Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions
Abstract
:1. Overview of Apoptotic Cell Clearance
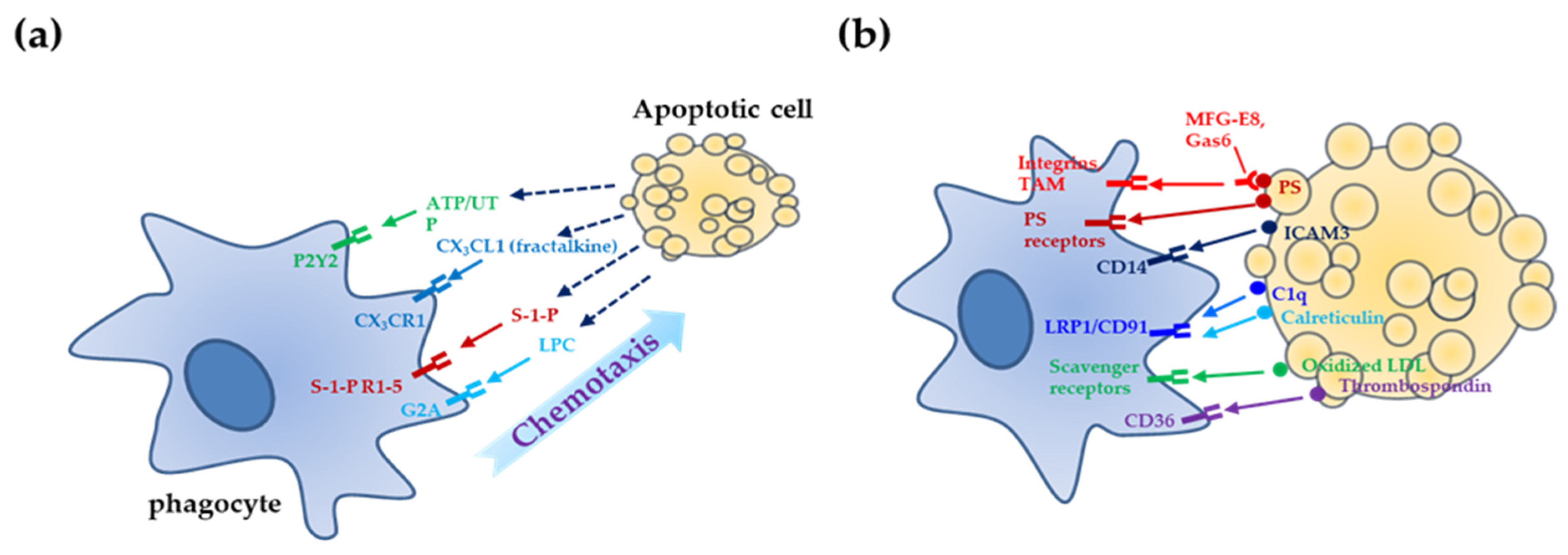
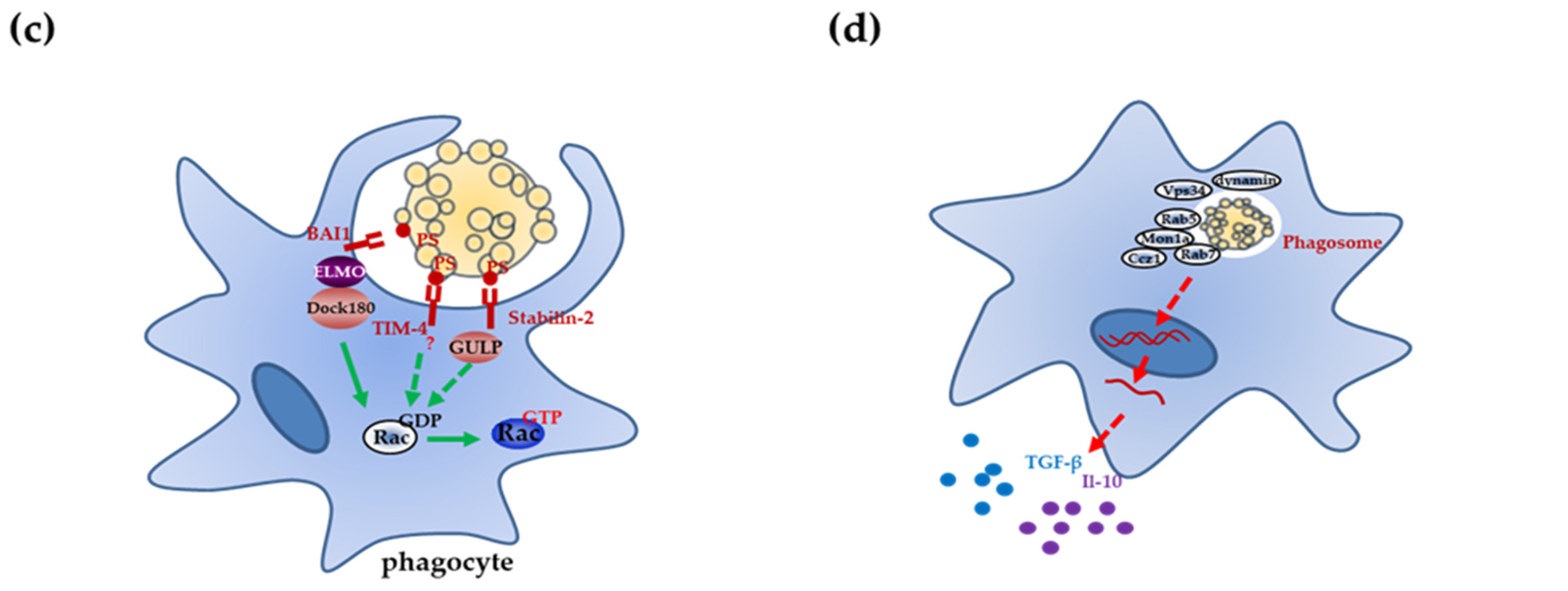
2. Mechanisms of Apoptotic Cell Clearance
3. Apoptotic Cell Clearance and Differentiation
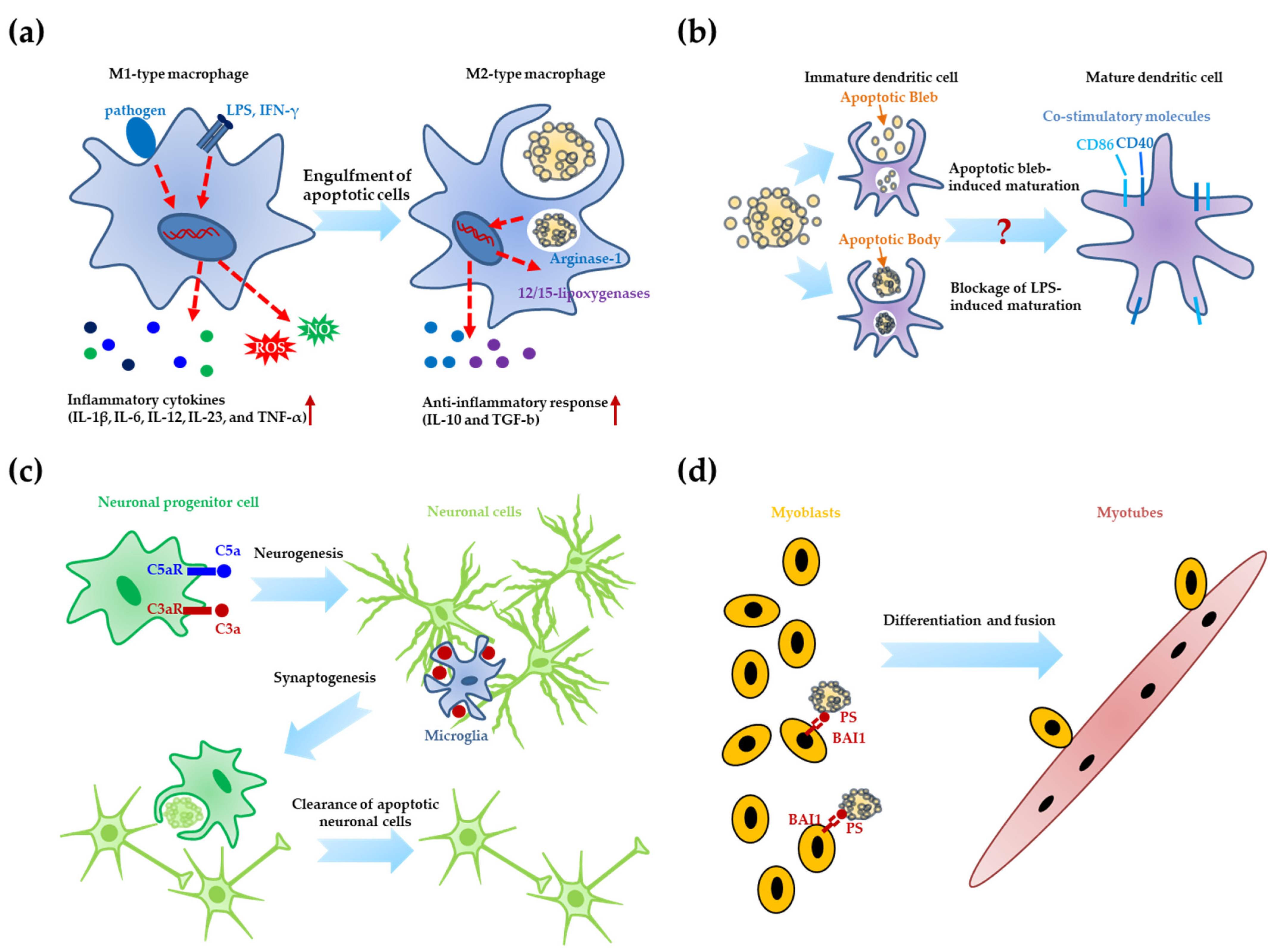
3.1. Differentiation of Immune Cells
3.2. Neurogenesis
3.3. Muscle Differentiation
4. Apoptotic Cell Clearance and Organogenesis
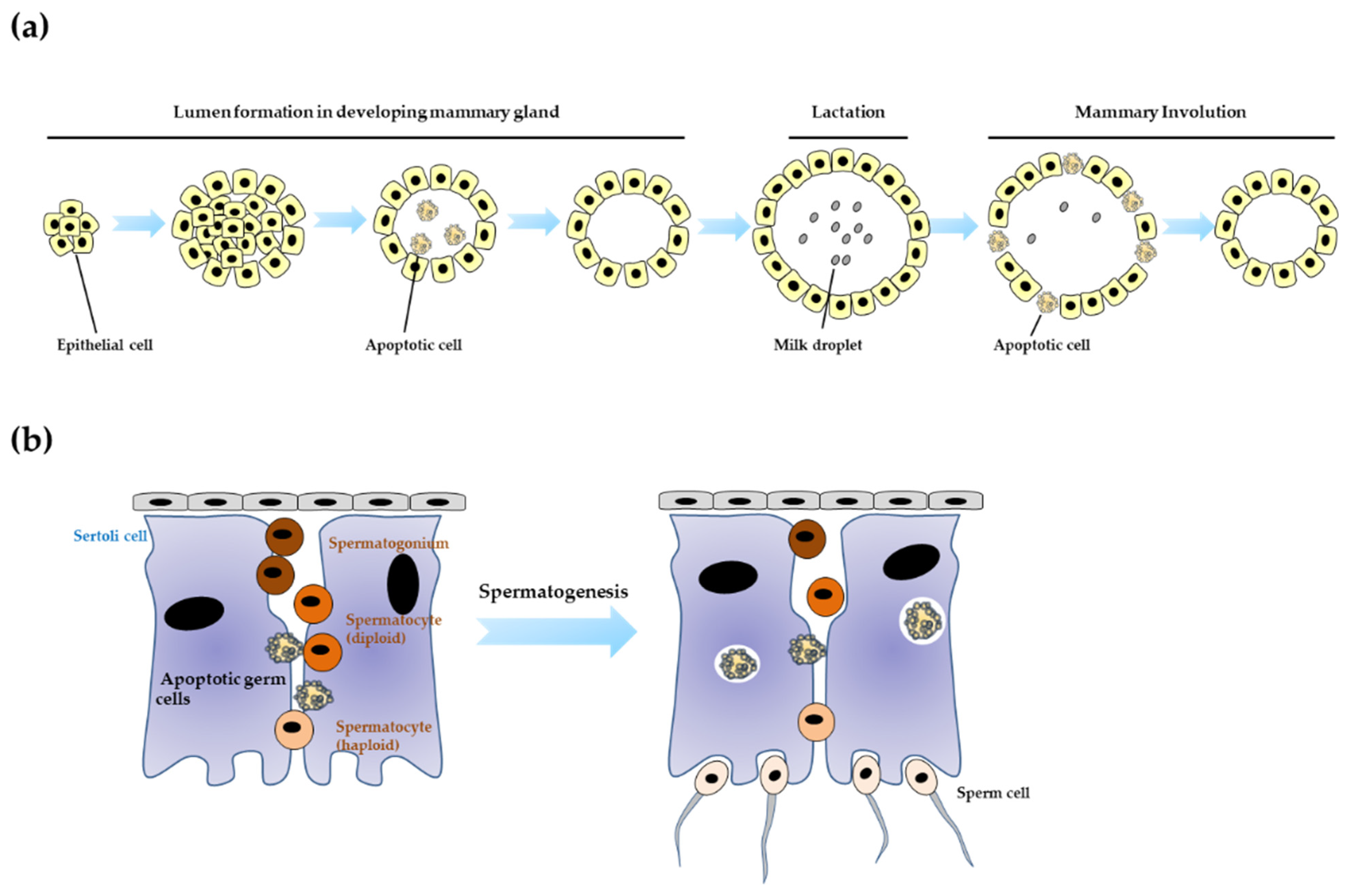
4.1. Mammary Development
4.2. Testis Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D.; Pound, J.D. Cell death in the neighbourhood: Direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J. Pathol. 2011, 223, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Calianese, D.; Birge, R.B. Efferocytosis of dying cells differentially modulate immunological outcomes in tumor microenvironment. Immunol. Rev. 2017, 280, 149–164. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of bcl-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payea, M.J.; Anerillas, C.; Tharakan, R.; Gorospe, M. Translational control during cellular senescence. Mol. Cell. Biol. 2021, 41, e00512-20. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Poon, I.K.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Gregory, C. Cell biology: Sent by the scent of death. Nature 2009, 461, 181–182. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K. Phagocytic clearance of apoptotic, necrotic, necroptotic and pyroptotic cells. Biochem. Soc. Trans. 2021, 49, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S.; Lorenz, U. Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Hajishengallis, G.; Chavakis, T. Phagocytosis of apoptotic cells in resolution of inflammation. Front. Immunol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Hussell, T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin. Immunopathol. 2016, 38, 409–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vre, E.A.; Ait-Oufella, H.; Tedgui, A.; Mallat, Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Schrijvers, D.M.; de Meyer, G.R.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Thorp, E.; Cui, D.; Schrijvers, D.M.; Kuriakose, G.; Tabas, I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Cohen, P.L.; Caricchio, R.; Abraham, V.; Camenisch, T.D.; Jennette, J.C.; Roubey, R.A.; Earp, H.S.; Matsushima, G.; Reap, E.A. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 2002, 196, 135–140. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miyasaka, K.; Aozasa, K.; Koike, M.; Uchiyama, Y.; Nagata, S. Autoimmune disease and impaired uptake of apoptotic cells in mfg-e8-deficient mice. Science 2004, 304, 1147–1150. [Google Scholar] [CrossRef] [Green Version]
