Effects of Magnitude of Leading Stimulus on Prepulse Inhibition of Auditory Evoked Cerebral Responses: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
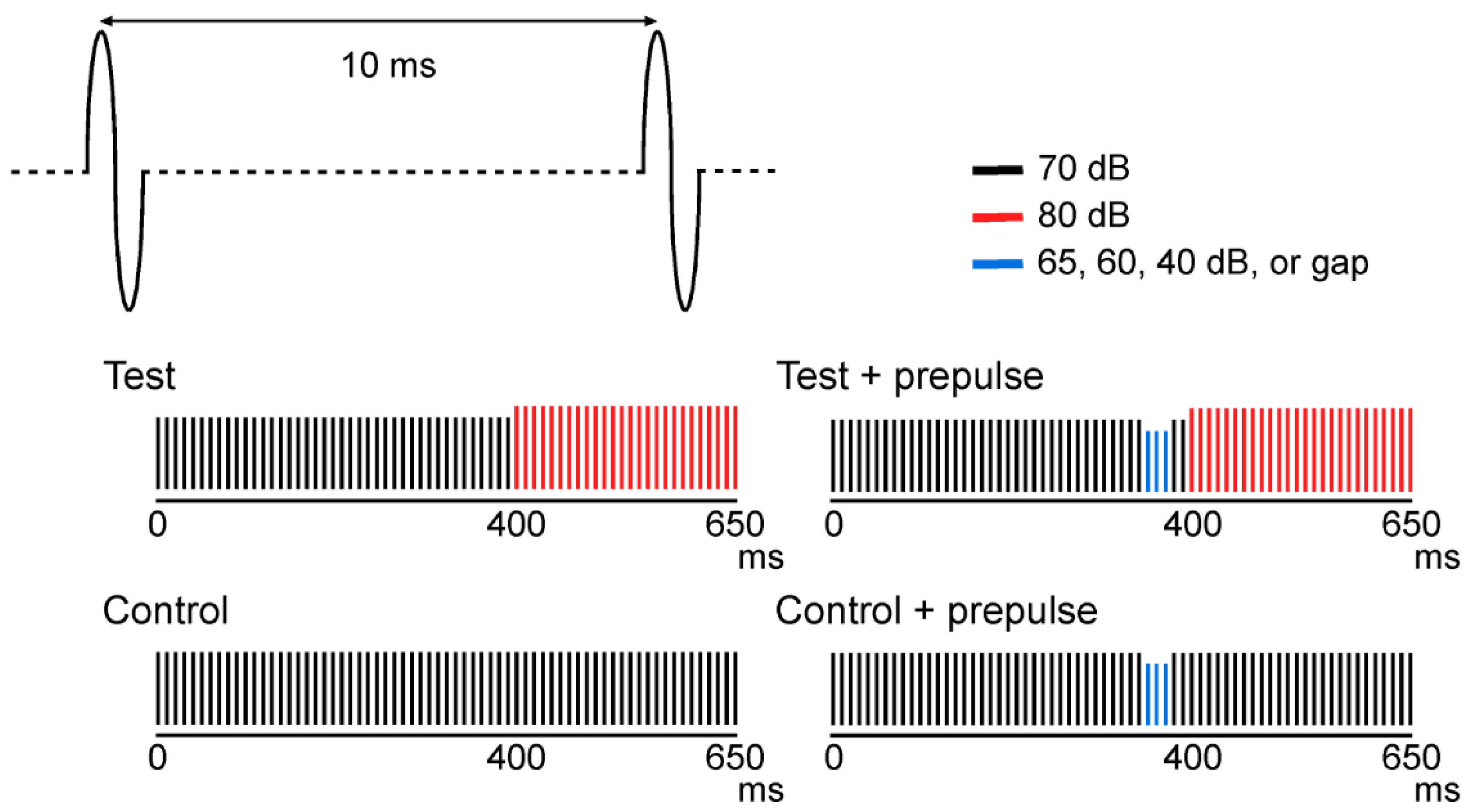
2.2. Stimuli
2.3. MEG Recordings
2.4. Analysis
3. Results
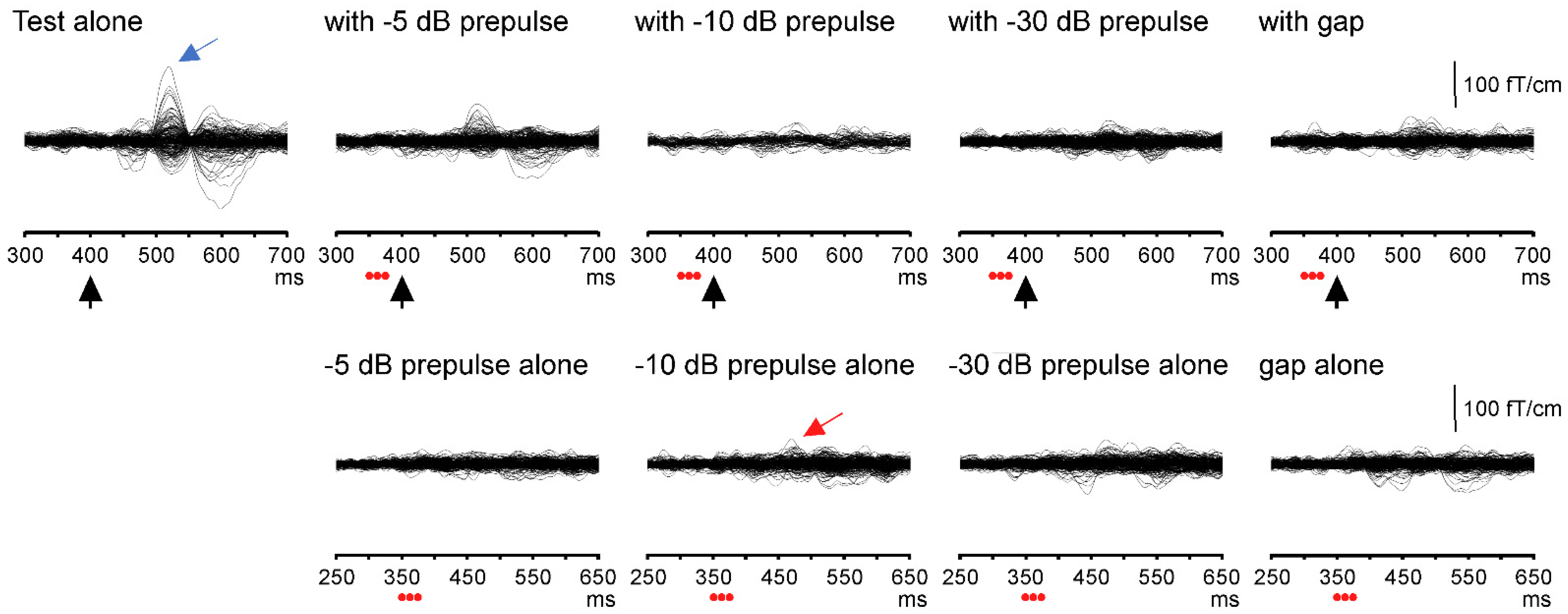
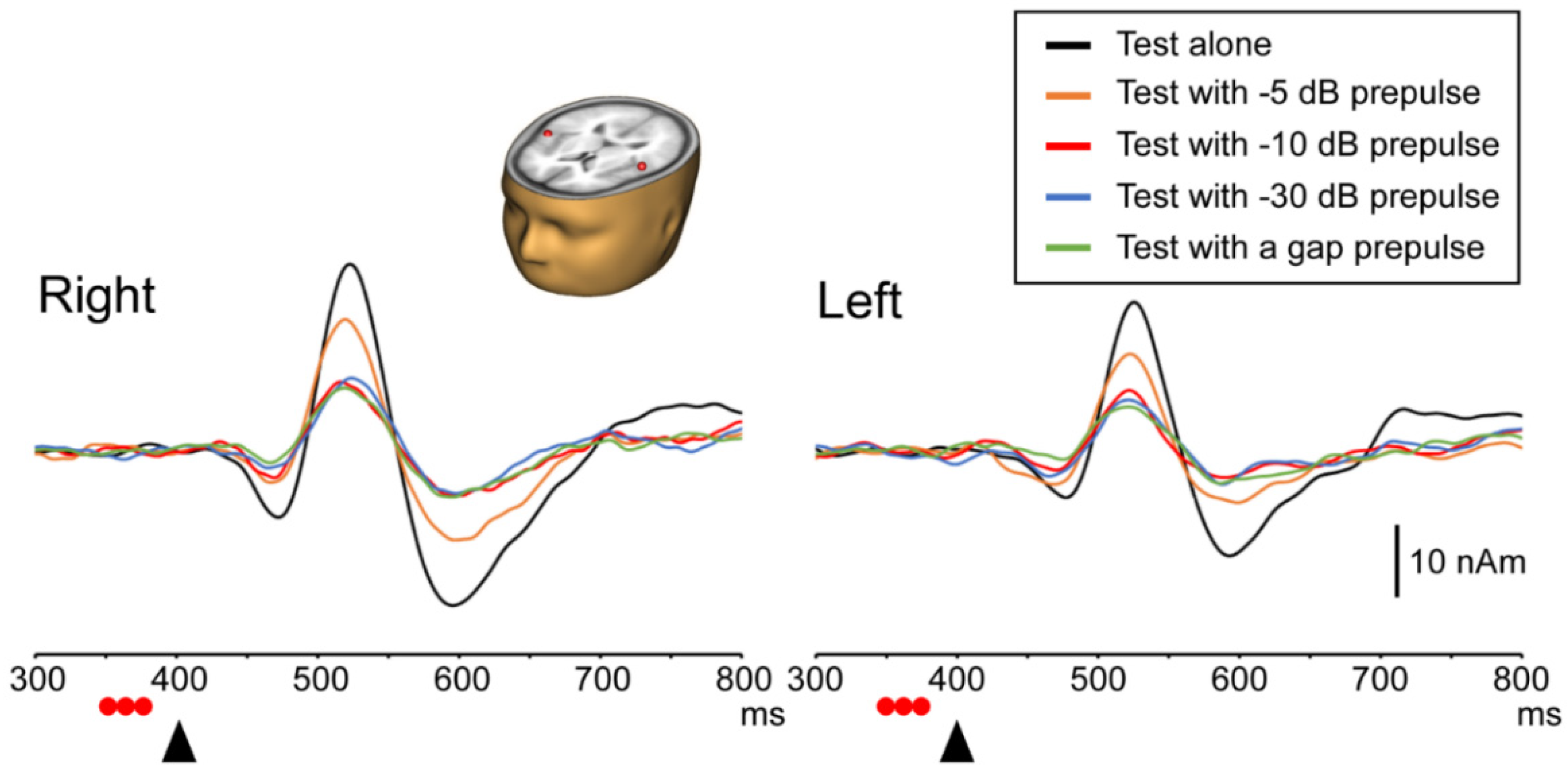
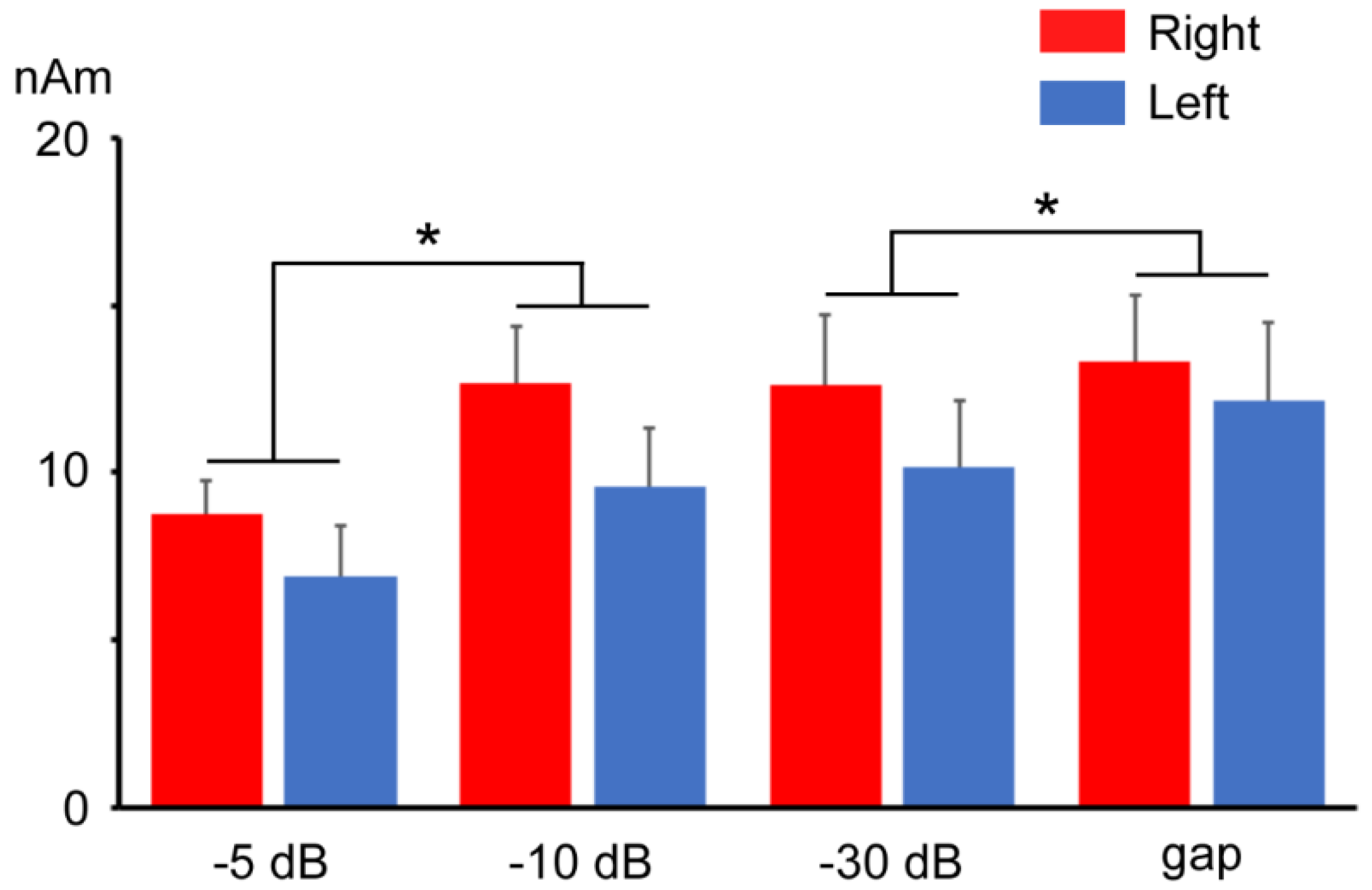
3.1. Test Response with and without a Prepulse
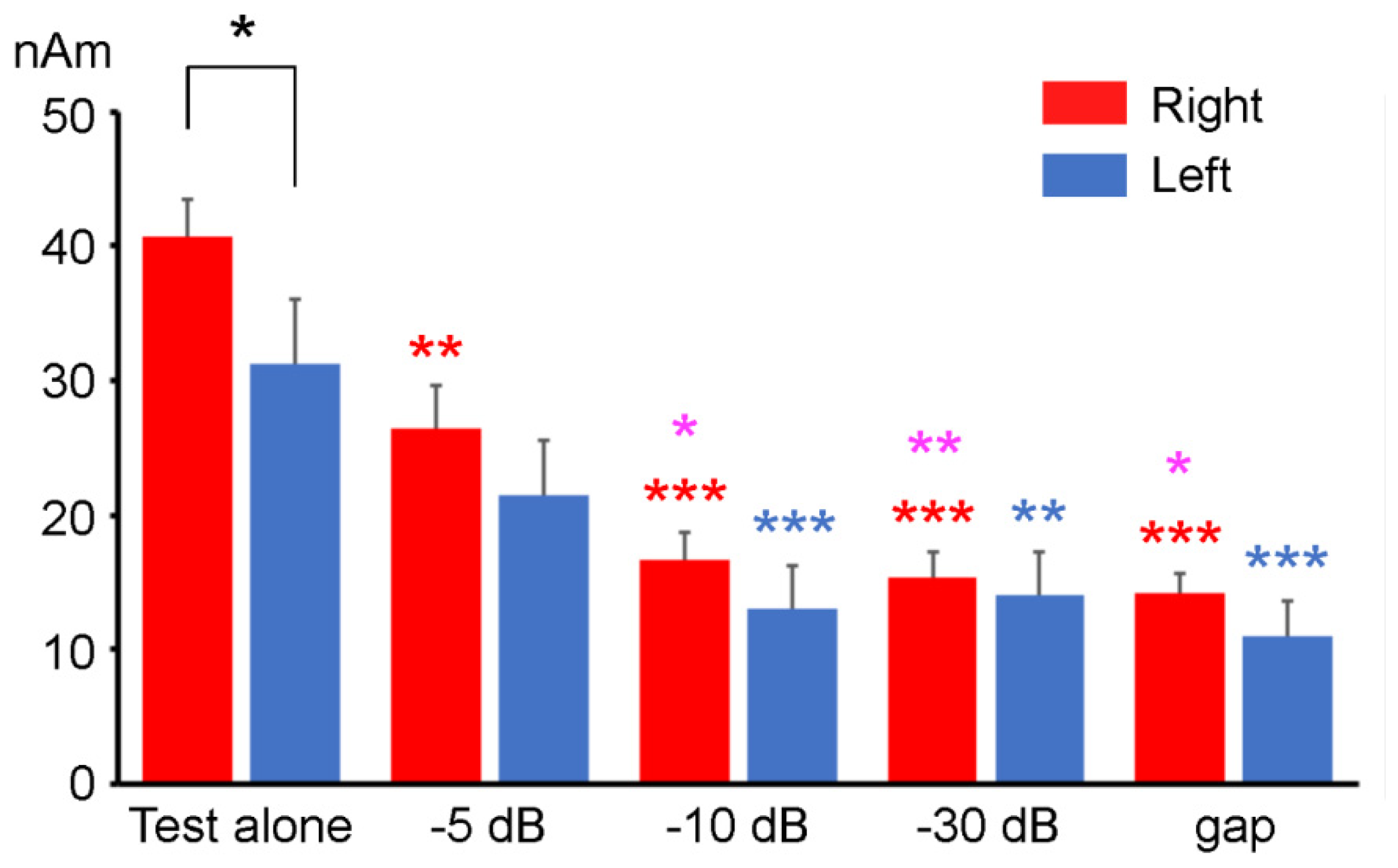
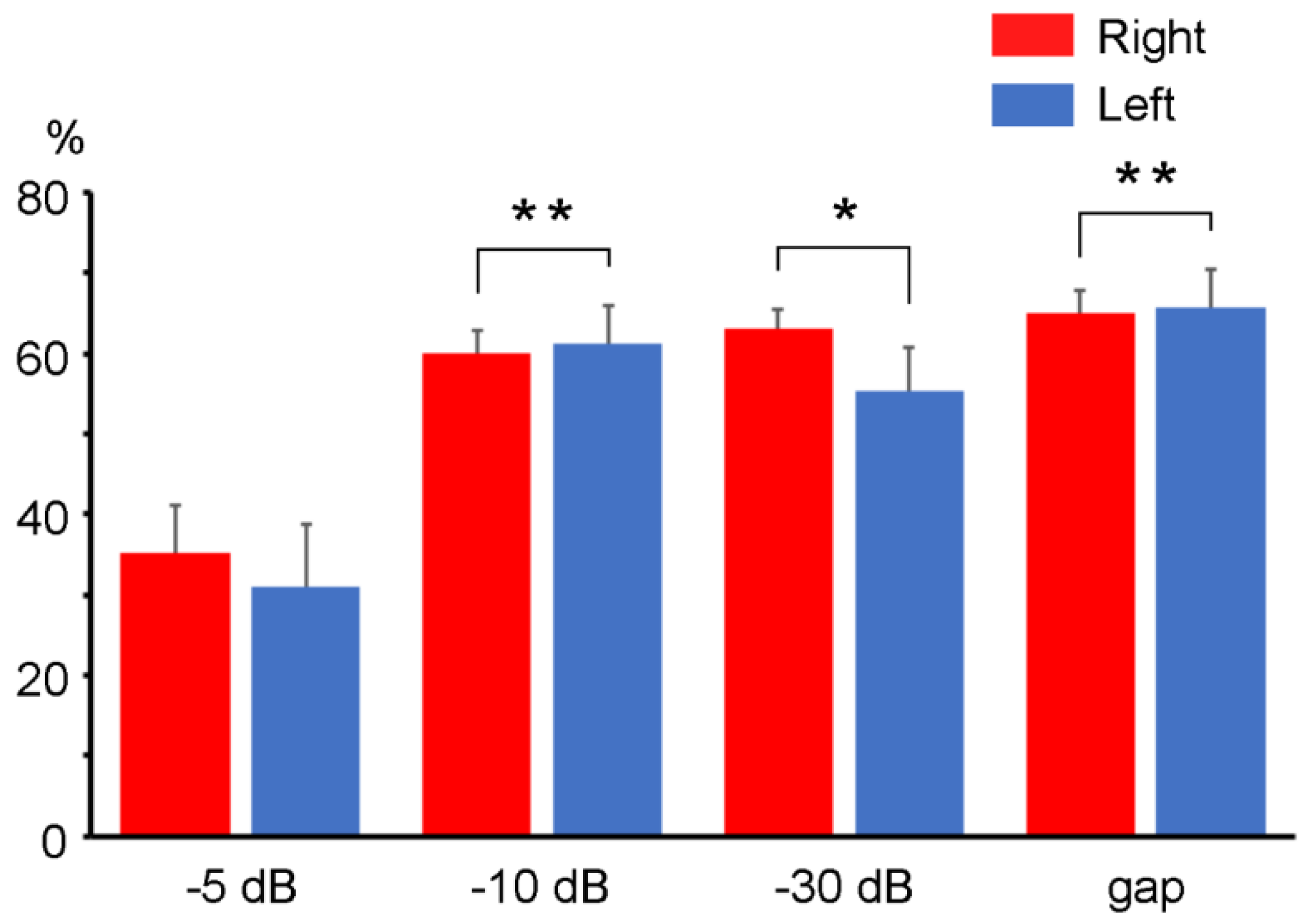
3.2. PPI
3.3. Responses to Prepulse Alone Stimuli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, F.K. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology 1975, 12, 238–248. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef]
- Kohl, S.; Heekeren, K.; Klosterkötter, J.; Kuhn, J. Prepulse inhibition in psychiatric disorders—Apart from schizophrenia. J. Psychiatr. Res. 2013, 47, 445–452. [Google Scholar] [CrossRef]
- Takahashi, H.; Hashimoto, R.; Iwase, M.; Ishii, R.; Kamio, Y.; Takeda, M. Prepulse inhibition of startle response: Recent advances in human studies of psychiatric disease. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2011, 9, 102–110. [Google Scholar] [CrossRef]
