Which PASI Outcome Is Most Relevant to the Patients in Real-World Care?
Abstract
:1. Background
- Which relative PASI outcomes and which DLQI endpoints are associated with maximum patient-reported outcomes in psoriasis routine care?
- To what extent are PASI 75, PASI 90, and PASI 100 differentiated after three and six months of treatment?
2. Methods
2.1. Patients
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients
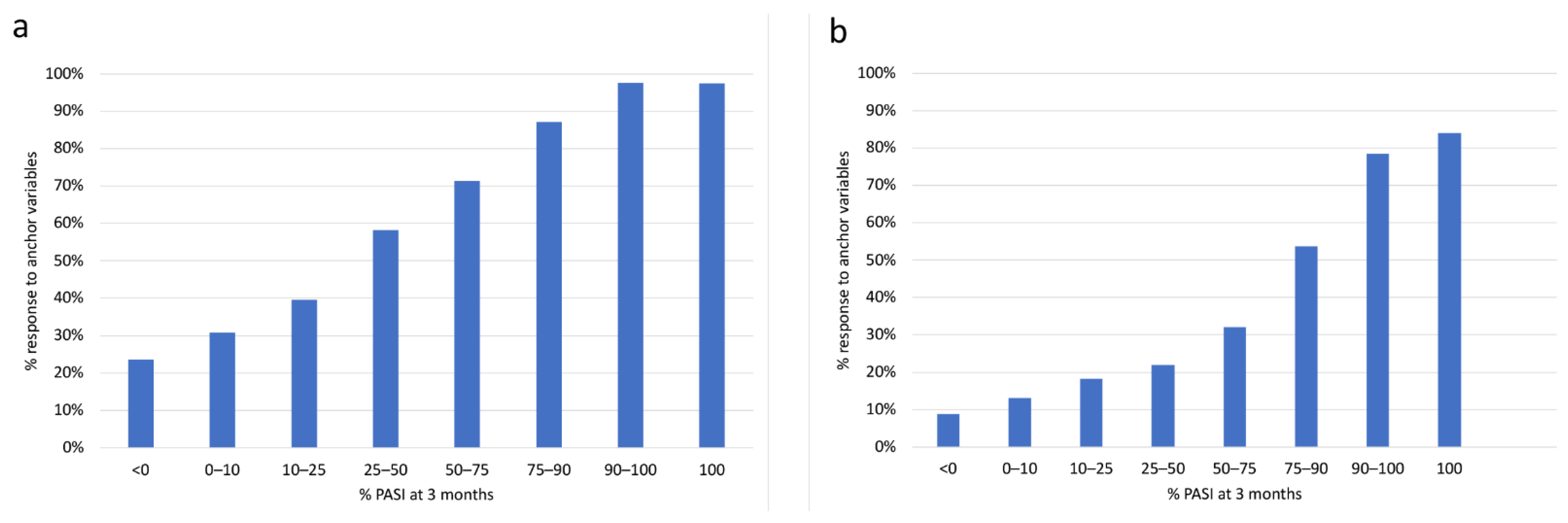
3.2. Cross-validation by Anchoring Variables
3.3. Characterization of PASI by Patient-Reported Outcomes
3.4. Association of the Relationship between PASI/DLQI and Anchor Variables to Baseline
4. Discussion
- (1)
- percentage PASI response and absolute target DLQI are visible but not optimum outcomes for measuring treatment success in psoriasis systemic treatment.
- (2)
- PASI 90 and PASI 100 reflect larger patient benefits from treatment than lower numbers.
- (3)
- PASI 90 and PASI 100 do not differentiate under real-world conditions of psoriasis care in Germany.
- (4)
- any improved PASI class between PASI 25 and PASI 90 as well as between DLQI > 20 and DLQI < 2 provides added value to the patients.
