Sorafenib Combined with Chemoembolization for Locally Advanced Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Propensity Score Analysis
Abstract
:1. Introduction
2. Materials and Methods
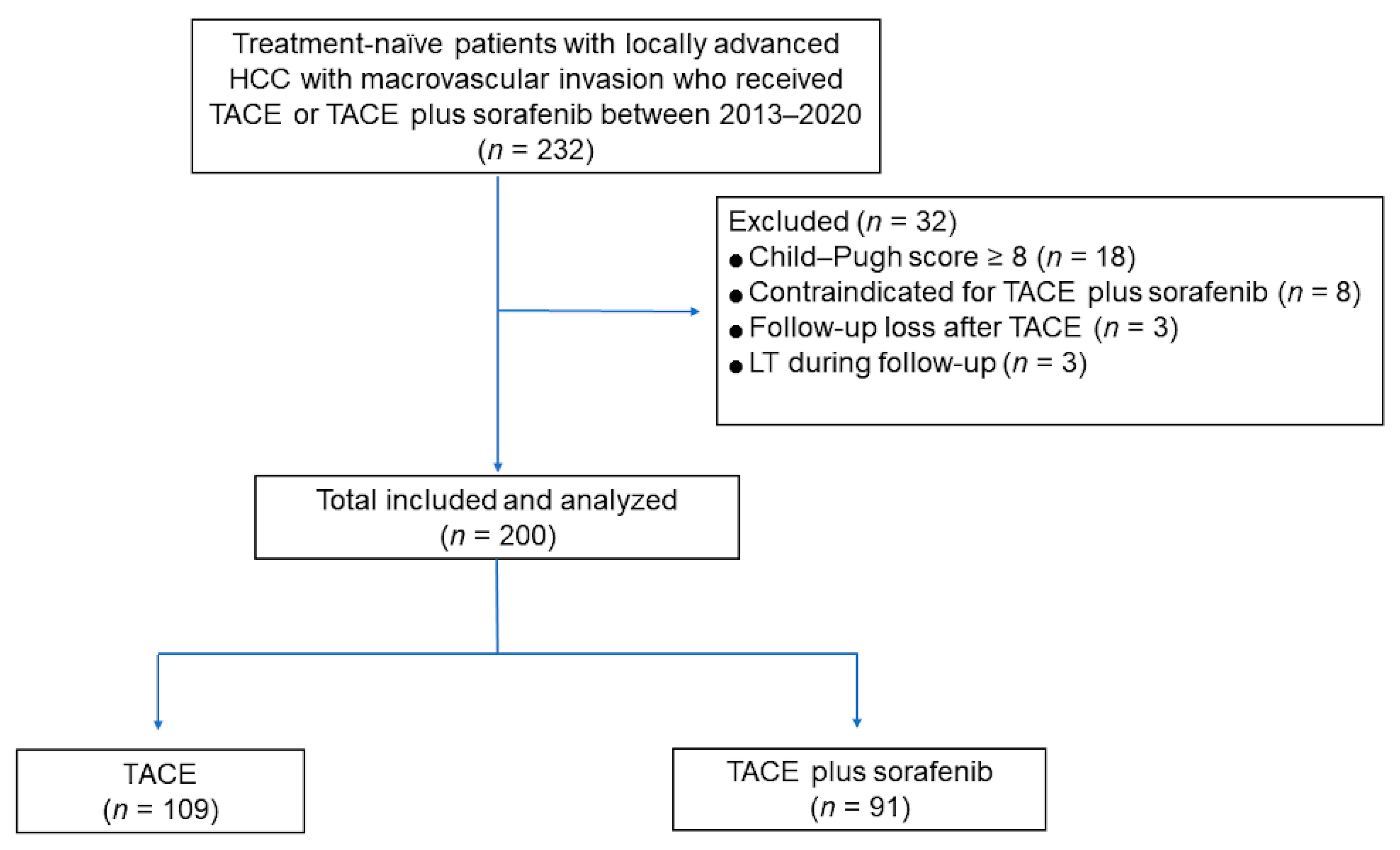
2.1. Study Patients
2.2. Transcatheter Arterial Chemoembolization
2.3. Sorafenib Therapy
2.4. Definitions and Statistical Analyses
3. Results
3.1. Patients
3.2. Radiologic Response after Treatment
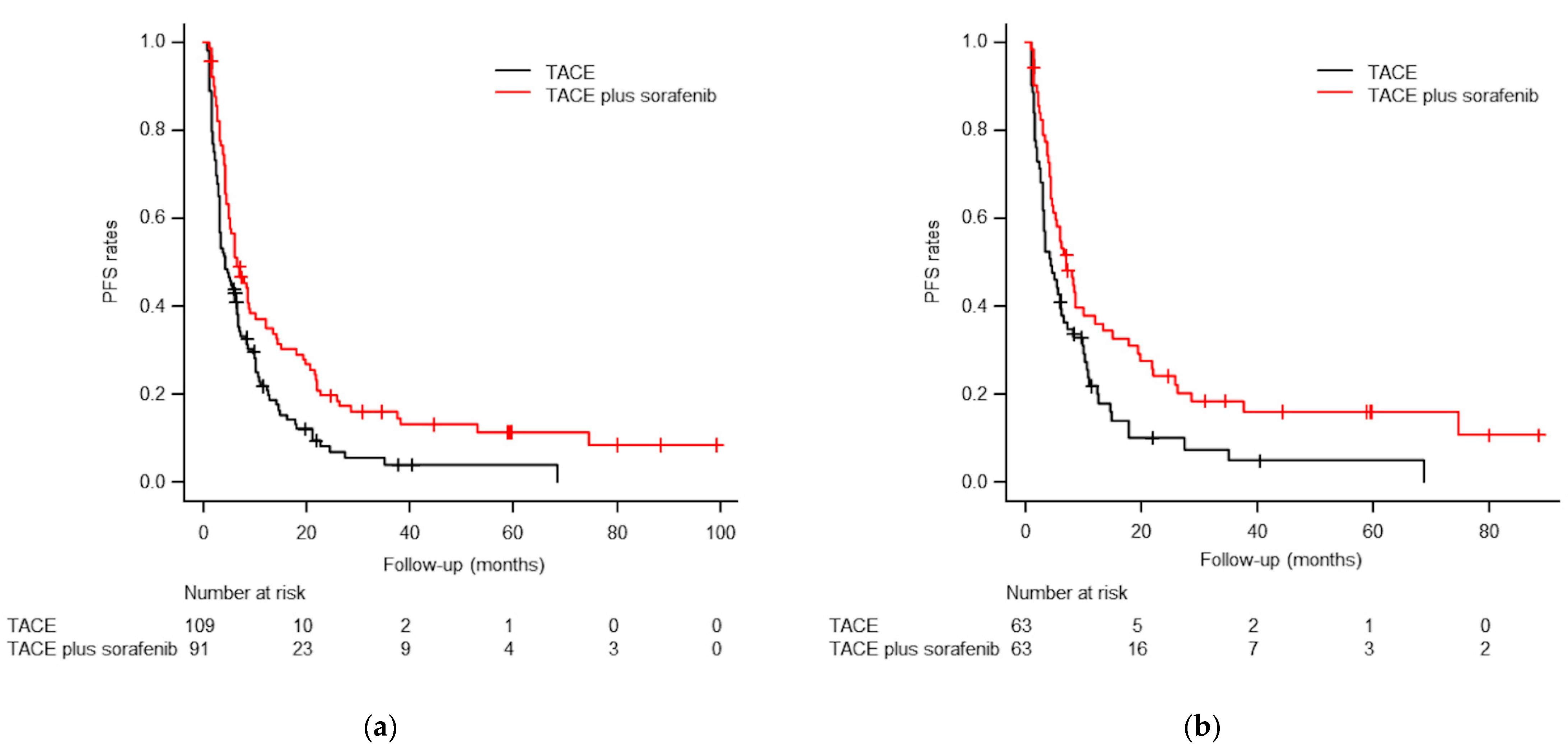
3.3. Progression-Free Survival Analyses
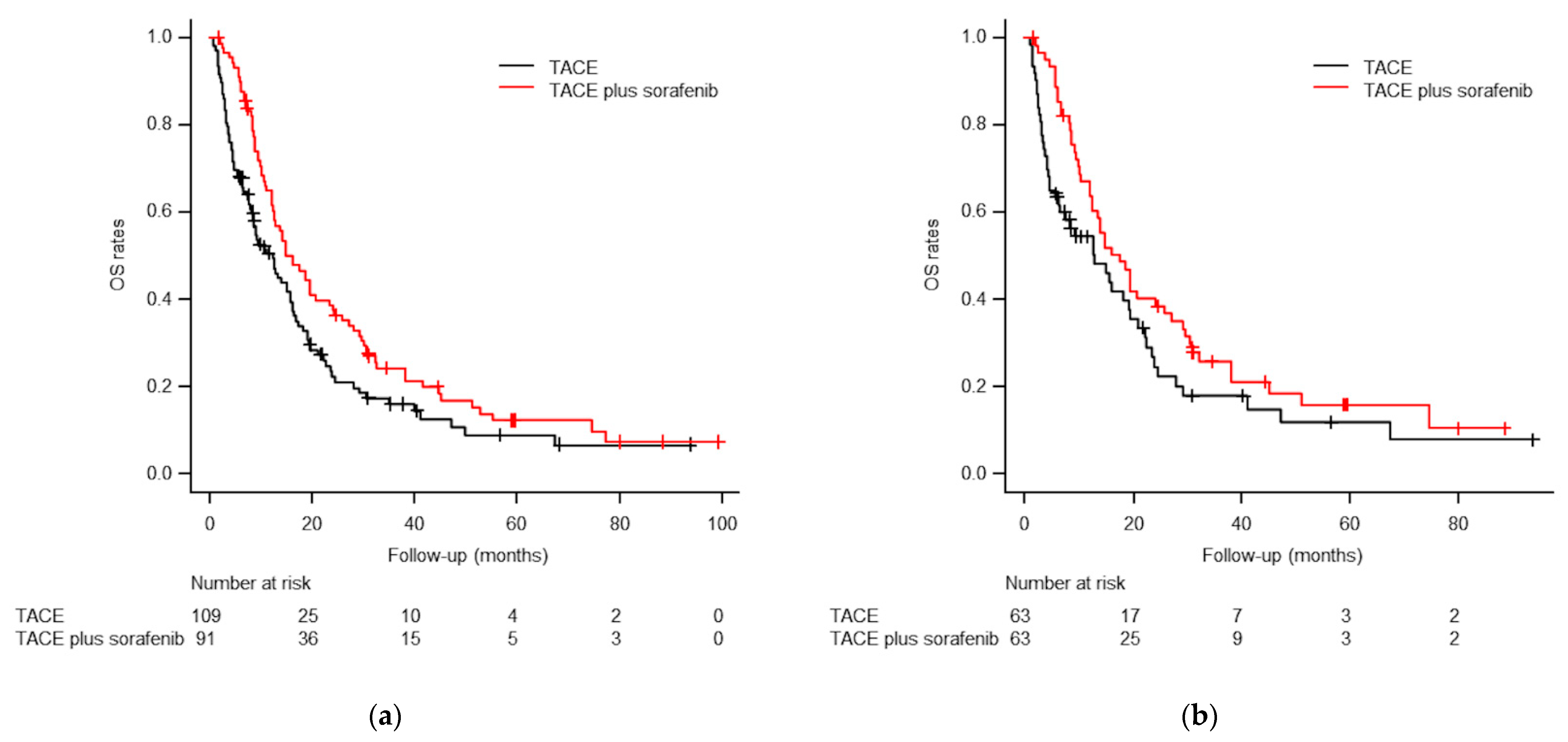
3.4. Overall Survival Analyses
3.5. Multivariable Analyses
3.6. Subgroup Analysis of OS Regarding Main PV or IVC Invasion
3.7. Major Complications (Grade 3 or 4 Toxicities)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Bouattour, M.; Mehta, N.; He, A.R.; Cohen, E.I.; Nault, J.C. Systemic Treatment for Advanced Hepatocellular Carcinoma. Liver Cancer 2019, 8, 341–358. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinter, M.; Hucke, F.; Graziadei, I.; Vogel, W.; Maieron, A.; Königsberg, R.; Stauber, R.; Grünberger, B.; Müller, C.; Kölblinger, C.; et al. Advanced-stage hepatocellular carcinoma: Transarterial chemoembolization versus sorafenib. Radiology 2012, 263, 590–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, G.E.; Lee, J.H.; Kim, H.Y.; Hwang, S.Y.; Kim, J.S.; Chung, J.W.; Yoon, J.H.; Lee, H.S.; Kim, Y.J. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011, 258, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoon, H.K.; Kim, S.Y.; Kim, K.M.; Ko, G.Y.; Gwon, D.I.; Sung, K.B. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment. Pharmacol. Ther. 2009, 29, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Guo, R.P.; Lai, E.C.; Zhang, Y.J.; Lau, W.Y.; Chen, M.S.; Shi, M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: A prospective comparative study. Ann. Surg. Oncol. 2011, 18, 413–420. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, J.H.; Yoon, H.K.; Ko, H.K.; Kim, J.W.; Gwon, D.I. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018, 38, 1646–1654. [Google Scholar] [CrossRef]