- Khan, A.; Powell, S.B. Sensorimotor gating deficits in “two-hit” models of schizophrenia risk factors. Schizophr. Res. 2018, 198, 68–83. [Google Scholar] [CrossRef]
- Powell, S.B.; Weber, M.; Geyer, M.A. Genetic models of sensorimotor gating: Relevance to neuropsychiatric disorders. Curr. Top. Behav. Neurosci. 2012, 12, 251–318. [Google Scholar] [CrossRef]
- Inui, K.; Urakawa, T.; Yamashiro, K.; Otsuru, N.; Nishihara, M.; Takeshima, Y.; Keceli, S.; Kakigi, R. Non-linear laws of echoic memory and auditory change detection in humans. BMC Neurosci. 2010, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Pratt, H.; Bleich, N.; Mittelman, N. The composite N1 component to gaps in noise. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2005, 116, 2648–2663. [Google Scholar] [CrossRef]
- Pratt, H.; Starr, A.; Michalewski, H.J.; Bleich, N.; Mittelman, N. The N1 complex to gaps in noise: Effects of preceding noise duration and intensity. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007, 118, 1078–1087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akiyama, L.F.; Yamashiro, K.; Inui, K.; Kakigi, R. Automatic cortical responses to sound movement: A magnetoencephalography study. Neurosci. Lett. 2011, 488, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ohoyama, K.; Motomura, E.; Inui, K.; Nishihara, M.; Otsuru, N.; Oi, M.; Kakigi, R.; Okada, M. Memory-based pre-attentive auditory N1 elicited by sound movement. Neurosci. Res. 2012, 73, 248–251. [Google Scholar] [CrossRef]
- Yamashiro, K.; Inui, K.; Otsuru, N.; Kakigi, R. Change-related responses in the human auditory cortex: An MEG study. Psychophysiology 2011, 48, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, A.; Lolli, B.; Michalewski, H.J.; Pratt, H.; Zeng, F.G.; Starr, A. Intensity changes in a continuous tone: Auditory cortical potentials comparison with frequency changes. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2009, 120, 374–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motomura, E.; Inui, K.; Kawano, Y.; Nishihara, M.; Okada, M. Effects of Sound-Pressure Change on the 40 Hz Auditory Steady-State Response and Change-Related Cerebral Response. Brain Sci. 2019, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Otsuru, N.; Inui, K.; Kakigi, R. Change-related