Serum Cytokine Profile, Beta-Hexosaminidase A Enzymatic Activity and GM2 Ganglioside Levels in the Plasma of a Tay-Sachs Disease Patient after Cord Blood Cell Transplantation and Curcumin Administration: A Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Plasma and Serum from Blood
2.2. Multiplex Analysis of Cytokine/Chemokine Levels
2.3. Determination of HexA Enzymatic Activity
2.4. Determination of HexA Concentration by ELISA
2.5. Mass Spectrometry Analysis of GM2 Plasma Levels
2.6. Statistical Analysis
3. Case Presentation
3.1. Patient Information
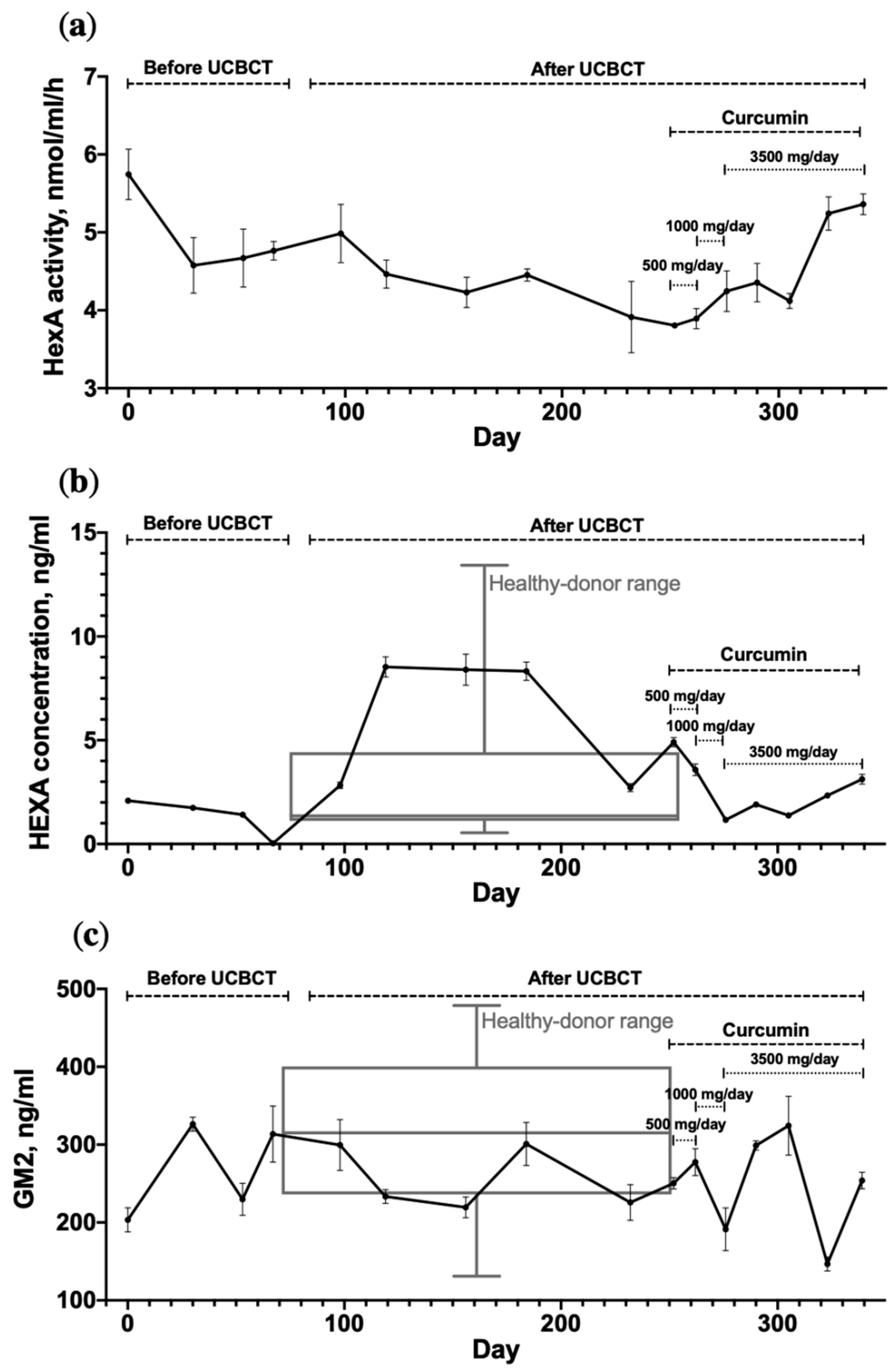
3.2. Dynamic Changes in HexA Activity and the GM2 Level in the TSD Patient’s Plasma
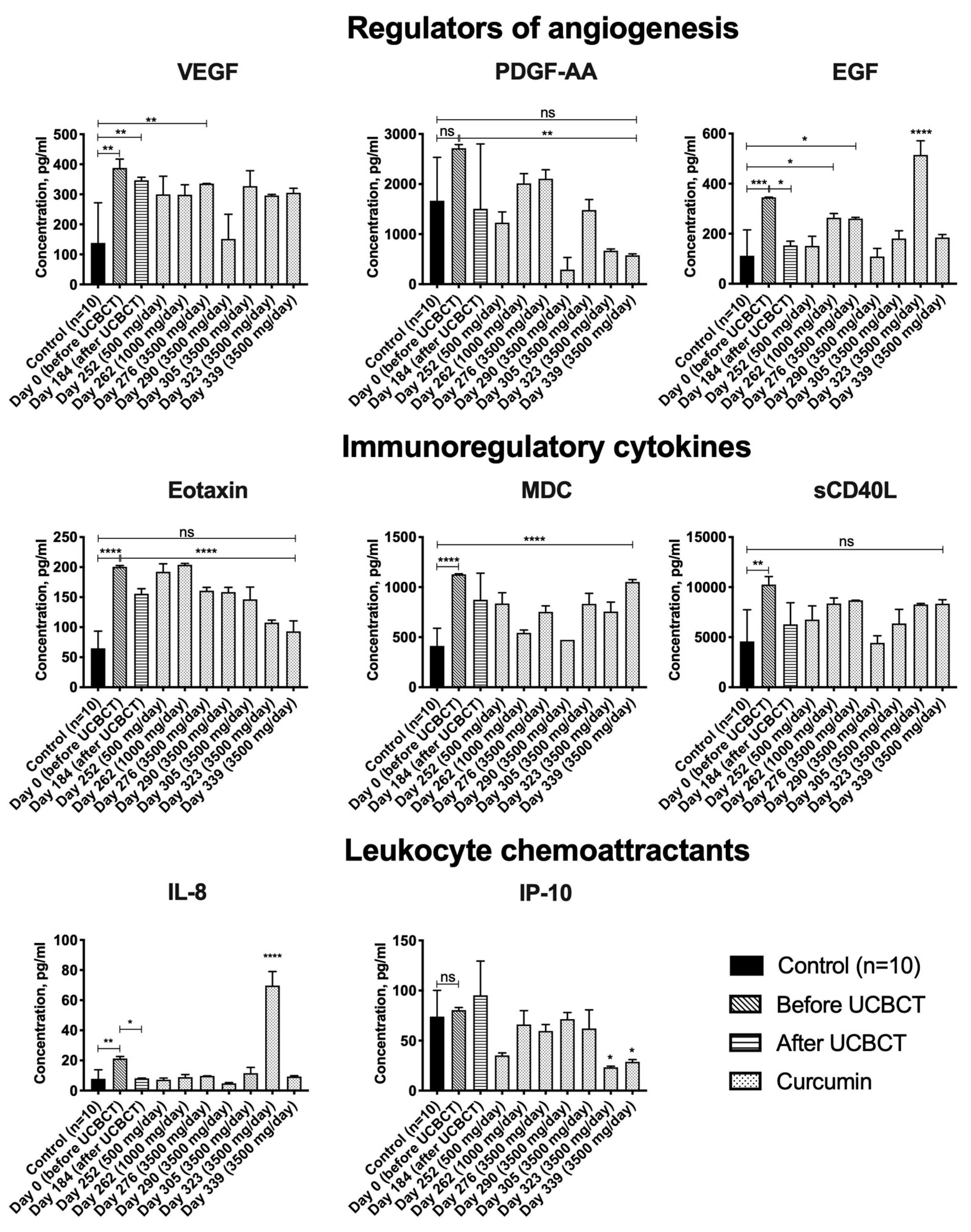
3.3. Cytokine Profile
4. Discussion
4.1. UCBCT and Curcumin Administration Fail to Affect GM2 Levels in the Patient’s Plasma
4.2. UCBCT and Curcumin Administration Fail to Affect the Activity of HexA in the Patient’s Plasma, but Patient’s Serum HexA Concentration Increases after UCBCT
4.3. The Levels of VEGF, EGF, Eotaxin-1, MDC, sCD40L and IL-8 Are Elevated in the Blood Serum of the Patient with TSD
4.4. EGF and IL-8 Levels Decrease in the TSD Patient’s Serum after UCBCT
4.5. PDGF-AA, Eotaxin-1, IP-10 and sCD40L Levels Are Reduced in the TSD Patient’s Serum after Administration of Curcumin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AACT | alpha-1-antichymotrypsin |
| Apo A-I | apolipoprotein A-I |
| BMT | bone marrow transplantation |
| CCL11 | C-C motif chemokine 11 |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CX3CL1 | C-X3-C motif ligand 1 |
| EGF | epidermal growth factor |
| ENA-78 | epithelial-neutrophil activating peptide 78 |
| FGF-2 | fibroblast growth factor 2 |
| Flt-3 ligand | fms-related tyrosine kinase 3 ligand |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GRO | growth-regulated oncogene |
| HB-EGF | heparin-binding EGF-like growth factor |
| Hex | hexosaminidase |
| HexA | hexosaminidase A |
| HGF | hepatocyte growth factor |
| HLA | human leukocyte antigens |
| IFN | interferon |
| IGFBP-2 | insulin-like growth factor-binding protein 2 |
| IL | interleukin |
| IP-10 | interferon gamma-induced protein 10 |
| LSD | lysosomal storage disorders |
| MCP | monocyte chemoattractant protein |
| MDC/CCL22 | macrophage-derived chemokine/C-C motif chemokine 22 |
| MIF | macrophage migration inhibitory factor |
| MIP | macrophage inflammatory protein |
| MMP-3 | matrix metalloproteinase 3 |
| MUGS | 4-methylumbelliferyl-beta-D-N-acetyl-glucosamine-6-sulfate |
| NMD | nonsense-mediated mRNA decay |
| PDGF | platelet-derived growth factor |
| RANTES/CCL5 | chemokine (C-C motif) ligand 5 |
| sCD40L | soluble CD40 ligand |
| SHBG | sex hormone binding globulin |
| TFEB | transcription factor EB |
| TGF | transforming growth factor |
| THP | tamm-horsfall urinary glycoprotein |
| TNF | tumor necrosis factor |
