Comparing Three Different Extraction Techniques on Essential Oil Profiles of Cultivated and Wild Lotus (Nelumbo nucifera) Flower
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Procedures for Sample Preparation
2.3. Apparatus and Operation Conditions
3. Results and Discussion
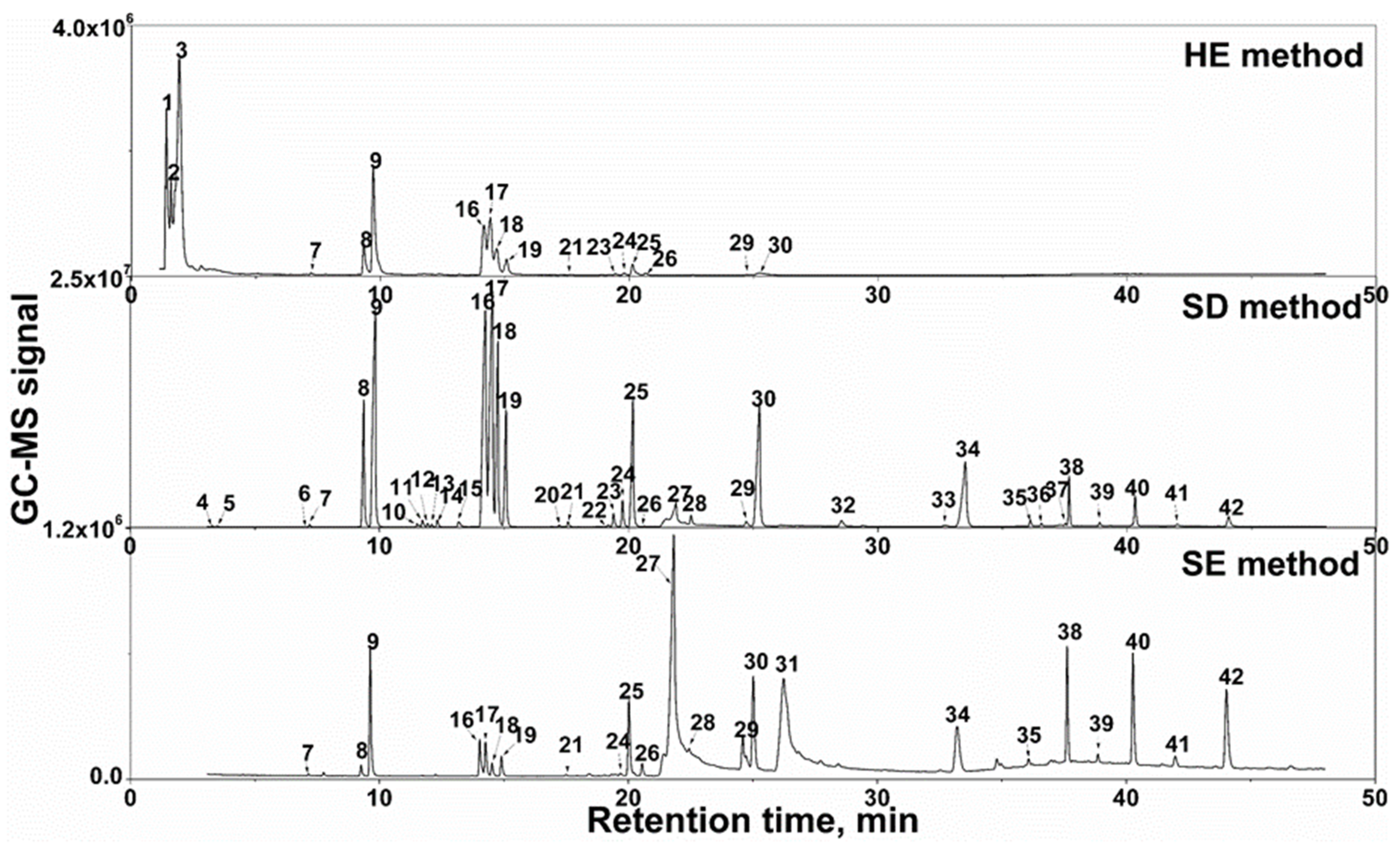
3.1. Chromatogram in the GC-MS Analysis of the Extracts
3.2. Identification of the Essential Oil Components
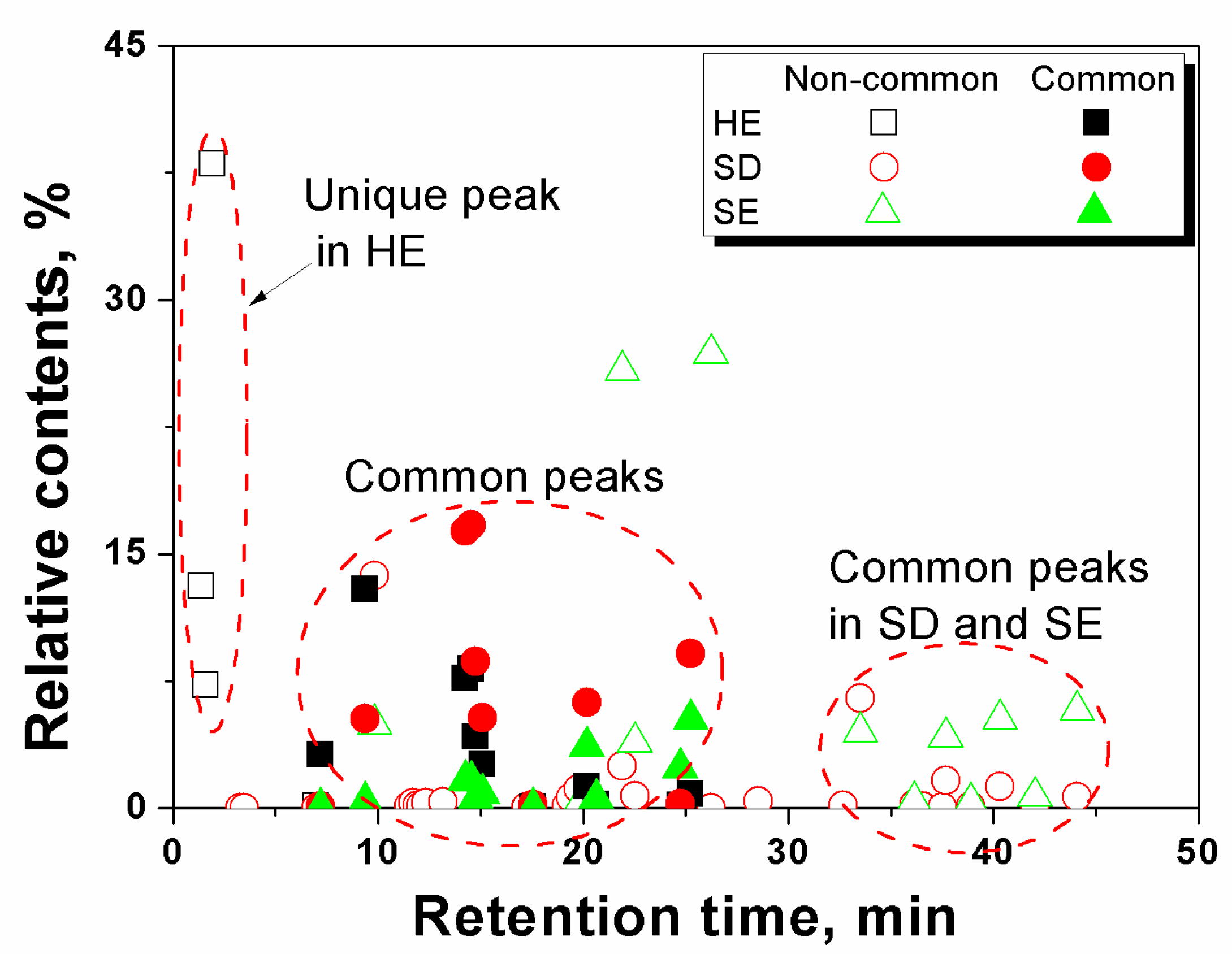
3.3. Comparison of the Essential Oil Components Extracted by Three Techniques
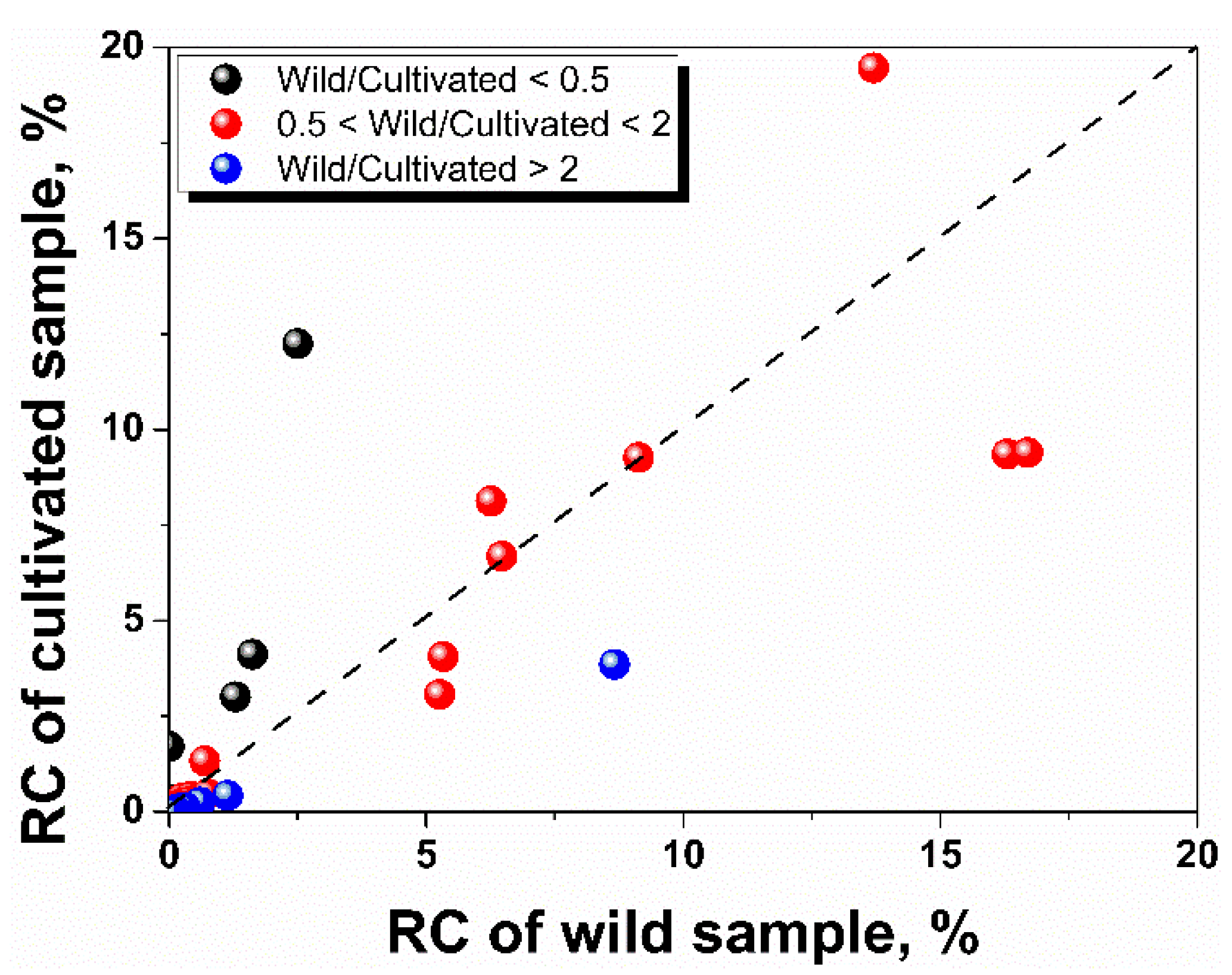
3.4. Comparison of the Essential Oil Components between Wild and Cultivated Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sridhar, K.R.; Rajeev, B. Lotus-A potential nutraceutical source. J. Agric. Technol. 2007, 3, 143–155. [Google Scholar]
- Brindha, B.; Arthi, D. Antimicrobial activity of white and pink Nelumbo nucifera Gaertn flowers. Asian J. Pharm. Res. Health Care 2010, 2, 147–155. [Google Scholar]
- Durairaj, B.; Dorai, A. Free radical scavenging potential of Nelumbo nucifera Gaertn flowers (white and pink). Int. J. Nat. Sci. Res. 2014, 2, 133–146. [Google Scholar]
- Zahid, Z.; Khan, S.W.; Patel, K.A.; Konale, A.G.; Lokre, S.S. Antimicrobial activity of essential oil of flower of Plumeria alba Linn. (Apocynaceae). Int. J. Pharm. Pharma. Sci. 2010, 2, 155–157. [Google Scholar]
- Huang, B.; Ban, X.Q.; He, J.S.; Tong, J.; Tian, J.; Wang, Y.W. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbo nucifera from various areas of China. J. Agric. Food Chem. 2010, 58, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Shin, M.K.; Lee, Y.J. Volatile aroma components of green tea scented with lotus (Nelumbo nucifera Gaertner) flower. Food Sci. Biotechnol. 2003, 12, 540–543. [Google Scholar]