auditory P50: A MEG study. NeuroImage 2014, 86, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Inui, K.; Motomura, E.; Otsuru, N.; Ushida, T.; Kakigi, R. Auditory N1 as a change-related automatic response. Neurosci. Res. 2011, 71, 145–148. [Google Scholar] [CrossRef]
- Inui, K.; Tsuruhara, A.; Kodaira, M.; Motomura, E.; Tanii, H.; Nishihara, M.; Keceli, S.; Kakigi, R. Prepulse inhibition of auditory change-related cortical responses. BMC Neurosci. 2012, 13, 135. [Google Scholar] [CrossRef]
- Motomura, E.; Inui, K.; Nishihara, M.; Tanahashi, M.; Kakigi, R.; Okada, M. Prepulse Inhibition of the Auditory Off-Response: A Magnetoencephalographic Study. Clin. EEG Neurosci. 2018, 49, 152–158. [Google Scholar] [CrossRef]
- Fujii, S.; Motomura, E.; Inui, K.; Watanabe, T.; Hakumoto, Y.; Higuchi, K.; Kawano, Y.; Morimoto, M.; Nakatani, K.; Okada, M. Weaker prepulse exerts stronger suppression of a change-detecting neural circuit. Neurosci. Res. 2021, 170, 195–200. [Google Scholar] [CrossRef]
