich, S.; Everett, K.L.; Wollerton, F.; Morrow, M.; Tuna, M.; Brewis, N. CD137/OX40 Bispecific Antibody Induces Potent Antitumor Activity that Is Dependent on Target Coengagement. Cancer Immunol. Res. 2020, 8, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Kraman, M.; Faroudi, M.; Allen, N.L.; Kmiecik, K.; Gliddon, D.; Seal, C.; Koers, A.; Wydro, M.M.; Batey, S.; Winnewisser, J.; et al. FS118, a Bispecific Antibody Targeting LAG-3 and PD-L1, Enhances T-Cell Activation Resulting in Potent Antitumor Activity. Clin. Cancer Res. 2020, 26, 3333–3344. [Google Scholar] [CrossRef] [Green Version]
- Lakins, M.A.; Koers, A.; Giambalvo, R.; Munoz-Olaya, J.; Hughes, R.; Goodman, E.; Marshall, S.; Wollerton, F.; Batey, S.; Gliddon, D.; et al. FS222, a CD137/PD-L1 Tetravalent Bispecific Antibody, Exhibits Low Toxicity and Antitumor Activity in Colorectal Cancer Models. Clin. Cancer Res. 2020, 26, 4154–4167. [Google Scholar] [CrossRef]
- Wozniak-Knopp, G.; Stadlmann, J.; Rüker, F. Stabilisation of the FC fragment of human IgG1 by engineered intradomain disulfide bonds. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Woisetschläger, M.; Antes, B.; Borrowdale, R.; Wiederkum, S.; Kainer, M.; Steinkellner, H.; Wozniak-Knopp, G.; Moulder, K.; Rüker, F.; Mudde, G.C.G.C. In vivo and in vitro activity of an immunoglobulin Fc fragment (Fcab) with engineered Her-2/neu binding sites. Biotechnol. J. 2014, 9, 844–851. [Google Scholar] [CrossRef]
- Edelman, G.M.; Cunningham, B.A.; Gall, W.E.; Gottlieb, P.D.; Rutishauser, U.; Waxdal, M.J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc. Natl. Acad. Sci. USA 1969, 63, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Traxlmayr, M.W.; Lobner, E.; Antes, B.; Kainer, M.; Wiederkum, S.; Hasenhindl, C.; Stadlmayr, G.; Rüker, F.; Woisetschläger, M.; Moulder, K.; et al. Directed evolution of Her2/neu-binding IgG1-Fc for improved stability and resistance to aggregation by using yeast surface display. Protein Eng. Des. Sel. 2013, 26, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, F.; Stracke, F.; Stadlmayr, G.; Stadlbauer, K.; Rüker, F.; Wozniak-Knopp, G. Bispecific antibodies with Fab-arms featuring exchanged antigen-binding constant domains. Biochem. Biophys. Reports 2021, 26. [Google Scholar] [CrossRef]
- Wozniak-Knopp, G.; Stadlmayr, G.; Perthold, J.W.; Stadlbauer, K.; Woisetschläger, M.; Sun, H.; Rüker, F. Designing Fcabs: Well-expressed and stable high affinity antigen-binding Fc fragments. Protein Eng. Des. Sel. 2017, 30, 567–581. [Google Scholar] [CrossRef]
- Nami, B.; Maadi, H.; Wang, Z. The Effects of Pertuzumab and Its Combination with Trastuzumab on HER2 Homodimerization and Phosphorylation. Cancers 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Lua, W.-H.; Gan, S.K.-E.; Lane, D.P.; Verma, C.S. A search for synergy in the binding kinetics of Trastuzumab and Pertuzumab whole and F(ab) to Her2. npj Breast Cancer 2015, 1, 15012. [Google Scholar] [CrossRef] [Green Version]
- Brockhoff, G.; Heckel, B.; Schmidt-Bruecken, E.; Plander, M.; Hofstaedter, F.; Vollmann, A.; Diermeier, S. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 2007, 40, 488–507. [Google Scholar] [CrossRef]
- Henson, E.S.; Hu, X.; Gibson, S.B. Herceptin Sensitizes ErbB2–Overexpressing Cells to Apoptosis by Reducing Antiapoptotic Mcl-1 Expression. Clin. Cancer Res. 2006, 12, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Austin, C.D.; Wen, X.; Gazzard, L.; Nelson, C.; Scheller, R.H.; Scales, S.J. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc. Natl. Acad. Sci. USA 2005, 102, 17987–17992. [Google Scholar] [CrossRef] [Green Version]
- Ram, S.; Kim, D.; Ober, R.J.; Ward, E.S. The level of HER2 expression is a predictor of antibody-HER2 trafficking behavior in cancer cells. MAbs 2014, 6, 1211. [Google Scholar] [CrossRef] [Green Version]