| TNFR2 | tumor necrosis factor receptor 2 |
| TSD | Tay-Sachs disease |
| UCBCT | umbilical cord blood cell transplantation |
| VEGF | vascular endothelial growth factor |
References
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New Approaches to Tay-Sachs Disease Therapy. Front. Physiol. 2018, 9, 1663. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Rizvanov, A.A. Tay-Sachs disease: Diagnostic, modeling and treatment approaches. Genes Cells 2020, 15, 17–22. [Google Scholar] [CrossRef]
- Lew, R.M.; Burnett, L.; Proos, A.L.; Delatycki, M.B. Tay-Sachs disease: Current perspectives from Australia. Appl. Clin. Genet. 2015, 8, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Dersh, D.; Iwamoto, Y.; Argon, Y. Tay-Sachs disease mutations in HEXA target the alpha chain of hexosaminidase A to endoplasmic reticulum-associated degradation. Mol. Biol. Cell 2016, 27, 3813–3827. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Magini, A.; Polchi, A.; Di Meo, D.; Buratta, S.; Chiaradia, E.; Germani, R.; Emiliani, C.; Tancini, B. Curcumin Analogue C1 Promotes Hex and Gal Recruitment to the Plasma Membrane via mTORC1-Independent TFEB Activation. Int. J. Mol. Sci. 2019, 20, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Kang, J.H.; Lee, S. Autophagy in Neurodegenerative Diseases: A Hunter for Aggregates. Int. J. Mol. Sci. 2020, 21, 3369. [Google Scholar] [CrossRef]
- Feng, D.; Su, R.C.; Zou, L.; Triggs-Raine, B.; Huang, S.; Xie, J. Increase of a group of PTC(+) transcripts by curcumin through inhibition of the NMD pathway. Biochim. Biophys. Acta 2015, 1849, 1104–1115. [Google Scholar] [CrossRef]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients with Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 430–438. [Google Scholar] [CrossRef] [Green Version]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.; Joseph, P.D.; Rose, K.J.; Ryan, M.M.; Ouvrier, R.A. Effect of oral curcumin on Dejerine-Sottas disease. Pediatr. Neurol. 2009, 41, 305–308. [Google Scholar] [CrossRef]
- Masoumi, A.; Goldenson, B.; Ghirmai, S.; Avagyan, H.; Zaghi, J.; Abel, K.; Zheng, X.; Espinosa-Jeffrey, A.; Mahanian, M.; Liu, P.T.; et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2009, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.L.; Carter, S.L.; Kernan, N.A.; Sahdev, I.; Wall, D.; Pietryga, D.; Wagner, J.E.; Kurtzberg, J. Results of the cord blood transplantation study (COBLT): Outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol. Blood Marrow Transpl. 2006, 12, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Stepien, K.M.; Lum, S.H.; Wraith, J.E.; Hendriksz, C.J.; Church, H.J.; Priestman, D.; Platt, F.M.; Jones, S.; Jovanovic, A.; Wynn, R. Haematopoietic Stem Cell Transplantation Arrests the Progression of Neurodegenerative Disease in Late-Onset Tay-Sachs Disease. JIMD Rep. 2018, 41, 17–23. [Google Scholar] [CrossRef]