- Watanabe, T.; Motomura, E.; Kawano, Y.; Fujii, S.; Hakumoto, Y.; Morimoto, M.; Nakatani, K.; Okada, M.; Inui, K. Electrical field distribution of Change-N1 and its prepulse inhibition. Neurosci. Lett. 2021, 751, 135804. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Ku, Y.; Ahn, J.W.; Kwon, C.; Kim, D.Y.; Suh, M.W.; Park, M.K.; Lee, J.H.; Oh, S.H.; Kim, H.C. The gap-prepulse inhibition deficit of the cortical N1-P2 complex in patients with tinnitus: The effect of gap duration. Hear Res. 2017, 348, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Ahn, J.W.; Kwon, C.; Suh, M.W.; Lee, J.H.; Oh, S.H.; Kim, H.C. Gap prepulse inhibition of the auditory late response in healthy subjects. Psychophysiology 2015, 52, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.Y.; Park, I. A Gap Prepulse with a Principal Stimulus Yields a Combined Auditory Late Response. J. Audiol. Otol. 2020, 24, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Nakagawa, K.; Nishihara, M.; Motomura, E.; Kakigi, R. Inhibition in the Human Auditory Cortex. PLoS ONE 2016, 11, e0155972. [Google Scholar] [CrossRef]
- Nakagawa, K.; Inui, K.; Yuge, L.; Kakigi, R. Inhibition of somatosensory-evoked cortical responses by a weak leading stimulus. NeuroImage 2014, 101, 416–424. [Google Scholar] [CrossRef]