- Tawada, A.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Saito, T.; Ogasawara, S.; Suzuki, E.; Maruyama, H.; Kanai, F.; et al. Efficacy of transarterial chemoembolization targeting portal vein tumor thrombus in patients with hepatocellular carcinoma. Anticancer Res. 2014, 34, 4231–4237. [Google Scholar]
- Sun, J.; Shi, J.; Huang, B.; Cheng, F.; Guo, W.; Lau, W.Y.; Cheng, S. The degree of hepatic arterial blood supply of portal vein tumor thrombus in patients with hepatocellular carcinoma and its impact on overall survival after transarterial chemoembolization. Oncotarget 2017, 8, 79816–79824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothary, N.; Weintraub, J.L.; Susman, J.; Rundback, J.H. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J. Vasc. Interv. Radiol. 2007, 18, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Alrashidi, I.; Chu, H.H.; Kim, J.H.; Shim, J.H.; Yoon, S.M.; Kim, P.H.; Gwon, D.I.; Ko, H.K. Combined Chemoembolization and Radiotherapy Versus Chemoembolization Alone for Hepatocellular Carcinoma Invading the Hepatic Vein or Inferior Vena Cava. Cardiovasc. Intervent. Radiol. 2021, 44, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Sergio, A.; Cristofori, C.; Cardin, R.; Pivetta, G.; Ragazzi, R.; Baldan, A.; Girardi, L.; Cillo, U.; Burra, P.; Giacomin, A.; et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am. J. Gastroenterol. 2008, 103, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Park, J.W.; Kim, J.H.; An, M.; Kong, S.Y.; Nam, B.H.; Choi, J.I.; Kim, H.B.; Lee, W.J.; Kim, C.M. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008, 99, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [Green Version]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Kim, D.Y.; Bae, S.H.; Paik, S.W.; Lee, Y.J.; Kim, H.Y.; Lee, H.C.; Han, S.Y.; Cheong, J.Y.; et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J. Hepatol. 