- Jeon, S.; Kim, N.H.; Koo, B.S.; Kim, J.Y.; Lee, A.Y. Lotus (Nelumbo nuficera) flower essential oil increased melanogenesis in normal human melanocytes. Exp. Mol. Med. 2009, 41, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ryman, D. The Aromatherapy Handbook: The Secret Healing Power of Essential Oils; Century Publishing CO Ltd.: Post Falls, ID, USA, 1984. [Google Scholar]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Cassel, E.; Vargas, R.M.F.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crop. Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Chemat, F.; Lcchesi, M.E.; Smadja, J.; Favretto, L.; Colnaghi, G.; Visinoni, F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal. Chim. Acta 2006, 555, 157–160. [Google Scholar] [CrossRef]
- Lam, K.C.; Nickerson, G.B.; Deinzer, M.L. A rapid solvent extraction method for hop essential oils. J. Agric. Food Chem. 1986, 34, 63–66. [Google Scholar] [CrossRef]
- Reverchon, E.; Porta, G.D.; Senatore, F. Supercritical CO2 extraction and fractionation of lavender essential oil and waxes. J. Agric. Food Chem. 1995, 43, 1654–1658. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Quantick, P.C. Method for the static headspace analysis of carrot volatiles. Food Chem. 1999, 65, 391–397. [Google Scholar] [CrossRef]
- Lorenzo, M.I.; Pavon, J.L.P.; Laespada, M.E.F.; Pinto, C.G.; Cordero, B.M.; Henriques, L.R.; Peres, M.F.; Simoes, M.P.; Lopes, P.S. Application ofheadspace-mass spectrometry for differentiating sources of olive oil. Anal. Bioanal. Chem. 2002, 374, 1205–1211. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Fernandez, X.; Lizzani-Cuveller, L.; Loiseau, A.M. Comparison of static headspace, headspace solid phase microextraction, headspace sorptiveextraction, and direct thermal desorption techniques on chemical composition of French olive oils. J. Agric. Food Chem. 2003, 51, 7709–7716. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Headspace solid-phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Yang, X.; Peppard, T. Solid-phase microextraction for flavor analysis. J. Agric. Food Chem. 1994, 42, 1925–1930. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography-Theory and Practice; Wiley-VCH Press: Hoboken, NJ, USA, 2006. [Google Scholar]
- Wang, L.M.; Li, M.T.; Jin, W.W.; Li, S.; Zhang, S.Q.; Yu, L.J. Variations in the components of Osmanthus fragrans Lour. essential oil at different stages of flowering. Food Chem. 2009, 114, 233–236. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some Greek Aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef]
- Ikawa, M.; Mosley, S.P.; Barbero, L.J. Inhibitory effects of terpene alcohols and aldehydes on growth algaChlorella pyrenoidosa. J. Chem. Ecol. 1992, 18, 1755–1760. [Google Scholar] [CrossRef]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stress on growth parameters and essential oil content of Matricaria chamomila. Int. J. Agri. Biol. 2008, 10, 451–454. [Google Scholar]
- Sangwan, R.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]

| Peak Number | Retention Time, Min | Components a | Relative Contents, % | Chemical Class | ||
|---|---|---|---|---|---|---|
| HE | SD | SE | ||||
| 7 | 7.188 | tetradecane | 3.16 | 0.0999 | 0.0696 | Alkanes |
| 9 | 9.832 | pentadecane | - | 13.7 | 4.98 | |