- Islamov, R.R.; Rizvanov, A.A.; Mukhamedyarov, M.A.; Ildusovich Salafutdinov, I.; Garanina, E.E.; Fedotova, V.Y.; Solovyeva, V.V.; Mukhamedshina, Y.O.; Safiullov, Z.Z.; Izmailov, A.A.; et al. Symptomatic improvement, increased life-span and sustained cell homing in amyotrophic lateral sclerosis after transplantation of human umbilical cord blood cells genetically modified with adeno-viral vectors expressing a neuro-protective factor and a neural cell adhesion molecule. Curr. Gene 2015, 15, 266–276. [Google Scholar]
- Mukhamedyarov, M.A.; Rizvanov, A.A.; Safiullov, Z.Z.; Izmailov, A.A.; Sharifullina, G.A.; Solovieva, V.V.; Fedotova, V.Y.; Ildusovich Salafutdinov, I.; Cherenkova, E.E.; Bashirov, F.V.; et al. Analysis of the efficiency of gene-cell therapy in transgenic mice with amyotrophic lateral sclerosis phenotype. Bull. Exp. Biol. Med. 2013, 154, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Escolar, M.L.; Poe, M.D.; Provenzale, J.M.; Richards, K.C.; Allison, J.; Wood, S.; Wenger, D.A.; Pietryga, D.; Wall, D.; Champagne, M.; et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N. Engl. J. Med. 2005, 352, 2069–2081. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.; Zeng, B.; Torres, P.A.; Pastores, G.M.; Kolodny, E.H.; Kurtzberg, J.; Krivit, W. Globoid cell leukodystrophy (Krabbe disease): Normal umbilical cord blood galactocerebrosidase activity and polymorphic mutations. J. Inherit. Metab. Dis. 2005, 28, 1005–1009. [Google Scholar] [CrossRef]
- Lehman, A.M.; Schultz, K.R.; Poskitt, K.; Bjornson, B.; Keyes, R.; Waters, P.J.; Clarke, L.A.; Everett, R.; McConnell, D.; Stockler, S. Intracranial calcification after cord blood neonatal transplantation for krabbe disease. Neuropediatrics 2009, 40, 189–191. [Google Scholar] [CrossRef]
- Escolar, M.L.; Poe, M.D.; Martin, H.R.; Kurtzberg, J. A staging system for infantile Krabbe disease to predict outcome after unrelated umbilical cord blood transplantation. Pediatrics 2006, 118, e879–e889. [Google Scholar] [CrossRef] [Green Version]
- Yagasaki, H.; Kato, M.; Ishige, M.; Shichino, H.; Chin, M.; Mugishima, H. Successful cord blood transplantation in a 42-day-old boy with infantile Krabbe disease. Int. J. Hematol. 2011, 93, 566–568. [Google Scholar] [CrossRef]
- Martin, H.R.; Poe, M.D.; Provenzale, J.M.; Kurtzberg, J.; Mendizabal, A.; Escolar, M.L. Neurodevelopmental outcomes of umbilical cord blood transplantation in metachromatic leukodystrophy. Biol. Blood Marrow Transplant. 2013, 19, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Pierson, T.M.; Bonnemann, C.G.; Finkel, R.S.; Bunin, N.; Tennekoon, G.I. Umbilical cord blood transplantation for juvenile metachromatic leukodystrophy. Ann. Neurol. 2008, 64, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Garty, B.Z.; Dror, Y.; Fenig, E.; Zeigler, M.; Yaniv, I. Successful treatment of Wolman disease by unrelated umbilical cord blood transplantation. Eur. J. Pediatr. 2007, 166, 663–666. [Google Scholar] [CrossRef]