2019, 70, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, G.H.; Shim, J.H.; Kim, M.J.; Ryu, M.H.; Ryoo, B.Y.; Kang, Y.K.; Shin, Y.M.; Kim, K.M.; Lim, Y.S.; Lee, H.C. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: Results of propensity score analyses. Radiology 2013, 269, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.C.; Chen, C.Y.; Cheng, P.N.; Liu, Y.S.; Cheng, H.C.; Chuang, C.H.; Chang, T.T.; Chiu, H.C.; Lin, Y.J.; Chiu, Y.C. Combined Transarterial Embolization/Chemoembolization-Based Locoregional Treatment with Sorafenib Prolongs the Survival in Patients with Advanced Hepatocellular Carcinoma and Preserved Liver Function: A Propensity Score Matching Study. Liver Cancer 2019, 8, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Chen, Y.C.; Chen, Y.Y.; Su, Y.C.; Ku, M.C.; Kuo, J.T.; Yoshida, G.J. Sorafenib with Transarterial Chemoembolization Achieves Improved Survival vs. Sorafenib Alone in Advanced Hepatocellular Carcinoma: A Nationwide Population-Based Cohort Study. Cancers 2019, 11, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Duan, Z.; Long, X.; Hertzanu, Y.; Shi, H.; Liu, S.; Yang, Z. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: A propensity score matching study. PLoS ONE 2014, 9, e96620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Chen, J.; Lai, L.; Meng, X.; Zhou, B.; Huang, W.; Cai, M.; Shan, H. Hepatocellular carcinoma with portal vein tumor thrombus: Treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology 2014, 272, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.M.; Ryoo, B.Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.H.; Lee, H.C.; Lim, Y.S. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 661–669. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, J.H.; Shim, J.H.; Yoon, S.M.; Kim, P.H.; Alrashidi, I. Chemoembolization Plus Radiotherapy Versus Chemoembolization Plus Sorafenib for the Treatment of Hepatocellular Carcinoma Invading the Portal Vein: A Propensity Score Matching Analysis. Cancers 2020, 12, 1116. [Google Scholar] [CrossRef]
