Platelet-Based Biomarkers for Diagnosis and Prognosis in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
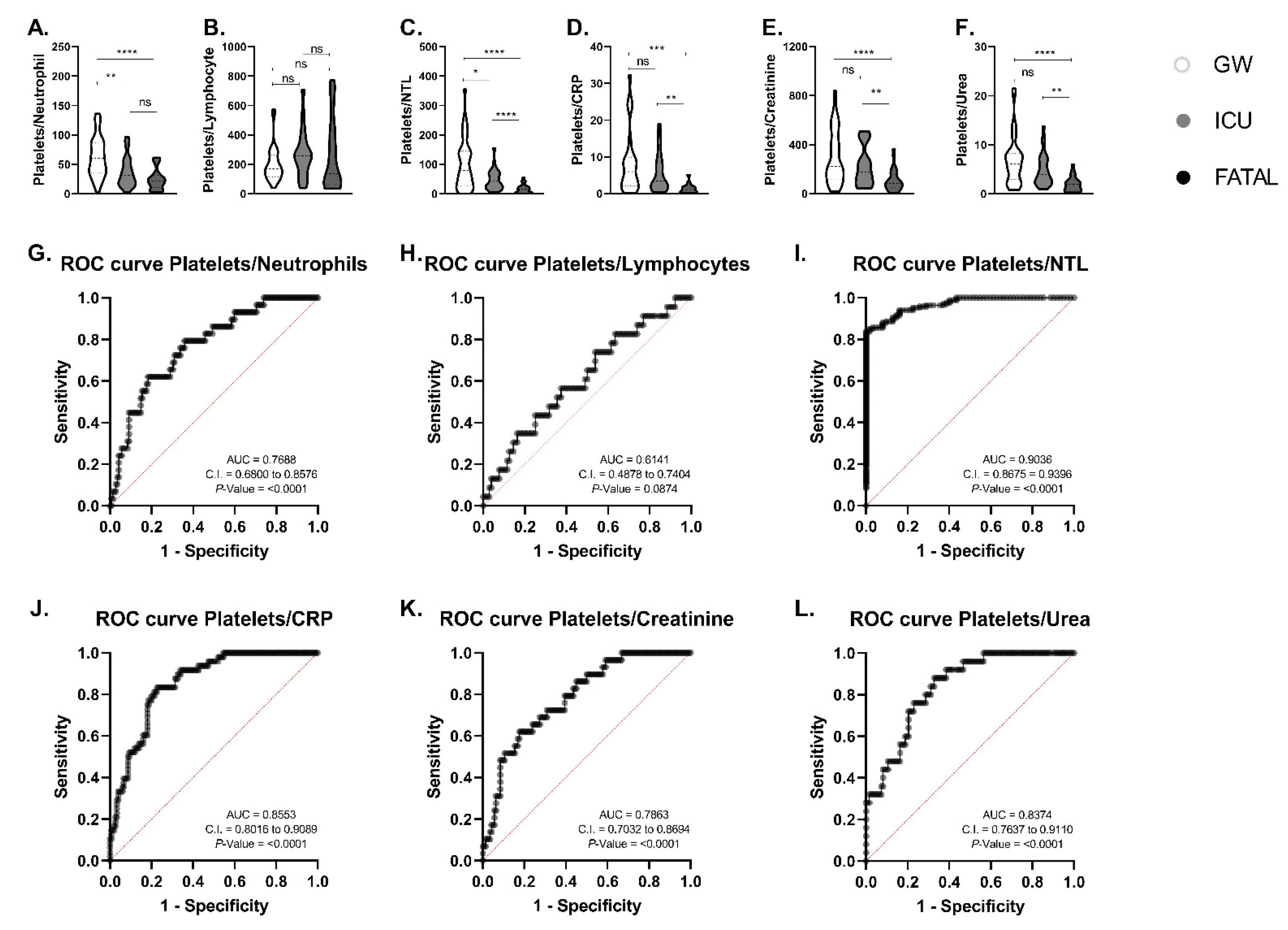
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhary, S.; Sreenivasulu, K.; Mitra, P.; Misra, S.; Sharma, P. Role of genetic variants and gene expression in the susceptibility and severity of COVID-19. Ann. Lab. Med. 2020, 41, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Alberca, R.W.; Lima, J.C.; Oliveira, E.A.; Gozzi-Silva, S.C.; Ramos, Y.Á.; Andrade, M.M.; Beserra, D.R.; Oliveira, L.D.; Branco, A.C.; Pietrobon, A.J.; et al. COVID-19 Disease Course in Former Smokers, Smokers and COPD Patients. Front. Physiol. 2021, 11, 1860. [Google Scholar] [CrossRef]
- Alberca, R.W.; Alberca, G.G.; Netto, L.C.; Orfali, R.L.; Gozzi-Silva, S.C.; da Silva Duarte, A.J.; Aoki, V.; Sato, M.N.; Benard, G. COVID-19 Severity and Mortality in Solid Organ Transplantation: Differences between Liver, Heart, and Kidney Recipients. Transplantology 2021, 2, 296–303. [Google Scholar] [CrossRef]
- D’Ardes, D.; Rossi, I.; Bucciarelli, B.; Allegra, M.; Bianco, F.; Sinjari, B.; Marchioni, M.; Di Nicola, M.; Santilli, F.; Guagnano, M.T.; et al. Metabolic Changes in SARS-CoV-2 Infection: Clinical Data and Molecular Hypothesis to Explain Alterations of Lipid Profile and Thyroid Function Observed in COVID-19 Patients. Life 2021, 11, 860. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Ding, F.; Han, L.; Yin, D.; Lu, H.; Zhang, M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy 2020, 76, 471–482. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Alberca, R.W.; Andrade, M.M.; Branco, A.C.; Pietrobon, A.J.; Pereira, N.Z.; Fernandes, I.G.; Oliveira, L.D.; Teixeira, F.M.; Beserra, D.R.; de Oliveira, E.A.; et al. Frequencies of CD33+ CD11b+ HLA-DR- CD14- CD66b+ and CD33+ CD11b+ HLA-DR- CD14+ CD66b- cells in peripheral blood as severity immune biomarkers in COVID-19. Front. Med. 2020, 7, 654. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef]
- Castelli, V.; Cimini, A.; Ferri, C. Cytokine Storm in COVID-19: When You Come Out of the Storm, You Won’t Be the Same Person Who Walked in. Front. Immunol. 2020, 11, 2132. [Google Scholar] [CrossRef]