- Shibazaki, T.; Hirabayashi, K.; Saito, S.; Shigemura, T.; Nakazawa, Y.; Sakashita, K.; Takagi, M.; Shiohara, M.; Adachi, K.; Nanba, E.; et al. Clinical and laboratory outcomes after umbilical cord blood transplantation in a patient with mucolipidosis II alpha/beta. Am. J. Med. Genet. Part A 2016, 170A, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.F.; Gassas, A.; Doyle, J.; Clarke, J.T. Unsuccessful treatment attempt: Cord blood stem cell transplantation in a patient with Niemann-Pick disease type A. J. Inherit. Metab. Dis. 2007, 30, 987. [Google Scholar] [CrossRef] [PubMed]
- Aldenhoven, M.; Kurtzberg, J. Cord blood is the optimal graft source for the treatment of pediatric patients with lysosomal storage diseases: Clinical outcomes and future directions. Cytotherapy 2015, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Aimaletdinov, A.M.; Rizvanov, A.A. Functionality of a bicistronic construction containing HEXA and HEXB genes encoding beta-hexosaminidase A for cell-mediated therapy of GM2 gangliosidoses. Neural Regen. Res. 2022, 17, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Tifft, C.J.; Soldin, S.J. Simultaneous quantification of GM1 and GM2 gangliosides by isotope dilution tandem mass spectrometry. Clin. Biochem. 2008, 41, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Duplock, S.; Hein, L.K.; Rigat, B.A.; Mahuran, D.J. Liquid chromatography/electrospray ionisation-tandem mass spectrometry quantification of GM2 gangliosides in human peripheral cells and plasma. Anal. Biochem. 2014, 458, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhou, X.; Liu, D.; Xin, B.; Cechner, K.; Wang, H.; Zhou, A. A new liquid chromatography/tandem mass spectrometry method for quantification of gangliosides in human plasma. Anal. Biochem. 2014, 455, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.D.; Chavez, J.L.; Mechref, Y. Rapid and sensitive LC-ESI-MS of gangliosides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 947–948, 1–7. [Google Scholar] [CrossRef]
- Hayase, T.; Shimizu, J.; Goto, T.; Nozaki, Y.; Mori, M.; Takahashi, N.; Namba, E.; Yamagata, T.; Momoi, M.Y. Unilaterally and rapidly progressing white matter lesion and elevated cytokines in a patient with Tay-Sachs disease. Brain Dev. 2010, 32, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Utz, J.R.; Crutcher, T.; Schneider, J.; Sorgen, P.; Whitley, C.B. Biomarkers of central nervous system inflammation in infantile and juvenile gangliosidoses. Mol. Genet. Metab. 2015, 114, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Khaiboullina, S.F.; Martynova, E.V.; Bardakov, S.N.; Mavlikeev, M.O.; Yakovlev, I.A.; Isaev, A.A.; Deev, R.V.; Rizvanov, A.A. Serum Cytokine Profile in a Patient Diagnosed with Dysferlinopathy. Case Rep. Med. 2017, 2017, 3615354. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Choi, E.N.; Jeon, Y.J.; Jung, S.C. Possible role of transforming growth factor-beta1 and vascular endothelial growth factor in Fabry disease nephropathy. Int. J. Mol. Med. 2012, 30, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Zampetti, A.; Gnarra, M.; Borsini, W.; Giurdanella, F.; Antuzzi, D.; Piras, A.; Smaldone, C.; Pieroni, M.; Cadeddu, C.; de Waure, C.; et al. Vascular endothelial growth factor (VEGF-a) in Fabry disease: Association with cutaneous and systemic manifestations with vascular involvement. Cytokine 2013, 61, 933–939. [Google Scholar] [CrossRef]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef] [Green Version]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Smagula, S.F.; Karim, H.T.; Lenze, E.J.; Butters, M.A.; Wu, G.F.; Mulsant, B.H.; Reynolds, C.F.; Aizenstein, H.J. Gray matter regions statistically mediating the cross-sectional association of eotaxin and set-shifting among older adults with major depressive disorder. Int. J. Geriatr. Psychiatry 2017, 32, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.R.; Sutton, J.M.; Belizaire, R.M.; Friend, L.A.; Schuster, R.M.; Johannigman, T.A.; Miller, S.G.; Lentsch, A.B.; Pritts, T.A. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock 2014, 42, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Itoh, M.; Okamura, T.; Kimura, M.; Yokoyama, A.; Yoshino, Y.; Makino, M.; Hayakawa, N.; Suzuki, A. Relative levels of the inflammatory cytokine TNFalpha and the soluble CD40 ligand profile in serum correlate with the thyrotoxic activity of Graves’ disease. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.P.; Ataga, K.I.; Orringer, E.P.; Phillips, D.R.; Parise, L.V. Biologically active CD40 ligand is elevated in sickle cell anemia: Potential role for platelet-mediated inflammation. Arter. Thromb. Vasc. Biol. 2006, 26, 1626–1631. [Google Scholar] [CrossRef]
- Giunta, B.; Rezai-Zadeh, K.; Tan, J. Impact of the CD40-CD40L dyad in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 149–155. [Google Scholar] [CrossRef]
- Thibert, K.A.; Raymond, G.V.; Tolar, J.; Miller, W.P.; Orchard, P.J.; Lund, T.C. Cerebral Spinal Fluid levels of Cytokines are elevated in Patients with Metachromatic Leukodystrophy. Sci. Rep. 2016, 6, 24579. [Google Scholar] [CrossRef] [Green Version]
- Raymond, G.V.; Pasquali, M.; Polgreen, L.E.; Dickson, P.I.; Miller, W.P.; Orchard, P.J.; Lund, T.C. Elevated cerebral spinal fluid biomarkers in children with mucopolysaccharidosis I-H. Sci. Rep. 2016, 6, 38305. [Google Scholar] [CrossRef] [PubMed]
- Campeau, P.M.; Rafei, M.; Boivin, M.N.; Sun, Y.; Grabowski, G.A.; Galipeau, J. Characterization of Gaucher disease bone marrow mesenchymal stromal cells reveals an altered inflammatory secretome. Blood 2009, 114, 3181–3190. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, E.V.; Deegan, P.B.; Tindall, J.; McFarlane, I.; Mehta, A.; Hughes, D.; Wraith, J.E.; Cox, T.M. Potential biomarkers of osteonecrosis in Gaucher disease. Blood Cells Mol. Dis. 2011, 46, 27–33. [Google Scholar] [CrossRef]