- Tas, S.W.; Quartier, P.; Botto, M.; Fossati-Jimack, L. Macrophages from patients with sle and rheumatoid arthritis have defective adhesion in vitro, while only sle macrophages have impaired uptake of apoptotic cells. Ann. Rheum. Dis. 2006, 65, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Grabiec, A.M.; Denny, N.; Doherty, J.A.; Happonen, K.E.; Hankinson, J.; Connolly, E.; Fife, M.E.; Fujimori, T.; Fujino, N.; Goenka, A.; et al. Diminished airway macrophage expression of the axl receptor tyrosine kinase is associated with defective efferocytosis in asthma. J. Allergy Clin. Immunol. 2017, 140, 1144–1146. [Google Scholar] [CrossRef] [Green Version]
- Gheibi Hayat, S.M.; Bianconi, V.; Pirro, M.; Sahebkar, A. Efferocytosis: Molecular mechanisms and pathophysiological perspectives. Immunol. Cell Biol. 2019, 97, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, Y.; Deng, Y.; Shao, A. Regulation of efferocytosis as a novel cancer therapy. Cell Commun. Signal. 2020, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.B.; Ravichandran, K.S. Do not let death do us part: ‘Find-me’ signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016, 23, 979–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, S.P.; Henry, C.M.; Kearney, C.J.; Logue, S.E.; Feoktistova, M.; Tynan, G.A.; Lavelle, E.C.; Leverkus, M.; Martin, S.J. Fas/cd95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol. Cell 2013, 49, 1034–1048. [Google Scholar] [CrossRef] [Green Version]
- Nagata, S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the clearance of dead cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, W.; Zhao, D.; Liu, B.; Shi, Y.; Chen, B.; Yang, H.; Guo, P.; Geng, X.; Shang, Z.; et al. Caenorhabditis elegans transthyretin-like protein ttr-52 mediates recognition of apoptotic cells by the ced-1 phagocyte receptor. Nat. Cell Biol. 2010, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Tibrewal, N.; Birge, R.B. Phosphatidylserine recognition by phagocytes: A view to a kill. Trends Cell Biol. 2006, 16, 189–197. [Google Scholar] [CrossRef]
- Kenis, H.; van Genderen, H.; Bennaghmouch, A.; Rinia, H.A.; Frederik, P.; Narula, J.; Hofstra, L.; Reutelingsperger, C.P. Cell surface-expressed phosphatidylserine and annexin a5 open a novel portal of cell entry. J. Biol. Chem. 2004, 279, 52623–52629. [Google Scholar] [CrossRef] [Green Version]
- Cory, S. Phosphatidylserine hide-and-seek. Proc. Natl. Acad. Sci. USA 2018, 115, 12092–12094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-dependent phospholipid scrambling by tmem16f. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Denning, D.P.; Imanishi, E.; Horvitz, H.R.; Nagata, S. Xk-related protein 8 and ced-8 promote phosphatidylserine exposure in apoptotic cells. Science 2013, 341, 403–406. [Google Scholar] [CrossRef] [Green Version]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Nagata, S. Phospholipid scrambling on the plasma membrane. Methods Enzymol. 2014, 544, 381–393. [Google Scholar]
