Haploid Embryogenesis in Isolated Microspore Culture of Carrots (Daucus carota L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Plants Growing Conditions
2.2. Microspore Culture
2.3. Plant Regeneration
2.4. Plantlets Growing
2.5. Cytological Analysis
2.6. Ploidy Level Determination
2.7. Statistical Analysis
3. Results
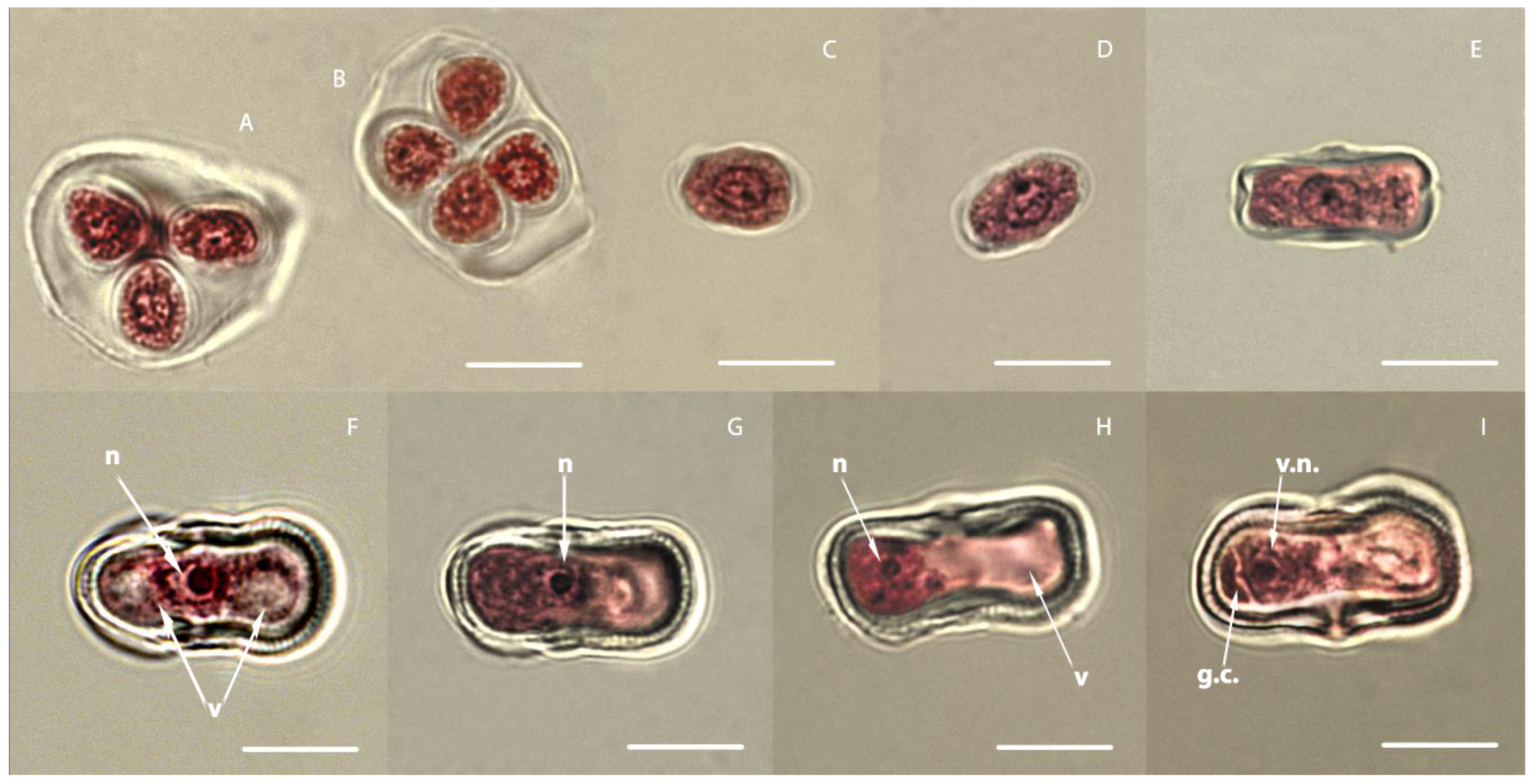
3.1. Study of the Development of Carrot Microspores
3.2. Embryogenesis of Carrots In Vitro Depending on the Stage of Development of Microspores
3.3. Secondary Embryogenesis in the Culture of Carrot Microspores
3.4. Development of Embryoids into Plant Regenerants Obtained in Culture of Carrot Microspores
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, S.B. Anther culture in carrot. Hered. Suppl. 1985, 3, 132. [Google Scholar]
- Tyukavin, G.B.; Tanganov, B.O.; Naumova, S.V. Induction of embryogenesis in the cultivation of carrot anthers. In Proceedings of the XI International Symposium, St. Petersburg, Russia, 3–7 July 1990; pp. 562–563. [Google Scholar]
- Hu, K.L.; Matsubara, S.; Murakami, K. Haploid Plant Production by Anther Culture in Carrot (Daucus carota L.). J. Jpn. Soc. Hortic. Sci. 1993, 62, 561–565. [Google Scholar] [CrossRef]
- Tyukavin, G.B.; Shmykova, N.A.; Monakhova, M.A. Cytological study of embryogenesis in cultured carrot anthers. Russ. J. Plant Physiol. 1999, 46, 767–773. [Google Scholar]
- Górecka, K.; Krzyzanowska, D.; Górecki, R. The influence of several factors on the efficiency of androgenesis in carrot. J. Appl. Genet. 2005, 46, 265–269. [Google Scholar] [PubMed]
- Zhuang, F.; Pei, H.; Ou, C.; Hu, H.; Zhao, Z.; Li, J. Induction of microspores-derived embryos and calli from anther culture in carrot. Acta Hortic. Sin. 2010, 37, 1613–1620. [Google Scholar]
- Shmykova, N.A.; Tiukavin, G.B. Specific Morphological and Genetic Changes in Carrot Anthers Cultivated in vitro. Biotechnol. Russ. 2002, 5, 75–81. [Google Scholar]
- Matsubara, S.; Dohya, N.; Murakami, K. Callus formation and regeneration of adventitious embryos from carrot, fennel and mitsuba microspores by anther and isolated microspore cultures. Acta Hortic. 1995, 392, 129–138. [Google Scholar] [CrossRef]
- Górecka, K.; Kowalska, U.; Krzyżanowska, D.; Kiszczak, W. Obtaining carrot (Daucus carota L.) plants in isolated microspore cultures. J. Appl. Genet. 2010, 51, 141–147. [Google Scholar] [CrossRef]
- Ferrie, A.M.R.; Möllers, C. Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell Tissue Organ. Cult. 2011, 104, 375–386. [Google Scholar] [CrossRef]
- Li, J.-R.; Zhuang, F.-Y.; Ou, C.-G.; Hu, H.; Zhao, Z.-W.; Mao, J.-H. Microspore embryogenesis and production of haploid and doubled haploid plants in carrot (Daucus carota L.). Plant Cell Tissue Organ. Cult. 2013, 112, 275–287. [Google Scholar] [CrossRef]
- Vjurtts, T.S.; Domblides, E.A.; Shmykova, N.A.; Fedorova, M.I.; Kan, L.J.; Domblides, A.S. Production of Dh-Plants in Culture of Isolated Microspore in Carrot. Veg. Crop. Russ. 2018, 5, 25–30. [Google Scholar] [CrossRef][Green Version]