| 14 | 12.319 | hexadecane | - | 0.309 | - | |
| 19 | 15.078 | heptadecane | 2.61 | 5.33 | 0.879 | |
| 21 | 17.566 | octadecane | 0.0718 | 0.242 | 0.0729 | |
| 25 | 20.171 | nonadecane | 1.26 | 6.26 | 3.63 | |
| 28 | 22.505 | eicosane | - | 0.749 | 3.89 | |
| 30 | 25.248 | heneicosane | 0.822 | 9.13 | 5.27 | |
| 32 | 28.541 | docosane | - | 0.426 | - | |
| 34 | 33.507 | tricosane | - | 6.47 | 4.51 | |
| 35 | 36.121 | trtracosane | - | 0.248 | 0.405 | |
| 38 | 37.672 | pentacocane | - | 1.62 | 4.21 | |
| 39 | 38.899 | hexacosane | - | 0.127 | 0.367 | |
| 40 | 40.324 | heptacosane | - | 1.29 | 5.29 | |
| 41 | 42.013 | octacosane | - | 0.106 | 0.691 | |
| 42 | 44.079 | noncosane | - | 0.690 | 5.76 | |
| 6 | 7.000 | 7-tetradecene | 0.128 | 0.00677 | - | Alkenes |
| 8 | 9.368 | 1-pentadecene | 12.9 | 5.26 | 0.410 | |
| 10 | 11.508 | E-1,9-hexadecadiene | - | 0.107 | - | |
| 11 | 11.725 | Z-7-hexadecene | - | 0.315 | - | |
| 12 | 11.931 | Z-3-hexadecene | - | 0.151 | - | |
| 13 | 12.094 | Z-8-hexadecene | - | 0.114 | - | |
| 18 | 14.752 | 8-heptadecene | 4.24 | 8.66 | 0.593 | |
| 20 | 17.213 | E-5octadecene | - | 0.0895 | - | |
| 23 | 19.390 | Z-5-nonadecene | 0.110 | 0.605 | - | |
| 24 | 19.753 | 1-nonadecene | - | 1.14 | 0.0973 | |
| 29 | 24.719 | 10-heneicosene | 0.191 | 0.267 | 2.40 | |
| 33 | 32.68 | Z-9-tricosene | - | 0.169 | - | |
| 36 | 36.561 | 1,12-docosadiene | - | 0.113 | - | |
| 37 | 37.526 | Z-12-pentacosene | - | 0.0658 | - | |
| 4 | 3.286 | terpinen-4-ol | - | 0.0328 | - | Alcohols |
| 5 | 3.471 | α-terpineol | - | 0.0414 | - | |
| 15 | 13.17 | γ-eudesmol | - | 0.388 | - | |
| 1 | 1.406 | furfural | 13.1 | - | - | Aldehydes |