- Inui, K.; Takeuchi, N.; Sugiyama, S.; Motomura, E.; Nishihara, M. GABAergic mechanisms involved in the prepulse inhibition of auditory evoked cortical responses in humans. PLoS ONE 2018, 13, e0190481. [Google Scholar] [CrossRef]
- Takeuchi, N.; Sugiyama, S.; Inui, K.; Kanemoto, K.; Nishihara, M. New paradigm for auditory paired pulse suppression. PLoS ONE 2017, 12, e0177747. [Google Scholar] [CrossRef]
- Jovanovic, T.; Szilagyi, S.; Chakravorty, S.; Fiallos, A.M.; Lewison, B.J.; Parwani, A.; Schwartz, M.P.; Gonzenbach, S.; Rotrosen, J.P.; Duncan, E.J. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology 2004, 41, 401–406. [Google Scholar] [CrossRef]
- Takeuchi, N.; Kinukawa, T.; Sugiyama, S.; Inui, K.; Nishihara, M. Test-retest reliability of prepulse inhibition paradigm using auditory evoked potentials. Neurosci. Res. 2021, 170, 187–194. [Google Scholar] [CrossRef]
- Kodaira, M.; Tsuruhara, A.; Motomura, E.; Tanii, H.; Inui, K.; Kakigi, R. Effects of acute nicotine on prepulse inhibition of auditory change-related cortical responses. Behav. Brain Res. 2013, 256, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Otsuru, N.; Tsuruhara, A.; Motomura, E.; Tanii, H.; Nishihara, M.; Inui, K.; Kakigi, R. Effects of acute nicotine on auditory change-related cortical responses. Psychopharmacology 2012, 224, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, M.; Motomura, E.; Inui, K.; Ohoyama, K.; Tanii, H.; Konishi, Y.; Shiroyama, T.; Nishihara, M.; Kakigi, R.; Okada, M. Auditory change-related cerebral responses and personality traits. Neurosci. Res. 2016, 103, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hormigo, S.; Cardoso, A.; Sancho, C.; López, D.E.; Moreno, C. Associations between sensorimotor gating mechanisms and athletic performance in a variety of physical conditioning tests. Eur. J. Appl. Physiol. 2019, 119, 921–932. [Google Scholar] [CrossRef]
| Test Alone | Test with a Prepulse | ||||
|---|---|---|---|---|---|
| −5 dB | −10 dB | −30 dB | gap | ||
| Test response | |||||
| Right | 116.2 ± 27.5 | 110.0 ± 25.8 | 116.7 ± 30.9 | 118.7 ± 29.1 | 123.4 ± 13.9 |
| Left | 126.4 ± 8.5 | 122.0 ± 8.4 | 121.4 ± 10.1 | 126.8 ± 16.4 | 125.2 ± 15.1 |
| Prepulse Alone | |||||
| −5 dB | −10 dB | −30 dB | gap | ||
| Prepulse response | |||||