- Caberoy, N.B.; Alvarado, G.; Bigcas, J.L.; Li, W. Galectin-3 is a new mertk-specific eat-me signal. J. Cell. Physiol. 2012, 227, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas, A.; Banerjee, S.; Xie, N.; Cui, H.; Davis, K.I.; Friggeri, A.; Fu, M.; Abraham, E.; Liu, G. Identification of tlt2 as an engulfment receptor for apoptotic cells. J. Immunol. 2012, 188, 6381–6388. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.; Ravichandran, K.S. Phosphatidylserine receptors: What is the new rage? EMBO Rep. 2011, 12, 287–288. [Google Scholar] [CrossRef] [Green Version]
- Yoon, K.W.; Byun, S.; Kwon, E.; Hwang, S.Y.; Chu, K.; Hiraki, M.; Jo, S.H.; Weins, A.; Hakroush, S.; Cebulla, A.; et al. Cell death. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 2015, 349, 1261669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.L.; Kim, J.I.; Birge, R.B. Alphavbeta5 integrin recruits the crkii-dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2000, 2, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Tosello-Trampont, A.C.; Elliott, M.R.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K.S. Bai1 is an engulfment receptor for apoptotic cells upstream of the elmo/dock180/rac module. Nature 2007, 450, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Abu-Thuraia, A.; Gauthier, R.; Chidiac, R.; Fukui, Y.; Screaton, R.A.; Gratton, J.P.; Cote, J.F. Axl phosphorylates elmo scaffold proteins to promote rac activation and cell invasion. Mol. Cell. Biol. 2015, 35, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kang, K.B.; Thapa, N.; Kim, S.Y.; Lee, S.J.; Kim, I.S. Requirement of adaptor protein gulp during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 2008, 283, 10593–10600. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, I.S. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp. Mol. Med. 2017, 49, e331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinchen, J.M.; Ravichandran, K.S. Journey to the grave: Signaling events regulating removal of apoptotic cells. J. Cell Sci. 2007, 120, 2143–2149. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.; Valdez, P.A.; Tan, C.; Yeh, S.; Hongo, J.A.; Ouyang, W. Phosphatidylserine receptor tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc. Natl. Acad. Sci. USA 2010, 107, 8712–8717. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Hochreiter-Hufford, A.; Ravichandran, K.S. The phosphatidylserine receptor tim-4 does not mediate direct signaling. Curr. Biol. 2009, 19, 346–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurwitz, M.E.; Vanderzalm, P.J.; Bloom, L.; Goldman, J.; Garriga, G.; Horvitz, H.R. Abl kinase inhibits the engulfment of apoptotic [corrected] cells in caenorhabditis elegans. PLoS Biol. 2009, 7, e99. [Google Scholar] [CrossRef]