- (5)
- in patients reaching less than PASI 75, still a marked group reaches good quality of life (DLQI 0–1) and high level of satisfaction with treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominguez-Rosado, I.; Moutinho, V.; DeMatteo, R.P.; Kingham, T.P.; D’Angelica, M.; Brennan, M.F. Outcomes of the Memorial Sloan Kettering Cancer Center International General Surgical Oncology Fellowship. J. Am. Coll. Surg. 2016, 222, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Villani, A.P.; Rouzaud, M.; Sevrain, M.; Barnetche, T.; Paul, C.; Richard, M.-A.; Beylot-Barry, M.; Misery, L.; Joly, P.; Le Maitre, M.; et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 2015, 73, 242–248. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Badaoui, A.; Tounian, P.; Mahé, E. Psoriasis and metabolic and cardiovascular comorbidities in children: A systematic review. Arch. Pediatr. 2019, 26, 86–94. [Google Scholar] [CrossRef]
- Augustin, M.; Krüger, K.; Radtke, M.A.; Schwippl, I.; Reich, K. Disease severity, quality of life and health care in plaque-type psoriasis: A multicenter cross-sectional study in Germany. Dermatology 2008, 216, 366–372. [Google Scholar] [CrossRef]
- Khan, J.M.; Rathore, M.U.; Tahir, M.; Abbasi, T. Dermatology Life Quality Index in Patients of Psoriasis and Its Correlation with Severity of Disease. J. Ayub Med. Coll. Abbottabad 2020, 32, 64–67. [Google Scholar] [PubMed]
- Puzenat, E.; Bronsard, V.; Prey, S.; Gourraud, P.-A.; Aractingi, S.; Bagot, M.; Cribier, B.; Joly, P.; Jullien, D.; Le Maitre, M.; et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2010, 24 (Suppl. 2), 10–16. [Google Scholar] [CrossRef] [PubMed]
- Puig, L. PASI90 response: The new standard in therapeutic efficacy for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 645–648. [Google Scholar] [CrossRef]
- Mrowietz, U. Implementing treatment goals for successful long-term management of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. 2), 12–20. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (accessed on 15 September 2021).
- National Institute for Health and Care Excellence. Psoriasis: Assessment and Management. Clinical Guideline [CG153] Published Date: October 2012 Last Updated: September 2017. Available online: www.nice.org.uk/guidance/cg153/chapter/1-guidance (accessed on 15 September 2021).
- Mrowietz, U.; Barker, J.; Boehncke, W.-H.; Iversen, L.; Kirby, B.; Naldi, L.; Reich, K.; Tanew, A.; van de Kerkhof, P.C.M.; Warren, R.B. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: A European expert consensus. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. 3), 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinsen, C.A.C.; de Korte, J.; Augustin, M.; Sampogna, F.; Salek, S.S.; Basra, M.K.A.; Holm, E.A.; Nijsten, T.E.C. Measurement of health-related quality of life in dermatological research and practice: Outcome of the EADV Taskforce on Quality of Life. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1195–1203. [Google Scholar] [CrossRef]
- Nijsten, T.; Meads, D.M.; de Korte, J.; Sampogna, F.; Gelfand, J.M.; Ongenae, K.; Evers, A.W.; Augustin, M. Cross-cultural inequivalence of dermatology-specific health-related quality of life instruments in psoriasis patients. J. Invest. Dermatol. 2007, 127, 2315–2322. [Google Scholar] [CrossRef]
- McLeod, L.D.; Coon, C.D.; Martin, S.A.; Fehnel, S.E.; Hays, R.D. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev. Pharmacoecon. Outcomes Res. 2011, 11, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson, T.; Pettersson, U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef]
- Naldi, L. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passé? facts and controversies. Clin. Dermatol. 2010, 28, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.M.; Cueva, A.C.; Vyas, J.; Atwan, A.A.; Salek, M.S.; Finlay, A.Y.; Piguet, V. A systematic review of the use of quality-of-life instruments in randomized controlled trials for psoriasis. Br. J. Dermatol. 2017, 176, 577–593. [Google Scholar] [CrossRef]
- Fernandez-Peñas, P.; Jones-Caballero, M.; Espallardo, O.; García-Díez, A. Comparison of Skindex-29, Dermatology Life Quality Index, Psoriasis Disability Index and Medical Outcome Study Short Form 36 in patients with mild to severe psoriasis. Br. J. Dermatol. 2012, 166, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medical Products for Human Use. Guideline on Clinical Investigation of Medical Products Indicated for the Treatment of Psoriasis. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-indicated-treatment-psoriasis_en.pdf (accessed on 15 September 2021).