- Chu, H.H.; Chun, S.Y.; Kim, J.H.; Kim, P.H.; Il Gwon, D.; Ko, H.K.; Kim, N. A prediction model for overall survival after transarterial chemoembolization for hepatocellular carcinoma invading the hepatic vein or inferior vena cava. Eur. Radiol. 2021, 31, 4232–4242. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Montal, R.; Torres, F.; Park, J.W.; Decaens, T.; Raoul, J.L.; Kudo, M.; Chang, C.; Ríos, J.; Boige, V.; et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J. Hepatol. 2017, 66, 1166–1172. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Chu, H.H.; Kim, J.H.; Kim, S.Y.; Alrashidi, I.; Gwon, D.I.; Yoon, H.K.; Kim, N. Clinical Significance of the Initial and Best Responses after Chemoembolization in the Treatment of Intermediate-Stage Hepatocellular Carcinoma with Preserved Liver Function. J. Vasc. Interv. Radiol. 2020, 31, 1998–2006.e1991. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Kudo, M.; Imanaka, K.; Chida, N.; Nakachi, K.; Tak, W.Y.; Takayama, T.; Yoon, J.H.; Hori, T.; Kumada, H.; Hayashi, N.; et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Aikata, H.; Izumi, N.; Yamasaki, T.; Hino, K.; Kuzuya, T.; Isoda, N.; et al. TACTICS: Final overall survival (OS) data from a randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy in combination with sorafenib as compared with TACE alone in patients (pts) with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 270. [Google Scholar]
- Silva, J.P.; Berger, N.G.; Tsai, S.; Christians, K.K.; Clarke, C.N.; Mogal, H.; White, S.; Rilling, W.; Gamblin, T.C. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: A systematic review and meta-analysis. HPB 2017, 19, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geschwind, J.F.; Kudo, M.; Marrero, J.A.; Venook, A.P.; Chen, X.P.; Bronowicki, J.P.; Dagher, L.; Furuse, J.; Ladrón de Guevara, L.; Papandreou, C.; et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology 2016, 279, 630–640. [Google Scholar] [CrossRef]