- Petito, E.; Falcinelli, E.; Paliani, U.; Cesari, E.; Vaudo, G.; Sebastiano, M.; Cerotto, V.; Guglielmini, G.; Gori, F.; Malvestiti, M.; et al. Association of Neutrophil Activation, More Than Platelet Activation, With Thrombotic Complications in Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 933–944. [Google Scholar] [CrossRef]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef] [PubMed]
- Comer, S.P.; Cullivan, S.; Szklanna, P.B.; Weiss, L.; Cullen, S.; Kelliher, S.; Smolenski, A.; Murphy, C.; Altaie, H.; Curran, J.; et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021, 19, e3001109. [Google Scholar] [CrossRef]
- Alberca, G.G.F.; Solis-Castro, R.L.; Solis-Castro, M.E.; Alberca, R.W. Coronavirus disease–2019 and the intestinal tract: An overview. World J. Gastroenterol. 2021, 27, 1255–1266. [Google Scholar] [CrossRef]
- Alberca, R.W.; Pereira, N.Z.; Oliveira, L.M.D.S.; Gozzi-Silva, S.C.; Sato, M.N. Pregnancy, Viral Infection, and COVID-19. Front. Immunol. 2020, 11, 1672. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, H.; Xie, X. Solid Organ Transplantation During the COVID-19 Pandemic. Front. Immunol. 2020, 11, 1392. [Google Scholar] [CrossRef]
- Jakhmola, S.; Indari, O.; Baral, B.; Kashyap, D.; Varshney, N.; Das, A.; Chatterjee, S.; Jha, H.C. Comorbidity Assessment Is Essential During COVID-19 Treatment. Front. Physiol. 2020, 11, 984. [Google Scholar] [CrossRef]
- Rossi, F.H. Venous thromboembolism in COVID-19 patients Tromboembolismo venoso em pacientes COVID-19. J. Vasc. Bras. 2020, 19, e20200107. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Mukaka, M. A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69. [Google Scholar] [PubMed]
- Alberca, R.W.; Yendo, T.; Aoki, V.; Sato, M.N. Asthmatic patients and COVID-19: Different disease course? Allergy 2020, 76, 963. [Google Scholar] [CrossRef]
- Alberca, R.W.; Rigato, P.O.; Ramos, Y.Á.; Teixeira, F.M.; Branco, A.C.; Fernandes, I.G.; Pietrobon, A.J.; da Silva Duarte, A.J.; Aoki, V.; Orfali, R.L.; et al. Clinical characteristics and survival analysis in frequent alcohol consumers with COVID-19. Front. Nutr. 2021, 8, 260. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid.-Based Med. 2020, 26, 107–108. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2020, 32, 314–324. [Google Scholar] [CrossRef]
- Hottz, E.D.; Monteiro, A.P.T.; Bozza, F.A.; Bozza, P.T. Inflammasome in platelets: Allying coagulation and inflammation in infectious and sterile diseases? Mediat. Inflamm. 2015, 2015, 435783. [Google Scholar] [CrossRef]