| 16 | 14.249 | Z,Z-10,12-hexadecadienal | 7.64 | 16.3 | 1.66 | |
| 17 | 14.534 | E-14-hexadecenal | 8.21 | 16.7 | 1.61 | |
| 22 | 19.180 | Z-9,17-octadecadienal | - | 0.101 | - | |
| 3 | 1.923 | acetic acid | 38.1 | - | - | Acids |
| 27 | 21.898 | n-hexadecanoic acid | - | 2.50 | 25.8 | |
| 31 | 26.229 | Z,Z-9,12-octadecadienoic acid | - | - | 26.8 | |
| 26 | 20.625 | hexadecanoic acid, methyl ester | 0.209 | 0.0311 | 0.541 | Esters |
| 2 | 1.587 | unidentified | 7.27 | - | - | - |
| Peak Number | Retention Time, Min | Components | Relative Contents b, % | |
|---|---|---|---|---|
| Cultivated | Wild | |||
| 4 | 3.286 | terpinen-4-ol | - | 0.0328 |
| 5 | 3.471 | α-terpineol | 0.311 | 0.0414 |
| 6 | 7.000 | 7-tetradecene | 0.145 | 0.00677 |
| 7 | 7.188 | tetradecane | 0.154 | 0.0999 |
| 8 | 9.368 | 1-pentadecene | 3.07 | 5.26 |
| 9 | 9.832 | Pentadecane | 19.47 | 13.7 |
| 10 | 11.508 | E-1,9-hexadecadiene | 0.0561 | 0.107 |
| 11 | 11.725 | Z-7-hexadecene | 0.118 | 0.315 |
| 12 | 11.931 | Z-3-hexadecene | 0.0807 | 0.151 |
| 13 | 12.094 | Z-8-hexadecene | 0.0356 | 0.114 |
| 14 | 12.319 | hexadecane | 0.244 | 0.309 |
| 15 | 13.17 | γ-eudesmol | 0.265 | 0.388 |
| 16 | 14.249 | Z,Z-10,12-hexadecadienal | 9.36 | 16.3 |
| 17 | 14.534 | E-14-hexadecenal | 9.40 | 16.7 |
| 18 | 14.752 | 8-heptadecene | 3.86 | 8.66 |
| 19 | 15.078 | heptadecane | 4.06 | 5.33 |
| 20 | 17.213 | E-5octadecene | 0.0231 | 0.0895 |
| 21 | 17.566 | Octadecane | 0.228 | 0.242 |
| 22 | 19.180 | Z-9,17-octadecadienal | 0.0232 | 0.101 |
| 23 | 19.390 | Z-5-nonadecene | 0.251 | 0.605 |
| 24 | 19.753 | 1-nonadecene | 0.426 | 1.14 |
| 25 | 20.171 | nonadecane | 8.13 | 6.26 |
| 26 | 20.625 | hexadecanoic acid, methyl ester | 0.0415 | 0.0311 |