- Nakaya, M.; Tajima, M.; Kosako, H.; Nakaya, T.; Hashimoto, A.; Watari, K.; Nishihara, H.; Ohba, M.; Komiya, S.; Tani, N.; et al. Grk6 deficiency in mice causes autoimmune disease due to impaired apoptotic cell clearance. Nat. Commun. 2013, 4, 1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinchen, J.M.; Ravichandran, K.S. Phagosome maturation: Going through the acid test. Nat. Rev. Mol. Cell Biol. 2008, 9, 781–795. [Google Scholar] [CrossRef]
- Korns, D.; Frasch, S.C.; Fernandez-Boyanapalli, R.; Henson, P.M.; Bratton, D.L. Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2011, 2, 57. [Google Scholar] [CrossRef] [Green Version]
- Mukundan, L.; Odegaard, J.I.; Morel, C.R.; Heredia, J.E.; Mwangi, J.W.; Ricardo-Gonzalez, R.R.; Goh, Y.P.; Eagle, A.R.; Dunn, S.E.; Awakuni, J.U.; et al. Ppar-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 2009, 15, 1266–1272. [Google Scholar] [CrossRef]
- Majai, G.; Sarang, Z.; Csomos, K.; Zahuczky, G.; Fesus, L. Ppargamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur. J. Immunol. 2007, 37, 1343–1354. [Google Scholar] [CrossRef]
- Mota, A.C.; Dominguez, M.; Weigert, A.; Snodgrass, R.G.; Namgaladze, D.; Brune, B. Lysosome-dependent lxr and ppardelta activation upon efferocytosis in human macrophages. Front. Immunol. 2021, 12, 637778. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Z.; Ravichandran, K.S. Metabolic connections during apoptotic cell engulfment. Cell 2011, 147, 1442–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Han, C.Z.; Elliott, M.R.; Kinchen, J.M.; Trampont, P.C.; Das, S.; Collins, S.; Lysiak, J.J.; Hoehn, K.L.; Ravichandran, K.S. Continued clearance of apoptotic cells critically depends on the phagocyte ucp2 protein. Nature 2011, 477, 220–224. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage m1/m2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in m1(lps+) vs. Classically and m2(lps−) vs. Alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Filardy, A.A.; Pires, D.R.; Nunes, M.P.; Takiya, C.M.; Freire-de-Lima, C.G.; Ribeiro-Gomes, F.L.; DosReis, G.A. Proinflammatory clearance of apoptotic neutrophils induces an il-12(low)il-10(high) regulatory phenotype in macrophages. J. Immunol. 2010, 185, 2044–2050. [Google Scholar] [CrossRef] [Green Version]