- Brack, S.; Attinger-Toller, I.; Schade, B.; Mourlane, F.; Klupsch, K.; Woods, R.; Hachemi, H.; von der Bey, U.; Koenig-Friedrich, S.; Bertschinger, J.; et al. A Bispecific HER2-Targeting FynomAb with Superior Antitumor Activity and Novel Mode of Action. Mol. Cancer Ther. 2014, 13, 2030–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Zha, Z.; Liu, Y.; Zhang, H.; Zhu, J.; Hu, S.; Shen, G.; Cheng, L.; Niu, L.; Greene, M.I.; et al. Structural insights into the down-regulation of overexpressed p185 her2/neu protein of transformed cells by the antibody chA21. J. Biol. Chem. 2011, 286, 31676–31683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Shen, L.; Zhang, J.; Su, J.; Shen, L.; Liu, X.; Han, H.; Han, W.; Yao, L. Degradation of HER2 by Cbl-based chimeric ubiquitin ligases. Cancer Res. 2007, 67, 8716–8724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuuchi, K.; Berezov, A.; Kumagai, T.; Greene, M.I. Targeted Antireceptor Therapy with Monoclonal Antibodies Leads to the Formation of Inactivated Tetrameric Forms of ErbB Receptors. J. Immunol. 2007, 178, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeie, M.; Nikolaysen, F.; Chitano, Y.; Stang, E. Hsp90 inhibition and co-incubation with pertuzumab induce internalization and degradation of trastuzumab: Implications for use of T-DM1. J. Cell. Mol. Med. 2020, 24, 10258–10262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Okollie, B.; Artemov, D. Controlled internalization of her-2/neu receptors by cross-linking for targeted delivery. Cancer Biol. Ther. 2007, 6, 1960–1966. [Google Scholar] [CrossRef]
- Ben-Kasus, T.; Schechter, B.; Lavi, S.; Yarden, Y.; Sela, M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: Relevance of receptor endocytosis. Proc. Natl. Acad. Sci. USA 2009, 106, 3294. [Google Scholar] [CrossRef] [Green Version]
- Moody, P.R.; Sayers, E.J.; Magnusson, J.P.; Alexander, C.; Borri, P.; Watson, P.; Jones, A.T. Receptor Crosslinking: A General Method to Trigger Internalization and Lysosomal Targeting of Therapeutic Receptor:Ligand Complexes. Mol. Ther. 2015, 23, 1888. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, M.; Malik, M.S.; Nikolaysen, F.; Skeie, M.; Stang, E. Protein kinase C mediated internalization of ErbB2 is independent of clathrin, ubiquitination and Hsp90 dissociation. Exp. Cell Res. 2018, 371, 139–150. [Google Scholar] [CrossRef]
- Radford, D.C.; Yang, J.; Doan, M.C.; Li, L.; Dixon, A.S.; Owen, S.C.; Kopeček, J. Multivalent HER2-binding polymer conjugates facilitate rapid endocytosis and enhance intracellular drug delivery. J. Control. Release 2020, 319, 285–299. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, F.; Stadlbauer, K.; Stadlmayr, G.; Rüker, F.; Wozniak-Knopp, G. A Tetravalent Biparatopic Antibody Causes Strong HER2 Internalization and Inhibits Cellular Proliferation. Life 2021, 11, 1157. https://doi.org/10.3390/life11111157
Benedetti F, Stadlbauer K, Stadlmayr G, Rüker F, Wozniak-Knopp G. A Tetravalent Biparatopic Antibody Causes Strong HER2 Internalization and Inhibits Cellular Proliferation. Life. 2021; 11(11):1157. https://doi.org/10.3390/life11111157
Chicago/Turabian StyleBenedetti, Filippo, Katharina Stadlbauer, Gerhard Stadlmayr, Florian Rüker, and Gordana Wozniak-Knopp. 2021. "A Tetravalent Biparatopic Antibody Causes Strong HER2 Internalization and Inhibits Cellular Proliferation" Life 11, no. 11: 1157. https://doi.org/10.3390/life11111157
APA StyleBenedetti, F., Stadlbauer, K., Stadlmayr, G., Rüker, F., & Wozniak-Knopp, G. (2021). A Tetravalent Biparatopic Antibody Causes Strong HER2 Internalization and Inhibits Cellular Proliferation. Life, 11(11), 1157. https://doi.org/10.3390/life11111157