| 27 | 21.898 | n-hexadecanoic acid | 12.25 | 2.50 |
| 28 | 22.505 | eicosane | 0.473 | 0.749 |
| 29 | 24.719 | 10-heneicosene | 0.149 | 0.267 |
| 30 | 25.248 | heneicosane | 9.27 | 9.13 |
| 31 | 26.229 | Z,Z-9,12-octadecadienoic acid | 1.71 | - |
| 32 | 28.541 | docosane | 0.406 | 0.426 |
| 33 | 32.68 | Z-9-tricosene | 0.0775 | 0.169 |
| 34 | 33.507 | tricosane | 6.688 | 6.47 |
| 35 | 36.121 | trtracosane | 0.356695 | 0.248 |
| 36 | 36.561 | 1,12-docosadiene | 0.020671 | 0.113 |
| 37 | 37.526 | Z-12-pentacosene | 0.0569 | 0.0658 |
| 38 | 37.672 | pentacocane | 4.12 | 1.62 |
| 39 | 38.899 | hexacosane | 0.276 | 0.127 |
| 40 | 40.324 | heptacosane | 3.01 | 1.29 |
| 41 | 42.013 | octacosane | 0.203 | 0.106 |
| 42 | 44.079 | noncosane | 1.33 | 0.690 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-Y.; Guo, M. Comparing Three Different Extraction Techniques on Essential Oil Profiles of Cultivated and Wild Lotus (Nelumbo nucifera) Flower. Life 2020, 10, 209. https://doi.org/10.3390/life10090209
Zhang C-Y, Guo M. Comparing Three Different Extraction Techniques on Essential Oil Profiles of Cultivated and Wild Lotus (Nelumbo nucifera) Flower. Life. 2020; 10(9):209. https://doi.org/10.3390/life10090209
Chicago/Turabian StyleZhang, Chun-Yun, and Mingquan Guo. 2020. "Comparing Three Different Extraction Techniques on Essential Oil Profiles of Cultivated and Wild Lotus (Nelumbo nucifera) Flower" Life 10, no. 9: 209. https://doi.org/10.3390/life10090209
APA StyleZhang, C.-Y., & Guo, M. (2020). Comparing Three Different Extraction Techniques on Essential Oil Profiles of Cultivated and Wild Lotus (Nelumbo nucifera) Flower. Life, 10(9), 209. https://doi.org/10.3390/life10090209