- Friedt, W.; Zarhloul, M.K. Haploids in the Improvement of Crucifers. In Haploids in Crop Improvement II; Springer: Berlin/Heidelberg, Germany, 2005; pp. 191–213. [Google Scholar]
- Asif, M. Progress and Opportunities of Doubled Haploid Production; SpringerBriefs in Plant Science; Springer International Publishing: Heidelberg, Germany, 2013; Volume 6, ISBN 978-3-319-00731-1. [Google Scholar]
- Heberle-Bors, E. Isolated pollen culture in tobacco: Plant reproductive development in a nutshell. Sex. Plant Reprod. 1989, 2, 1–10. [Google Scholar] [CrossRef]
- Zaki, M.A.M.; Dickinson, H.G. Structural changes during the first divisions of embryos resulting from anther and free microspore culture in Brassica napus. Protoplasma 1990, 156, 149–162. [Google Scholar] [CrossRef]
- González-Melendi, P.; Testillano, P.S.; Ahmadian, P.; Fadón, B.; Vicente, O.; Risueño, M.C. In situ characterization of the late vacuolate microspore as a convenient stage to induce embryogenesis in Capsicum. Protoplasma 1995, 187, 60–71. [Google Scholar] [CrossRef]
- Hu, T.; Kasha, K.J. A cytological study of pretreatments used to improve isolated microspore cultures of wheat (Triticum aestivum L.) cv. Chris. Genome 1999, 42, 432–441. [Google Scholar] [CrossRef]
- Lichter, R. Induction of Haploid Plants from Isolated Pollen of Brassica napus. Z. Für Pflanzenphysiol. 1982, 105, 427–434. [Google Scholar] [CrossRef]
- Masuda, K.; Kikuta, Y.; Okazawa, Y. A revision of the medium for somatic embryogenesis in carrot suspension culture. J. Fac. Agric. Hokkaido Univ. 1981, 60, 183–193. [Google Scholar]
- Skaptsov, M.V.; Smirnov, S.V.; Kutsev, M.G.; Shmakov, A.I. Problems of a standardization in plant flow cytometry. Turczaninowia 2016, 19, 120–122. [Google Scholar]
- Shmykova, N.A.; Tiukavin, G.B. Pollen Dimorphism and evaluation of the embryogenic capacity of microspores of various carrot genotypes. Agric. Biol. 2001, 5, 53–61. [Google Scholar]
- Touraev, A.; Ilham, A.; Vicente, O.; Heberle-Bors, E. Stress-induced microspore embryogenesis in tobacco: An optimized system for molecular studies. Plant Cell Rep. 1996, 15, 561–565. [Google Scholar] [CrossRef]
- Supena, E.D.J.; Suharsono, S.; Jacobsen, E.; Custers, J.B.M. Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep. 2006, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parra-Vega, V.; González-García, B.; Seguí-Simarro, J.M. Morphological markers to correlate bud and anther development with microsporogenesis and microgametogenesis in pepper (Capsicum annuum L.). Acta Physiol. Plant. 2013, 35, 627–633. [Google Scholar] [CrossRef]
- Otani, M.; Shimada, T. High frequency of pollen embryo formation in Triticum aestivum L. on maltose containing medium. Cereal Res. Commun. 1993, 21, 11–15. [Google Scholar]
- Simonson, R.L.; Beanziger, P.S.; Gustafson, V.D. Wheat anther culture as affected by various cultural changes and supplements. J. Appl. Genet. 1997, 4, 381–392. [Google Scholar]