- Ren, B.; Wang, W.; Shen, J.; Li, W.; Ni, C.; Zhu, X. Transarterial Chemoembolization (TACE) Combined with Sorafenib versus TACE Alone for Unresectable Hepatocellular Carcinoma: A Propensity Score Matching Study. J. Cancer 2019, 10, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.H.; Kim, P.H.; Chu, H.H.; Gwon, D.I.; Ko, H.K. Emerging Trends in the Treatment of Advanced Hepatocellular Carcinoma: A Radiological Perspective. Korean J. Radiol. 2021, 22, e110. [Google Scholar] [CrossRef] [PubMed]

| Study Population before PSM | Study Population after PSM | |||||
|---|---|---|---|---|---|---|
| TACE + Sorafenib | TACE | p-Value | TACE + Sorafenib | TACE | p-Value | |
| Patients | 91 | 109 | 63 | 63 | ||
| Age (mean ± SD) | 56.2 ± 10.6 | 60.5 ± 11.1 | 0.005 | 58.0 ± 10.0 | 58.1 ± 10.4 | 0.941 |
| Male sex, n (%) | 78 (86) | 87 (80) | 0.351 | 52 (83) | 51 (81) | >0.999 |
| Etiology | 0.819 | 0.866 | ||||
| HBV | 71 (78) | 81 (74) | 48 (76) | 48 (76) | ||
| HCV | 6 (7) | 9 (8) | 3 (5) | 4 (6) | ||
| Others | 14 (15) | 19 (18) | 12 (19) | 11 (17) | ||
| ECOG PS | 0.225 | 0.838 | ||||
| 0 | 57 (63) | 78 (72) | 44 (70) | 42 (67) | ||
| 1 | 34 (37) | 31 (28) | 19 (30) | 21 (33) | ||
| Maximal tumor size (cm, mean ± SD) | 9.4 ± 3.5 | 9.0 ± 4.5 | 0.412 | 9.1 ± 3.4 | 9.1 ± 4.4 | 0.996 |
| Tumor number ≥ 4, n (%) | 18 (20) | 40 (37) | 0.012 | 15 (24) | 14 (22) | >0.999 |
| Tumor extent | 0.054 | >0.999 | ||||
| Unilobar | 70 (77) | 69 (63) | 47 (75) | 46 (73) | ||
| Bilobar | 21 (23) | 40 (37) | 16 (25) | 17 (27) | ||
| Bilirubin (mg/dL, mean ± SD) | 0.85 ± 0.47 | 0.84 ± 0.40 | 0.866 | 0.86 ± 0.49 | 0.86 ± 0.40 | 0.953 |
| Albumin ≤ 3.5 mg/dL, n (%) | 44 (48) | 56 (51) | 0.777 | 31 (49) | 34 (54) | 0.735 |
| AFP ≥ 400 ng/mL, n (%) | 51 (57) | 64 (59) | 0.775 | 37 (59) | 36 (57) | >0.999 |
| Child-Pugh score B, n (%) | 7 (8) | 17 (16) | 0.135 | 6 (10) | 6 (10) | >0.999 |
| Tumor type | 0.257 | 0.265 | ||||
| Nodular | 36 (40) | 52 (48) | 26 (41) | 19 (30) | ||