- Nitsche, A.; Zhang, M.; Clauss, T.; Siegert, W.; Brune, K.; Pahl, A. Cytokine profiles of cord and adult blood leukocytes: Differences in expression are due to differences in expression and activation of transcription factors. BMC Immunol. 2007, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V.K.; Mendizabal, A.; Parikh, S.H.; Szabolcs, P.; Driscoll, T.A.; Page, K.; Lakshminarayanan, S.; Allison, J.; Wood, S.; Semmel, D.; et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: Influence of cellular composition of the graft on transplantation outcomes. Blood 2008, 112, 2979–2989. [Google Scholar] [CrossRef]
- Konuma, T.; Kohara, C.; Watanabe, E.; Mizukami, M.; Nagai, E.; Oiwa-Monna, M.; Tanoue, S.; Isobe, M.; Kato, S.; Tojo, A.; et al. Cytokine Profiles of Pre-Engraftment Syndrome after Single-Unit Cord Blood Transplantation for Adult Patients. Biol. Blood Marrow Transplant. 2017, 23, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Kim, H.T.; Li, L.Q.; Cutler, C.; Antin, J.H.; Ballen, K.; Ritz, J.; Politikos, I.; Boussiotis, V.A. Assessment of a Multi-Cytokine Profile by a Novel Biochip-Based Assay Reveals Unique Correlation Patterns with Immune Reconstitution after Cord Blood Transplantation in Adults. Blood 2017, 130, 1980. [Google Scholar]
- Guo, D.B.; Zhu, X.Q.; Li, Q.Q.; Liu, G.M.; Ruan, G.P.; Pang, R.Q.; Chen, Y.H.; Wang, Q.; Wang, J.X.; Liu, J.F.; et al. Efficacy and mechanisms underlying the effects of allogeneic umbilical cord mesenchymal stem cell transplantation on acute radiation injury in tree shrews. Cytotechnology 2018, 70, 1447–1468. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Bullock, M.R.; Altememi, N.; Zhou, Z.; Hagood, S.; Rolfe, A.; McGinn, M.J.; Hamm, R.; Colello, R.J. The effect of epidermal growth factor in the injured brain after trauma in rats. J. Neurotrauma 2010, 27, 923–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, B. Epidermal growth factor and necrotizing enterocolitis. Clin. Perinatol. 2004, 31, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Thomas, D.P.; Zhang, X.; Culver, B.W.; Alexander, B.M.; Murdoch, W.J.; Rao, M.N.; Tulis, D.A.; Ren, J.; Sreejayan, N. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arter. Thromb. Vasc. Biol. 2006, 26, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.D.; Jung, J.H.; Lee, H.W.; Kwon, Y.M.; Chung, K.H.; Kim, M.G.; Kim, C.H. Zedoariae rhizoma and curcumin inhibits platelet-derived growth factor-induced proliferation of human hepatic myofibroblasts. Int. Immunopharmacol. 2005, 5, 555–569. [Google Scholar] [CrossRef]
- Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004, 15, 215–228. [Google Scholar] [CrossRef]
- Shin, S.D.; Shin, A.; Mayagoitia, K.; Siebold, L.; Rubini, M.; Wilson, C.G.; Bellinger, D.L.; Soriano, S. Loss of amyloid precursor protein exacerbates early inflammation in Niemann-Pick disease type C. J. Neuroinflamm. 2019, 16, 269. [Google Scholar] [CrossRef] [Green Version]