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): Results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): Results from two randomised controlled, phase 3 trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Krenzer, S.; Radtke, M.; Schmitt-Rau, K.; Augustin, M. Characterization of patient-reported outcomes in moderate to severe psoriasis. Dermatology 2011, 223, 80–86. [Google Scholar] [CrossRef]
- Çakmur, H.; Derviş, E. The relationship between quality of life and the severity of psoriasis in Turkey. Eur. J. Dermatol. 2015, 25, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.P.d.; Fortes, M.R.P.; Miot, L.D.B.; Marques, S.A. Psoriasis: Correlation between severity index (PASI) and quality of life index (DLQI) in patients assessed before and after systemic treatment. An. Bras. Dermatol. 2013, 88, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Mattei, P.L.; Corey, K.C.; Kimball, A.B. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The correlation between disease severity and psychological burden in patients treated with biological therapies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 333–337. [Google Scholar] [CrossRef]
- Schäfer, I.; Hacker, J.; Rustenbach, S.J.; Radtke, M.; Franzke, N.; Augustin, M. Concordance of the Psoriasis Area and Severity Index (PASI) and patient-reported outcomes in psoriasis treatment. Eur. J. Dermatol. 2010, 20, 62–67. [Google Scholar] [CrossRef] [PubMed]

| Parameter | T1 | T2 | T3 |
|---|---|---|---|
| Inclusion | 3 Months | 6 Months | |
| Primary Time Point | Secondary Time Point | ||
| General | |||
| Patient socioeconomic data | X | ||
| Clinical history | X | ||
| Clinical Outcomes | |||
| PASI | X | X | X |
| PGA | X | X | X |
| BSA | X | X | X |
| Patient-Reported Outcomes | |||
| DLQI | X | X | X |
| PBI | X | X | X |
| Anchoring Variable: Satisfaction | X | X | |
| Anchoring Variable: Complete Healing | X | X |
| PASI 75 Achieved after 3 Months | |||||
| Omnibus Test | n | PASI 75 Achieved | Chi-square | df | Sig.≤ |
| 2240 | 756 | 82.008 | 3 | 0.000 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.169 | 0.093 | 1.184 | 1 | 0.069 |
| Age | 0.006 | 0.003 | 1.006 | 1 | 0.087 |
| PASI | 0.041 | 0.005 | 1.042 | 1 | 0.000 |
| Constant | −1.629 | 0.183 | 0.196 | 1 | 0.000 |
| PASI 90 Achieved after 3 Months | |||||
| Omnibus Test | n | PASI 90 Achieved | Chi-square | df | Sig.≤ |
| 2240 | 325 | 53.662 | 3 | 0.000 | |
| Baseline predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.339 | 0.123 | 1.404 | 1 | 0.006 |
| Age | 0.007 | 0.004 | 1.007 | 1 | 0.118 |
| PASI | 0.039 | 0.006 | 1.040 | 1 | 0.000 |
| Constant | −2.867 | 0.246 | 0.057 | 1 | 0.000 |
| “Satisfaction” Achieved after 3 Months | |||||
| Omnibus Test | n | “Satisfaction” Achieved | Chi-square | df | Sig.≤ |
| 2229 | 1096 | 19.830 | 3 | 0.000 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | −0.028 | 0.087 | 0.973 | 1 | 0.751 |
| Age | 0.008 | 0.003 | 1.008 | 1 | 0.010 |
| PASI | 0.016 | 0.004 | 1.016 | 1 | 0.000 |
| Constant | −0.629 | 0.170 | 0.533 | 1 | 0.000 |
| “All Leasons Healed” Achieved after 3 Months | |||||
| Omnibus Test | n | PNQ 4 Achieved | Chi-square | df | Sig.≤ |
| 2120 | 391 | 14.572 | 3 | 0.002 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.100 | 0.114 | 1.106 | 1 | 0.381 |