- Schif-Zuck, S.; Gross, N.; Assi, S.; Rostoker, R.; Serhan, C.N.; Ariel, A. Saturated-efferocytosis generates pro-resolving cd11b low macrophages: Modulation by resolvins and glucocorticoids. Eur. J. Immunol. 2011, 41, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage polarization comes of age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Ivashkiv, L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013, 34, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The jmjd3-irf4 axis regulates m2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of macrophage polarization and wound healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracini, M.; Rios, F.J.; Pecenin, M.; Jancar, S. Clearance of apoptotic cells by macrophages induces regulatory phenotype and involves stimulation of cd36 and platelet-activating factor receptor. Mediat. Inflamm. 2013, 2013, 950273. [Google Scholar] [CrossRef]
- Stuart, L.M.; Lucas, M.; Simpson, C.; Lamb, J.; Savill, J.; Lacy-Hulbert, A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J. Immunol. 2002, 168, 1627–1635. [Google Scholar] [CrossRef]
- Fransen, J.H.; Hilbrands, L.B.; Ruben, J.; Stoffels, M.; Adema, G.J.; van der Vlag, J.; Berden, J.H. Mouse dendritic cells matured by ingestion of apoptotic blebs induce t cells to produce interleukin-17. Arthritis Rheum. 2009, 60, 2304–2313. [Google Scholar] [CrossRef]
- Fransen, J.H.; van der Vlag, J.; Ruben, J.; Adema, G.J.; Berden, J.H.; Hilbrands, L.B. The role of dendritic cells in the pathogenesis of systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, 207. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.H.; Zhen, Y.; Finkelman, F.D.; Cohen, P.L. The mertk receptor tyrosine kinase promotes t-b interaction stimulated by igd b-cell receptor cross-linking. J. Autoimmun. 2014, 53, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Inokuchi, K. Adult neurogenesis and modulation of neural circuit function. Curr. Opin. Neurobiol. 2011, 21, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kouser, L.; Madhukaran, S.P.; Shastri, A.; Saraon, A.; Ferluga, J.; Al-Mozaini, M.; Kishore, U. Emerging and novel functions of complement protein c1q. Front. Immunol. 2015, 6, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crehan, H.; Hardy, J.; Pocock, J. Microglia, Alzheimer’s disease, and complement. Int. J. Alzheimer’s Dis. 2012, 2012, 983640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aburto, M.R.; Sanchez-Calderon, H.; Hurle, J.M.; Varela-Nieto, I.; Magarinos, M. Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death Dis. 2012, 3, e394. [Google Scholar] [CrossRef] [Green Version]
- Mellen, M.A.; de la Rosa, E.J.; Boya, P. The autophagic machinery is necessary for removal of cell corpses from the developing retinal neuroepithelium. Cell Death Differ. 2008, 15, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.H.; Bellmunt, E.; Scheib, J.L.; Venegas, V.; Burkert, C.; Reichardt, L.F.; Zhou, Z.; Farinas, I.; Carter, B.D. Glial precursors clear sensory neuron corpses during development via jedi-1, an engulfment receptor. Nat. Neurosci. 