- Zheng, M.Y.; Konzak, C.F. Effect of 2,4-dichlorophenoxyacetic acid on callus induction and plant regeneration in anther culture of wheat (Triticum aestivum L.). Plant Cell Rep. 1999, 19, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Redha, A.; Islam, S.M.S.; Büter, B.; Stamp, P.; Schmid, J.E. The improvement in regenerated doubled haploids from anther culture of wheat by anther transfer. Plant Cell. Tissue Organ. Cult. 2000, 63, 167–172. [Google Scholar] [CrossRef]
- Pauk, J.; Hasan, M.S.; Puolimatka, M.; Lantos, C.; Mihály, R.; Mesterhazy, A.; Kertész, Z.; Matuz, J. Microspore and Anther Culture Improvements for Wheat Breeding. In In Vitro Application in Crop Improvement; CRC Press: Boca Raton, FI, USA, 2004; pp. 151–172. [Google Scholar]
- Pauk, J.; Mihály, R.; Monostori, T.; Puolimatka, M. Protocol of triticale (x Triticosecale Wittmack) microspore culture. In Doubled Haploid Production in Crop Plants; Springer: Dordrecht, The Netherlands, 2003; pp. 129–134. [Google Scholar]
- Andersen, S.B.; Christiansen, I.; Farestveit, B. Carrot (Daucus carota L.): In Vitro Production of Haploids and Field Trials. In Haploids in Crop Improvement I; SpringerLink: New York, NY, USA, 1990; pp. 393–402. [Google Scholar]
- Kiszczak, W.; Kowalska, U.; Kapuścińska, A.; Burian, M.; Górecka, K. Comparison of methods for obtaining doubled haploids of carrot. Acta Soc. Bot. Pol. 2017, 86, 35–47. [Google Scholar] [CrossRef][Green Version]
- Cho, M.S.; Zapata, F.J. Callus formation and plant regeneration in isolated pollen culture of rice (Oryza sativa L. cv. Taipei 309). Plant Sci. 1988, 58, 239–244. [Google Scholar] [CrossRef]
- Cho, M.S.; Zapata, F.J. Plant Regeneration from Isolated Microspore of Indica Rice. Plant Cell Physiol. 1990, 31, 881–885. [Google Scholar] [CrossRef]
- Sunderland, N. Haploids in Higher Plants: Advances and Potential; Kasha, K.J., Ed.; University of Guelph Press: Guelph, ON, Canada, 1974; pp. 91–122. [Google Scholar]
- Indrianto, A.; Heberle-Bors, E.; Touraev, A. Assessment of various stresses and carbohydrates for their effect on the induction of embryogenesis in isolated wheat microspores. Plant Sci. 1999, 143, 71–79. [Google Scholar] [CrossRef]
- Li, H.; Devaux, P. High frequency regeneration of barley doubled haploid plants from isolated microspore culture. Plant Sci. 2003, 164, 379–386. [Google Scholar] [CrossRef]
- Bishnoi, U.; Jain, R.K.; Rohilla, J.S.; Chowdhury, V.K.; Gupta, K.R.; Chowdhury, J.B. Anther culture of recalcitrant indica× Basmati rice hybrids. Euphytica 2000, 114, 93–101. [Google Scholar] [CrossRef]
- Sunderland, N.; Roberts, M. Cold-pretreatment of Excised Flower Buds in Float Culture of Tobacco Anthers. Ann. Bot. 1979, 43, 405–414. [Google Scholar] [CrossRef]
- Sato, S.; Katoh, N.; Iwai, S.; Hagimori, M. Effect of Low Temperature Pretreatment of Buds or Inflorescence on Isolated Microspore Culture in Brassica rapa (syn. B. campestris). Breed. Sci. 2002, 52, 23–26. [Google Scholar] [CrossRef]
- Binarova, P.; Hause, G.; Cenklová, V.; Cordewener, J.H.G.; Campagne, M.M.L. A short severe heat shock is required to induce embryogenesis in late bicellular pollen of Brassica napus L. Sex. Plant Reprod. 1997, 10, 200–208. [Google Scholar] [CrossRef]