| Right | 145.4 ± 25.5 | 127.8 ± 25.1 | 134.7 ± 20.0 | 138.2 ± 23.5 | |
| Left | 151.5 ± 33.1 | 133.2 ± 22.1 | 134.5 ± 18.1 | 141.5 ± 23.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, Y.; Motomura, E.; Inui, K.; Okada, M. Effects of Magnitude of Leading Stimulus on Prepulse Inhibition of Auditory Evoked Cerebral Responses: An Exploratory Study. Life 2021, 11, 1024. https://doi.org/10.3390/life11101024
Kawano Y, Motomura E, Inui K, Okada M. Effects of Magnitude of Leading Stimulus on Prepulse Inhibition of Auditory Evoked Cerebral Responses: An Exploratory Study. Life. 2021; 11(10):1024. https://doi.org/10.3390/life11101024
Chicago/Turabian StyleKawano, Yasuhiro, Eishi Motomura, Koji Inui, and Motohiro Okada. 2021. "Effects of Magnitude of Leading Stimulus on Prepulse Inhibition of Auditory Evoked Cerebral Responses: An Exploratory Study" Life 11, no. 10: 1024. https://doi.org/10.3390/life11101024
APA StyleKawano, Y., Motomura, E., Inui, K., & Okada, M. (2021). Effects of Magnitude of Leading Stimulus on Prepulse Inhibition of Auditory Evoked Cerebral Responses: An Exploratory Study. Life, 11(10), 1024. https://doi.org/10.3390/life11101024