2009, 12, 1534–1541. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Elliott, M.R.; Chen, Y.; Walsh, J.T.; Klibanov, A.L.; Ravichandran, K.S.; Kipnis, J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat. Cell Biol. 2011, 13, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Lovelace, M.D.; Gu, B.J.; Eamegdool, S.S.; Weible, M.W., 2nd; Wiley, J.S.; Allen, D.G.; Chan-Ling, T. P2x7 receptors mediate innate phagocytosis by human neural precursor cells and neuroblasts. Stem Cells 2015, 33, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Endo, T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone 2015, 80, 2–13. [Google Scholar] [CrossRef]
- Tu, M.K.; Levin, J.B.; Hamilton, A.M.; Borodinsky, L.N. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 2016, 59, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Abmayr, S.M.; Pavlath, G.K. Myoblast fusion: Lessons from flies and mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.F.; Baylies, M.K. Cell biology: Death brings new life to muscle. Nature 2013, 497, 196–197. [Google Scholar] [CrossRef] [Green Version]
- Van den Eijnde, S.M.; van den Hoff, M.J.; Reutelingsperger, C.P.; van Heerde, W.L.; Henfling, M.E.; Vermeij-Keers, C.; Schutte, B.; Borgers, M.; Ramaekers, F.C. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J. Cell Sci. 2001, 114, 3631–3642. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Pombo, I.; Poncet, P.; David, B.; Arock, M.; Blank, U. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. Int. Arch. Allergy Immunol. 2000, 123, 249–258. [Google Scholar] [CrossRef]
- Rysavy, N.M.; Shimoda, L.M.; Dixon, A.M.; Speck, M.; Stokes, A.J.; Turner, H.; Umemoto, E.Y. Beyond apoptosis: The mechanism and function of phosphatidylserine asymmetry in the membrane of activating mast cells. Bioarchitecture 2014, 4, 127–137. [Google Scholar]
- Jeong, J.; Conboy, I.M. Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem. Biophys. Res. Commun. 2011, 414, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, P.; Kelly, J.F.; Balazsi, K.; Slack, R.S.; Megeney, L.A. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 2002, 99, 11025–11030. [Google Scholar] [CrossRef] [Green Version]
- Hochreiter-Hufford, A.E.; Lee, C.S.; Kinchen, J.M.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.A.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K.S. Phosphatidylserine receptor bai1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Hamoud, N.; Tran, V.; Croteau, L.P.; Kania, A.; Cote, J.F. G-protein coupled receptor bai3 promotes myoblast fusion in vertebrates. Proc. Natl. Acad. Sci. USA 2014, 111, 3745–3750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwig, L.P.; Henson, P.M. Immunological consequences of apoptotic cell phagocytosis. Am. J. Pathol. 