- Pechan, P.M.; Smykal, P. Androgenesis: Affecting the fate of the male gametophyte. Physiol. Plant. 2001, 111, 1–8. [Google Scholar] [CrossRef]
- Seguí-Simarro, J.; Testillano, P.; Risueño, M. Hsp70 and Hsp90 change their expression and subcellular localization after microspore embryogenesis induction in Brassica napus L. J. Struct. Biol. 2003, 142, 379–391. [Google Scholar] [CrossRef]
- Barany, I.; Testillano, P.S.; Mityko, J.; Risueño, M.D. The switch of the microspore developmental program in Capsicum involves HSP70 expression and leads to the production of haploid plants. Int. J. Dev. Biol. 2001, 45, 39–40. [Google Scholar]
- Ashok Kumar, H.G.; Murthy, H.N.; Paek, K.Y. Embryogenesis and plant regeneration from anther cultures of Cucumis sativus L. Sci. Hortic. (Amst.) 2003, 98, 213–222. [Google Scholar] [CrossRef]
- Ashok Kumar, H.G.; Murthy, H.N. Effect of sugars and amino acids on androgenesis of Cucumis sativus. Plant Cell. Tissue Organ. Cult. 2004, 78, 201–208. [Google Scholar] [CrossRef]
- Song, H.; Lou, Q.F.; Luo, X.D.; Wolukau, J.N.; Diao, W.P.; Qian, C.T.; Chen, J.F. Regeneration of doubled haploid plants by androgenesis of cucumber (Cucumis sativus L.). Plant Cell. Tissue Organ. Cult. 2007, 90, 245–254. [Google Scholar] [CrossRef]
- Tyukavin, G.B. Osnovy Biotehnologii Morkovi; Pivovarov, V.F., Ed.; VNIISSOK: Moscow, Russia, 2007; ISBN 978-5-901695-19-7. [Google Scholar]
- Jähne, A.; Lörz, H. Cereal microspore culture. Plant. Sci. 1995, 109, 1–12. [Google Scholar] [CrossRef]
- Ferrie, A.M.R.; Epp, D.J.; Keller, W.A. Evaluation of Brassica rapa L. genotypes for microspore culture response and identification of a highly embryogenic line. Plant Cell Rep. 1995, 14, 580–584. [Google Scholar] [CrossRef]
- Kuginuki, Y.; Nakamura, K.; Hida, K.; Yosikawa, H. Varietal Differences in Embryogenic and Regenerative Ability in Microspore Culture of Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Ikushugaku Zasshi 1997, 47, 341–346. [Google Scholar] [CrossRef]
- Phippen, C.; Ockendon, D.J. Genotype, plant, bud size and media factors affecting anther culture of cauliflowers (Brassica oleracea var. botrytis). Theor. Appl. Genet. 1990, 79, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Caredda, S.; Clément, C. Androgenesis and albinism in Poaceae: Influence of genotype and carbohydrates. In Anther and Pollen; Springer: Berlin/Heidelberg, Germany, 1999; pp. 211–228. [Google Scholar]
- Kumari, M.; Clarke, H.J.; Small, I.; Siddique, K.H.M. Albinism in Plants: A Major Bottleneck in Wide Hybridization, Androgenesis and Doubled Haploid Culture. CRC Crit. Rev. Plant Sci. 2009, 28, 393–409. [Google Scholar] [CrossRef]
- Krzewska, M.; Czyczyło-Mysza, I.; Dubas, E.; Gołębiowska-Pikania, G.; Żur, I. Identification of QTLs associated with albino plant formation and some new facts concerning green versus albino ratio determinants in triticale (×Triticosecale Wittm.) anther culture. Euphytica 2015, 206, 263–278. [Google Scholar] [CrossRef]