| Age | 0.014 | 0.004 | 1.014 | 1 | 0.001 |
| PASI | 0.008 | 0.006 | 1.008 | 1 | 0.167 |
| Constant | −2.325 | 0.231 | 0.098 | 1 | 0.000 |
| DLQI after 3 Months | |||||
| Omnibus Test | n | df | F | Sig.≤ | |
| 2240 | 3 | 15.458 | 0.000 | ||
| Baseline Predictors | B | SE | Beta | t | Sig.≤ |
| Constant | 6.138 | 0.472 | 13.013 | 0.000 | |
| Sex | 0.972 | 0.244 | 0.084 | 3.979 | 0.000 |
| Age | −0.037 | 0.009 | −0.091 | −4.342 | 0.000 |
| PASI | 0.049 | 0.012 | 0.084 | 3.981 | 0.000 |
| PASI 75 Achieved after 6 Months | |||||
| Omnibus Test | n | PASI 75 Achieved | Chi-square | df | Sig.≤ |
| 1933 | 919 | 100.645 | 3 | 0.000 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.231 | 0.096 | 1.260 | 1 | 0.016 |
| Age | 0.012 | 0.003 | 1.012 | 1 | 0.000 |
| PASI | 0.045 | 0.005 | 1.047 | 1 | 0.000 |
| Constant | −1.453 | 0.193 | 0.234 | 1 | 0.000 |
| PASI 90% Achieved after 6 Months | |||||
| Omnibus Test | n | PASI 90 Achieved | Chi-square | df | Sig.≤ |
| 1933 | 500 | 78.066 | 3 | 0.000 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.433 | 0.108 | 1.543 | 1 | 0.000 |
| Age | 0.011 | 0.004 | 1.011 | 1 | 0.005 |
| PASI | 0.040 | 0.005 | 1.040 | 1 | 0.000 |
| Constant | −2.378 | 0.221 | 0.093 | 1 | 0.000 |
| “Satisfation” Achieved after 6 Months | |||||
| Omnibus Test | n | “Satisfaction” Achieved | Chi-square | df | Sig.≤ |
| 1905 | 1030 | 3.043 | 3 | 0.385 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.041 | 0.094 | 1.042 | 1 | 0.663 |
| Age | 0.003 | 0.003 | 1.003 | 1 | 0.305 |
| Pasi | 0.006 | 0.005 | 1.006 | 1 | 0.167 |
| Constant | −0.113 | 0.185 | 0.893 | 1 | 0.541 |
| “All Leasons Healed” Achieved after 6 Months | |||||
| Omnibus Test | n | PNQ 4 Achieved | Chi-square | df | Sig.≤ |
| 1831 | 389 | 15.530 | 3 | 0.001 | |
| Baseline Predictors | B | SE | Exp(B) | df | Sig.≤ |
| Sex | 0.163 | 0.117 | 1.177 | 1 | 0.163 |
| Age | 0.010 | 0.004 | 1.010 | 1 | 0.013 |
| PASI | 0.016 | 0.005 | 1.016 | 1 | 0.004 |
| Constant | −2.115 | 0.235 | 0.121 | 1 | 0.000 |
| DLQI after 6 months | |||||
| Omnibus test | n | df | F | Sig.≤ | |
| 1920 | 3 | 6,173 | 0.000 | ||
| Baseline predictors | B | SE | Beta | t | Sig.≤ |
| Constant | 5.219 | 0.487 | 10.723 | 0.000 | |
| Sex | 0.257 | 0.249 | 0.024 | 1.030 | 0.303 |
| Age | −0.029 | 0.009 | −0.074 | −3.258 | 0.001 |
| PASI | 0.033 | 0.012 | 0.061 | 2.681 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsten, N.; Rustenbach, S.; von Kiedrowski, R.; Sorbe, C.; Reich, K.; Augustin, M. Which PASI Outcome Is Most Relevant to the Patients in Real-World Care? Life 2021, 11, 1151. https://doi.org/10.3390/life11111151
Kirsten N, Rustenbach S, von Kiedrowski R, Sorbe C, Reich K, Augustin M. Which PASI Outcome Is Most Relevant to the Patients in Real-World Care? Life. 2021; 11(11):1151. https://doi.org/10.3390/life11111151
Chicago/Turabian StyleKirsten, Natalia, Stephan Rustenbach, Ralph von Kiedrowski, Christina Sorbe, Kristian Reich, and Matthias Augustin. 2021. "Which PASI Outcome Is Most Relevant to the Patients in Real-World Care?" Life 11, no. 11: 1151. https://doi.org/10.3390/life11111151
APA StyleKirsten, N., Rustenbach, S., von Kiedrowski, R., Sorbe, C., Reich, K., & Augustin, M. (2021). Which PASI Outcome Is Most Relevant to the Patients in Real-World Care? Life, 11(11), 1151. https://doi.org/10.3390/life11111151