- Bi, X.; Su, Z.; Yan, H.; Du, J.; Wang, J.; Chen, L.; Peng, M.; Chen, S.; Shen, B.; Li, J. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets 2020, 31, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, W.; Guo, Y.; Chen, L.; Zhang, L.; Zhao, S.; Long, D.; Yu, L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 2020, 31, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Chao, C.H.; Wu, W.C.; Lai, Y.C.; Tsai, P.J.; Perng, G.C.; Lin, Y.S.; Yeh, T.M. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019, 15, e1007625. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Shipe, M.E.; Deppen, S.A.; Farjah, F.; Grogan, E.L. Developing prediction models for clinical use using logistic regression: An overview. J. Thorac. Dis. 2019, 11, S574. [Google Scholar] [CrossRef]
- Jamal, M.H.; Doi, S.A.; AlYouha, S.; Almazeedi, S.; Al-Haddad, M.; Al-Muhaini, A.; Al-Ghimlas, F.; Chowdhury, M.E.; Al-Sabah, S. A biomarker based severity progression indicator for COVID-19: The Kuwait prognosis indicator score. Biomarkers 2020, 25, 641–648. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J. Gastrointest. Oncol. 2019, 10, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci. Rep. 2019, 9, 3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douedi, S.; Chaudhri, M.; Miskoff, J. Anti-interleukin-6 monoclonal antibody for cytokine storm in COVID-19. Ann. Thorac. Med. 2020, 15, 171–173. [Google Scholar] [CrossRef]
- Siess, W.; Hundelshausen, P.V.; Lorenz, R. Selective inhibition of thromboinflammation in COVID-19 by Btk inhibitors. Platelets 2020, 31, 989–992. [Google Scholar] [CrossRef] [PubMed]
| Male/Female | 91/78 | 40/22 | 48/27 | |||||
|---|---|---|---|---|---|---|---|---|
| Laboratory Data | MEAN | SEM | MEAN | SEM | MEAN | SEM | Reference Numbers | p-Value |
| Age (years) | 56.24 | 0.98 | 56.93 | 1.3 | 60.19 | 1.39 | 0.0736 | |
| Neutrophils (×103/mm3) | 5.805 | 0.5578 | 9.621 | 1.172 | 11.11 *,# | 1.165 | 2.5–7.5 | 0.0002 |
| Lymphocytes (×103/mm3) | 1.438 | 0.1312 | 1.329 | 0.1266 | 1.019 * | 0.1609 | 1.5–3.5 | 0.0266 |
| Neutrophil-to-lymphocyte ratio (NTL) | 4.566 | 0.3937 | 8.432 | 0.8036 | 11.55 *,# | 1.159 | 4–11 | <0.001 |
| Creatinine (mg/dL) | 1.68 | 0.2789 | 2.085 | 0.3353 | 2.851 * | 0.4301 | 0.7–1.2 | 0.0197 |
| Urea (mg/dL) | 58.02 | 6.372 | 80.68 | 11.25 | 116.7 * | 13.45 | 10–50 | 0.0003 |
| C-reactive protein (CRP) (mg/L) | 68.18 | 15.16 | 104.9 | 21.15 | 146 * | 26.42 | <5.0 | 0.0490 |