- Lantos, C.; Purgel, S.; Ács, K.; Langó, B.; Bóna, L.; Boda, K.; Békés, F.; Pauk, J. Utilization of in Vitro Anther Culture in Spelt Wheat Breeding. Plants 2019, 8, 436. [Google Scholar] [CrossRef]
- Kozar, E.V.; Korottseva, K.S.; Romanova, O.V.; Chichvarina, O.A.; Kan, L.Y.; Ahramenko, V.A.; Domblides, E.A. Production of doubled haplois in Brassica purpuraria. Veg. Crop. Russ. 2019, 10–18. [Google Scholar] [CrossRef]
- Hagemann, R.; Schröder, M.B. The cytological basis of the plastid inheritance in angiosperms. Protoplasma 1989, 152, 57–64. [Google Scholar] [CrossRef]
- Hause, G. Ultrastructural investigations of mature embryo sacs of Daaucus carota, D. aureus and D. muricatus—Possible cytological explanations of paternal plastid inheritance. Sex. Plant Reprod. 1991, 4, 288–292. [Google Scholar] [CrossRef]
- Pacini, E.; Taylor, P.E.; Singh, M.B.; Knox, R.B. Development of Plastids in Pollen and Tapetum of Rye-grass, Lolium perenne L. Ann. Bot. 1992, 70, 179–188. [Google Scholar] [CrossRef]







| Accession | Abnormal Pollen Grains before Cultivation, (%) | The Number of Embryoids, Pcs/1 Petri Dish * | Embryoids Forming Green Seedlings/Albino Seedlings | |
|---|---|---|---|---|
| Maximum | Average | |||
| Responsive Genotypes | ||||
| Nantskaya 4 | 5.7 ± 2.5 | 53 | 34.7 ± 11.3 a | 94/162 |
| Chantenay | 3.6 ± 1.5 | 37 | 25.2 ± 7.4 b | 52/45 |
| Weakly Responsive Genotypes | ||||
| Scarlet Nantes (K-2030) | 2 ± 1.0 | 5 | 2.5 ± 1.2 c | 0 |
| Nantes Red ( Bp.k-2566) | 0 | 3 | 1.3 ± 0.8 c | 0 |
| Imperator (Bp.k.2569) | 0 | 3 | 1.8 ± 0.9 c | 0 |
| Reluctant Genotypes | ||||
| Naga (K-845) | 0 | 0 | 0 d | 0 |
| Tip Top (K-2332) | 0 | 0 | 0 d | 0 |
| Scarlet (Bp.k 2568) | 0 | 0 | 0 d | 0 |
| LSD = 3.45 | ||||
| Accession | No. of Plants | Ploidy Level | |||||
|---|---|---|---|---|---|---|---|
| Haploid | Diploid | Polyploids | |||||
| No. | % | No. | % | No. | % | ||
| Nantskaya 4 | 50 | 7 | 14.0 | 37 | 74.0 | 6 | 12.0 |
| Chantenay | 30 | 8 | 26.6 | 20 | 66.7 | 2 | 6.7 |
| Total | 80 | 15 | 57 | 8 | |||
| Mean, % | 18.7 | 71.3 | 10.0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmykova, N.; Domblides, E.; Vjurtts, T.; Domblides, A. Haploid Embryogenesis in Isolated Microspore Culture of Carrots (Daucus carota L.). Life 2021, 11, 20. https://doi.org/10.3390/life11010020
Shmykova N, Domblides E, Vjurtts T, Domblides A. Haploid Embryogenesis in Isolated Microspore Culture of Carrots (Daucus carota L.). Life. 2021; 11(1):20. https://doi.org/10.3390/life11010020
Chicago/Turabian StyleShmykova, Natalia, Elena Domblides, Tatiana Vjurtts, and Arthur Domblides. 2021. "Haploid Embryogenesis in Isolated Microspore Culture of Carrots (Daucus carota L.)" Life 11, no. 1: 20. https://doi.org/10.3390/life11010020
APA StyleShmykova, N., Domblides, E., Vjurtts, T., & Domblides, A. (2021). Haploid Embryogenesis in Isolated Microspore Culture of Carrots (Daucus carota L.). Life, 11(1), 20. https://doi.org/10.3390/life11010020