- Smagula, S.F.; Lotrich, F.E.; Aizenstein, H.J.; Diniz, B.S.; Krystek, J.; Wu, G.F.; Mulsant, B.H.; Butters, M.A.; Reynolds, C.F., 3rd; Lenze, E.J. Immunological biomarkers associated with brain structure and executive function in late-life depression: Exploratory pilot study. Int. J. Geriatr. Psychiatry 2017, 32, 692–699. [Google Scholar] [CrossRef]
- Chandra, G.; Rangasamy, S.B.; Roy, A.; Kordower, J.H.; Pahan, K. Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease. J. Biol. Chem. 2016, 291, 15267–15281. [Google Scholar] [CrossRef] [Green Version]
- Shahid, H.; Shahzad, M.; Shabbir, A.; Saghir, G. Immunomodulatory and Anti-Inflammatory Potential of Curcumin for the Treatment of Allergic Asthma: Effects on Expression Levels of Pro-inflammatory Cytokines and Aquaporins. Inflammation 2019, 42, 2037–2047. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Dash, D.; Singh, R. Intranasal Curcumin Inhibits Pulmonary Fibrosis by Modulating Matrix Metalloproteinase-9 (MMP-9) in Ovalbumin-Induced Chronic Asthma. Inflammation 2017, 40, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. J. Clin. Ther. 2010, 15, 337–344. [Google Scholar]
- Lei, J.; Yin, X.; Shang, H.; Jiang, Y. IP-10 is highly involved in HIV infection. Cytokine 2019, 115, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, D.; Hooper, D.C.; Roberts, A.L.; Harshyne, L.A.; Nagurney, M.; Curtis, M.T. Potential role of CSF cytokine profiles in discriminating infectious from non-infectious CNS disorders. PLoS ONE 2018, 13, e0205501. [Google Scholar] [CrossRef] [Green Version]
- Matta, M.C.; Vairo, F.; Torres, L.C.; Schwartz, I. Could enzyme replacement therapy promote immune tolerance in Gaucher disease type 1? Blood Cells Mol. Dis. 2018, 68, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.D.; Shin, A.; Mayagoitia, K.; Wilson, C.G.; Bellinger, D.L.; Soriano, S. Interferon downstream signaling is activated early in pre-symptomatic Niemann-Pick disease type C. Neurosci. Lett. 2019, 706, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Pindolia, K.R.; Janakiraman, N.; Noth, C.J.; Chapman, R.A.; Gautam, S.C. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 1997, 25, 413–422. [Google Scholar] [PubMed]
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Munch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharm. 2012, 69, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharm. 2013, 71, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, R.X.; Liu, Z. A high-throughput quantification method of curcuminoids and curcumin metabolites in human plasma via high-performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 949–950, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef] [Green Version]
| Q1 | Q3 | Fragment | Declustering Potential | Entrance Potential | Collision Energy |