| Infiltrative | 55 (60) | 57 (52) | 37 (59) | 44 (70) | ||
| Main PV or IVC invasion | 0.016 | 0.773 | ||||
| Yes | 20 (22) | 10 (9) | 7 (11) | 9 (14) | ||
| No | 71 (78) | 99 (91) | 56 (89) | 54 (86) | ||
| Analysis | HR | 95% CI | p-Value | |
|---|---|---|---|---|
| PFS | ||||
| Unadjusted | 0.622 | 0.459 | 0.842 | 0.002 |
| Adjusted | 0.651 | 0.476 | 0.890 | 0.007 |
| Propensity score matched * | 0.463 | 0.310 | 0.693 | <0.001 |
| Propensity score matched and adjusted for selected variables † | 0.443 | 0.286 | 0.687 | <0.001 |
| OS | ||||
| Unadjusted | 0.701 | 0.514 | 0.955 | 0.024 |
| Adjusted | 0.750 | 0.543 | 1.035 | 0.080 |
| Propensity score matched * | 0.583 | 0.399 | 0.853 | 0.005 |
| Propensity score matched and adjusted for selected variables ‡ | 0.574 | 0.377 | 0.872 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.H.; Choi, S.L.; Kim, J.H.; Shim, J.H.; Alali, M.; Kim, N. Sorafenib Combined with Chemoembolization for Locally Advanced Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Propensity Score Analysis. Life 2021, 11, 1066. https://doi.org/10.3390/life11101066
Kim GH, Choi SL, Kim JH, Shim JH, Alali M, Kim N. Sorafenib Combined with Chemoembolization for Locally Advanced Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Propensity Score Analysis. Life. 2021; 11(10):1066. https://doi.org/10.3390/life11101066
Chicago/Turabian StyleKim, Gun Ha, Sang Lim Choi, Jin Hyoung Kim, Ju Hyun Shim, Meshari Alali, and Nayoung Kim. 2021. "Sorafenib Combined with Chemoembolization for Locally Advanced Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Propensity Score Analysis" Life 11, no. 10: 1066. https://doi.org/10.3390/life11101066
APA StyleKim, G. H., Choi, S. L., Kim, J. H., Shim, J. H., Alali, M., & Kim, N. (2021). Sorafenib Combined with Chemoembolization for Locally Advanced Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Propensity Score Analysis. Life, 11(10), 1066. https://doi.org/10.3390/life11101066