| Platelets (×103/mm3) | 296.7 | 15.79 | 297.1 | 21.99 | 229.9 * | 22.58 | 150–400 | 0.0131 |
| Alanine aminotransferase (U/L) | 36.59 | 5.593 | 68.46 | 13.52 | 39.47 | 6.877 | <41 | 0.1206 |
| Aspartate aminotransferase (U/L) | 37.88 | 6.964 | 44.23 | 7.943 | 49.07 | 6.916 | <37 | 0.3123 |
| Glutamyl transferase gamma (U/L) | 115.3 | 43.86 | 334.8 | 164.1 | 295.0 | 77.64 | 8–61 | 0.0689 |
| Glucose (mg/dL) | 160.8 | 45.49 | 172.1 # | 39.99 | 288.2 # | 32.03 | 70–100 | 0.0073 |
| Lactate dehydrogenase (U/L) | 280 | 26.41 | 505.2 | 53.78 | 515 *,# | 48.03 | 135–225 | 0.0038 |
| D-dimer (ng/mL) | 1443 | 281.5 | 6590 | 2642 | 6191 * | 2461 | <500 | 0.0237 |
| Comorbidities | ||||||||
| Diabetes mellitus/ Metabolic syndrome | 76 | 46 | 27 | |||||
| Systemic Arterial Hypertension | 77 | 49 | 35 | |||||
| Heart disease | 15 | 10 | 6 | |||||
| Hepatic disease | 10 | 4 | 3 | |||||
| Renal disease | 19 | 7 | 12 | |||||
| No disease | 7 | 3 | 4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberca, R.W.; Solis-Castro, R.L.; Solis-Castro, M.E.; Cardoso, F.; Duarte, A.J.d.S.; Oliveira, L.d.M.; Pereira, N.Z.; Gozzi-Silva, S.C.; Oliveira, E.A.d.; Aoki, V.; et al. Platelet-Based Biomarkers for Diagnosis and Prognosis in COVID-19 Patients. Life 2021, 11, 1005. https://doi.org/10.3390/life11101005
Alberca RW, Solis-Castro RL, Solis-Castro ME, Cardoso F, Duarte AJdS, Oliveira LdM, Pereira NZ, Gozzi-Silva SC, Oliveira EAd, Aoki V, et al. Platelet-Based Biomarkers for Diagnosis and Prognosis in COVID-19 Patients. Life. 2021; 11(10):1005. https://doi.org/10.3390/life11101005
Chicago/Turabian StyleAlberca, Ricardo Wesley, Rosa Liliana Solis-Castro, Maria Edith Solis-Castro, Fernanda Cardoso, Alberto Jose da Silva Duarte, Luana de Mendonça Oliveira, Nátalli Zanete Pereira, Sarah Cristina Gozzi-Silva, Emily Araujo de Oliveira, Valeria Aoki, and et al. 2021. "Platelet-Based Biomarkers for Diagnosis and Prognosis in COVID-19 Patients" Life 11, no. 10: 1005. https://doi.org/10.3390/life11101005
APA StyleAlberca, R. W., Solis-Castro, R. L., Solis-Castro, M. E., Cardoso, F., Duarte, A. J. d. S., Oliveira, L. d. M., Pereira, N. Z., Gozzi-Silva, S. C., Oliveira, E. A. d., Aoki, V., Orfali, R. L., Beserra, D. R., Andrade, M. M. d. S., & Sato, M. N. (2021). Platelet-Based Biomarkers for Diagnosis and Prognosis in COVID-19 Patients. Life, 11(10), 1005. https://doi.org/10.3390/life11101005