|---|---|---|---|---|---|
| 1382.785 | 290.1 | * GM2_1 | −175 | −10 | −70 |
| 1382.785 | 1091.5 | GM2_2 | −175 | −10 | −72 |
| 1382.785 | 888.6 | GM2_3 | −175 | −10 | −70 |
| 1382.785 | 564.3 | GM2_4 | −175 | −10 | −92 |
| 1385.723 | 290.0 | * GM2_d3_1 | −198 | −10 | −68 |
| 1385.723 | 1094.5 | GM2_d3_2 | −198 | −10 | −64 |
| 1385.723 | 567.6 | GM2_d3_3 | −198 | −10 | −84 |
| 1385.723 | 891.5 | GM2_d3_4 | −198 | −10 | −76 |
| 1385.723 | 729.5 | GM2_d3_5 | −198 | −10 | −82 |
| Q1 | before the UCBCT | 6 Months after the UCBCT |
|---|---|---|
| Strength of the arm muscles | 4 points | 4 points (significant reduction in tremor and dynamic ataxia was noted) |
| Strength of the proximal muscles of the left leg | 1 point | 2.5 points |
| Strength of the proximal muscles of the right leg | 2 points | 2.5 points |
| Strength of the distal muscles of the left leg | 2.5 points | 3 points |
| Strength of the distal muscles of the right leg | 3 points | 3 points |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Garanina, E.E.; Salafutdinov, I.I.; Laikov, A.V.; Kursenko, V.V.; Chakrabarti, L.; Zakharova, E.Y.; Bukina, T.M.; et al. Serum Cytokine Profile, Beta-Hexosaminidase A Enzymatic Activity and GM2 Ganglioside Levels in the Plasma of a Tay-Sachs Disease Patient after Cord Blood Cell Transplantation and Curcumin Administration: A Case Report. Life 2021, 11, 1007. https://doi.org/10.3390/life11101007
Shaimardanova AA, Chulpanova DS, Solovyeva VV, Garanina EE, Salafutdinov II, Laikov AV, Kursenko VV, Chakrabarti L, Zakharova EY, Bukina TM, et al. Serum Cytokine Profile, Beta-Hexosaminidase A Enzymatic Activity and GM2 Ganglioside Levels in the Plasma of a Tay-Sachs Disease Patient after Cord Blood Cell Transplantation and Curcumin Administration: A Case Report. Life. 2021; 11(10):1007. https://doi.org/10.3390/life11101007
Chicago/Turabian StyleShaimardanova, Alisa A., Daria S. Chulpanova, Valeriya V. Solovyeva, Ekaterina E. Garanina, Ilnur I. Salafutdinov, Alexander Vladimirovich Laikov, Vadim V. Kursenko, Lisa Chakrabarti, Ekaterina Yu. Zakharova, Tatiana M. Bukina, and et al. 2021. "Serum Cytokine Profile, Beta-Hexosaminidase A Enzymatic Activity and GM2 Ganglioside Levels in the Plasma of a Tay-Sachs Disease Patient after Cord Blood Cell Transplantation and Curcumin Administration: A Case Report" Life 11, no. 10: 1007. https://doi.org/10.3390/life11101007
APA StyleShaimardanova, A. A., Chulpanova, D. S., Solovyeva, V. V., Garanina, E. E., Salafutdinov, I. I., Laikov, A. V., Kursenko, V. V., Chakrabarti, L., Zakharova, E. Y., Bukina, T. M., Baydakova, G. V., & Rizvanov, A. A. (2021). Serum Cytokine Profile, Beta-Hexosaminidase A Enzymatic Activity and GM2 Ganglioside Levels in the Plasma of a Tay-Sachs Disease Patient after Cord Blood Cell Transplantation and Curcumin Administration: A Case Report. Life, 11(10), 1007. https://doi.org/10.3390/life11101007