2007, 171, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Mailleux, A.A.; Overholtzer, M.; Brugge, J.S. Lumen formation during mammary epithelial morphogenesis: Insights from in vitro and in vivo models. Cell Cycle 2008, 7, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Monks, J.; Henson, P.M. Differentiation of the mammary epithelial cell during involution: Implications for breast cancer. J. Mammary Gland Biol. Neoplasia 2009, 14, 159–170. [Google Scholar] [CrossRef]
- Fornetti, J.; Flanders, K.C.; Henson, P.M.; Tan, A.C.; Borges, V.F.; Schedin, P. Mammary epithelial cell phagocytosis downstream of tgf-beta3 is characterized by adherens junction reorganization. Cell Death Differ. 2015, 23, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanayama, R.; Nagata, S. Impaired involution of mammary glands in the absence of milk fat globule egf factor 8. Proc. Natl. Acad. Sci. USA 2005, 102, 16886–16891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Moore, R.; Smith, E.R.; Xu, X.X. Hormonal induction and roles of disabled-2 in lactation and involution. PLoS ONE 2014, 9, e110737. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Li, W.; Mironov, A.; Streuli, C.H. Rac1 controls both the secretory function of the mammary gland and its remodeling for successive gestations. Dev. Cell 2016, 38, 522–535. [Google Scholar] [CrossRef] [Green Version]
- Bagci, H.; Laurin, M.; Huber, J.; Muller, W.J.; Cote, J.F. Impaired cell death and mammary gland involution in the absence of dock1 and rac1 signaling. Cell Death Dis. 2014, 5, e1375. [Google Scholar] [CrossRef] [Green Version]
- Teplova, I.; Lozy, F.; Price, S.; Singh, S.; Barnard, N.; Cardiff, R.D.; Birge, R.B.; Karantza, V. Atg proteins mediate efferocytosis and suppress inflammation in mammary involution. Autophagy 2013, 9, 459–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. The central role of sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, Spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754. [Google Scholar] [CrossRef]
- Elliott, M.R.; Ravichandran, K.S. Elmo1 signaling in apoptotic germ cell clearance and spermatogenesis. Ann. N. Y. Acad. Sci. 2010, 1209, 30–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Chen, Q.; Yan, K.; Liu, Z.; Zhang, X.; Liu, P.; Chen, Y.; Han, D. Breakdown of immune homeostasis in the testis of mice lacking tyro3, axl and mer receptor tyrosine kinases. Immunol. Cell Biol. 2013, 91, 416–426. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Qi, N.; Wu, H.; Xiong, W.; Ma, J.; Lu, Q.; Han, D. Functions of tam rtks in regulating spermatogenesis and male fertility in mice. Reproduction 2009, 138, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Gore, M.; Zhang, Q.; Camenisch, T.; Boast, S.; Casagranda, F.; Lai, C.; Skinner, M.K.; Klein, R.; Matsushima, G.K.; et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 1999, 398, 723–728. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, Y.; Wang, H.; Wang, H.; Wu, H.; Lu, Q.; Han, D. Gas6 and the tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of sertoli cells. Reproduction 2008, 135, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Gillot, I.; Jehl-Pietri, C.; Gounon, P.; Luquet, S.; Rassoulzadegan, M.; Grimaldi, P.; Vidal, F. Germ cells and fatty acids induce translocation of cd36 scavenger receptor to the plasma membrane of sertoli cells. J. Cell Sci. 2005, 118, 3027–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, M.R.; Zheng, S.; Park, D.; Woodson, R.I.; Reardon, M.A.; Juncadella, I.J.; Kinchen, J.M.; Zhang, J.; Lysiak, J.J.; Ravichandran, K.S. Unexpected requirement for elmo1 in clearance of apoptotic germ cells in vivo. Nature 2010, 467, 333–337. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Messaddeq, N.; Harnois, T.; Meunier, A.C.; Clarhaut, J.; Noblanc, A.; Weickert, J.L.; Cantereau, A.; Philippe, M.; Bourmeyster, N.; et al. A chimerical phagocytosis model reveals the recruitment by sertoli cells of autophagy for the degradation of ingested illegitimate substrates. Autophagy 2013, 9, 653–666. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.A.; Moon, S.Y.; Park, D.; Park, J.B.; Lee, C.S. Apoptotic cell clearance in the tumor microenvironment: A potential cancer therapeutic target. Arch. Pharmacal Res. 2019, 42, 658–671. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Tumor-associated macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Kale, A. Cellular cannibalism. J. Oral Maxillofac. Pathol. 2015, 19, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, F.; Fais, S. Cancer cell cannibalism: A primeval option to survive. Curr. Mol. Med. 2015, 15, 836–841. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Ryu, G.; Shin, S.-A.; An, J.; Kim, H.; Park, D.; Lee, D.-H.; Lee, C.S. Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions. Life 2021, 11, 1141. https://doi.org/10.3390/life11111141
Han M, Ryu G, Shin S-A, An J, Kim H, Park D, Lee D-H, Lee CS. Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions. Life. 2021; 11(11):1141. https://doi.org/10.3390/life11111141
Chicago/Turabian StyleHan, Minjoo, Gyoungah Ryu, Seong-Ah Shin, Jangeun An, Huiji Kim, Daeho Park, Dae-Hee Lee, and Chang Sup Lee. 2021. "Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions" Life 11, no. 11: 1141. https://doi.org/10.3390/life11111141
APA StyleHan, M., Ryu, G., Shin, S.-A., An, J., Kim, H., Park, D., Lee, D.-H., & Lee, C. S. (2021). Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions. Life, 11(11), 1141. https://doi.org/10.3390/life11111141





