From Bench to Bedside in Tongue Muscle Cancer Invasion and Back again: Gross Anatomy, Microanatomy, Surgical Treatments and Basic Research
Abstract
1. Introduction
2. Bedside to the Bench in Tongue Muscle Invasion: Arising Aspects from Gross Anatomy of the Tongue
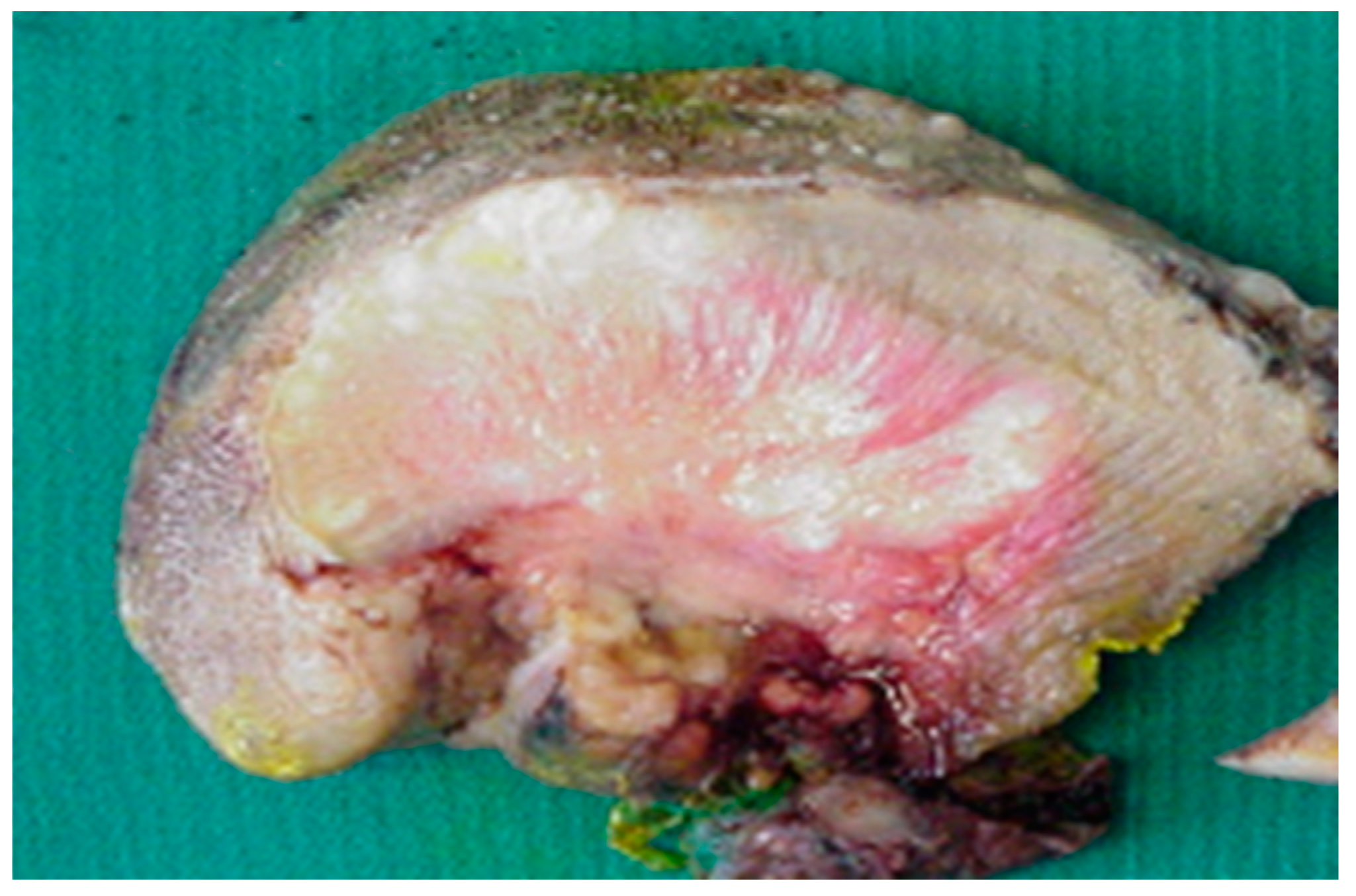
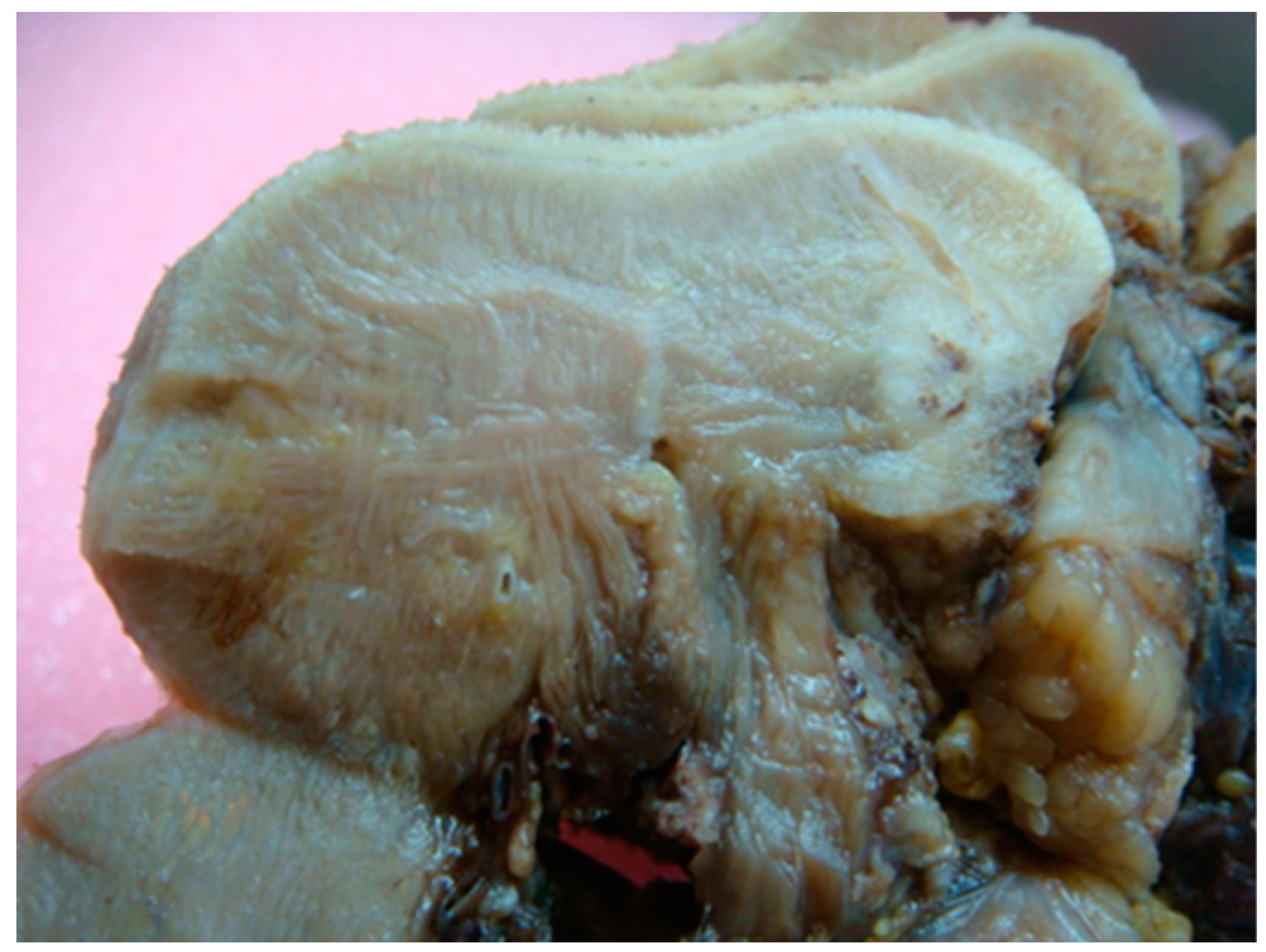
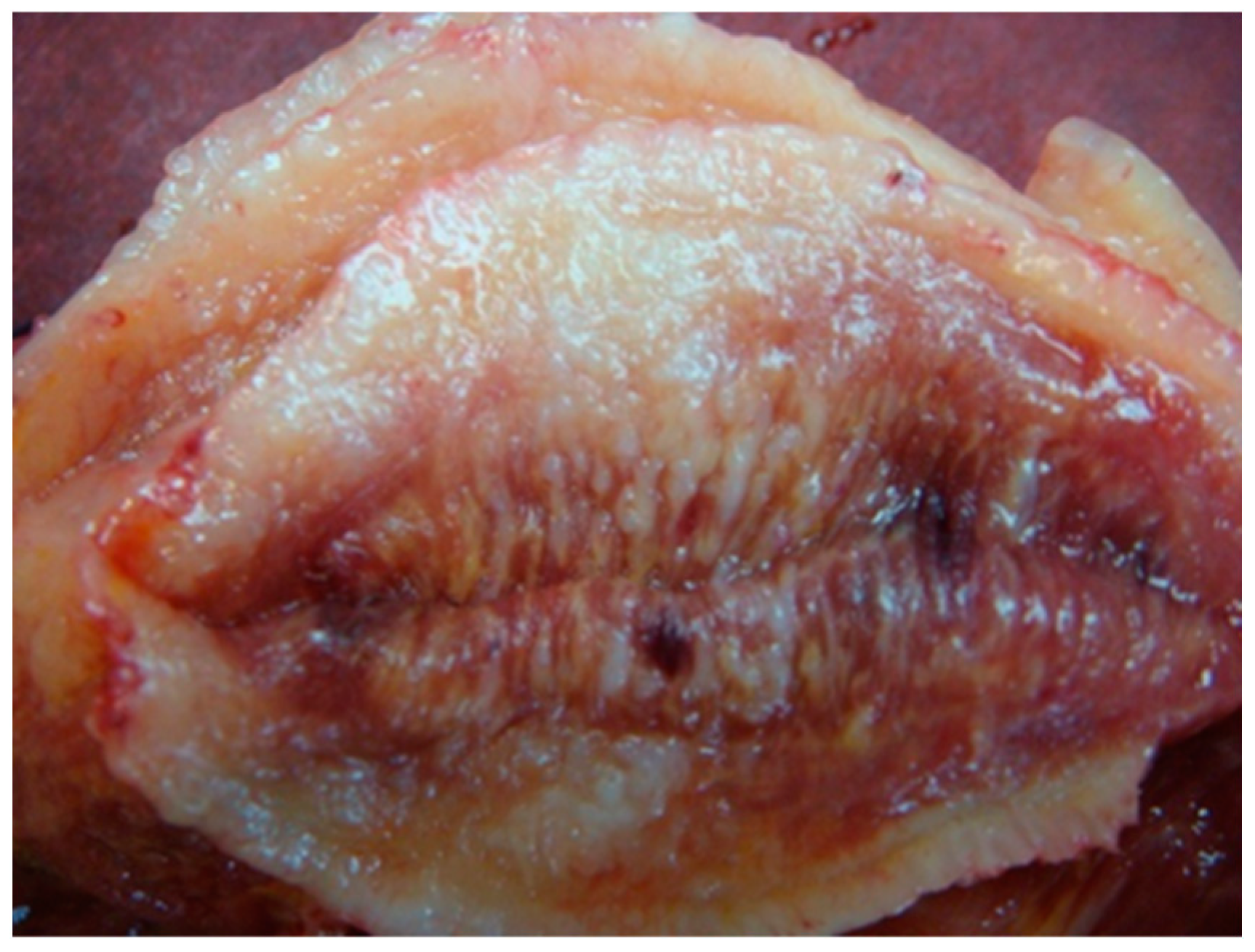
3. Bedside to the Bench in Tongue Muscle Invasion: Arising Aspects from Surgery Specimens
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yosefof, E.; Hilly, O.; Stern, S.; Bachar, G.; Shpitzer, T.; Mizrachi, A. Squamous cell carcinoma of the oral tongue: Distinct epidemiological profile disease. Head Neck 2020, 42, 2316–2320. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.R.; Kemmer, J.; Formeister, E.; El-Sayed, I.; Ha, P.; George, J.; Ryan, W.; Chan, E.; Heaton, C. Beyond Depth of Invasion: Adverse Pathologic Tumor Features in Early Oral Tongue Squamous Cell Carcinoma. Laryngoscope 2020, 130, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, Y.; Yoshida, S.; Nakahira, M.; Kogashiwa, Y.; Enoki, Y.; Kuba, K.; Inoue, H.; Minami, K.; Yasuda, M.; Sugasawa, M. Importance of tumor budding grade as independent prognostic factor for early tongue squamous cell carcinoma. Head Neck 2019, 41, 1809–1815. [Google Scholar] [CrossRef]
- Yang, W.; Sun, M.; Jie, Q.; Zhou, H.; Zhang, P.; Zhu, J. Lingual Lymph Node Metastasis in cT1-2N0 Tongue Squamous Cell Carcinoma: Is It an Indicator for Elective Neck Dissection. Front. Oncol. 2020, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, H.; Zhan, Y.; Fan, S. An emerging tumor invasion mechanism about the collective cell migration. Am. J. Transl. Res. 2019, 11, 5301–5312. [Google Scholar] [PubMed]
- Tinhofer, I.; Staudte, S. Circulating tumor cells as biomarkers in head and neck cancer: Recent advances and future outlook. Expert Rev. Mol. Diagn. 2018, 18, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.; Vance, C.; Budnick, S.; Muller, S. Muscle invasion in oral tongue squamous cell carcinoma as a predictor of nodal status and local recurrence: Just as effective as depth of invasion? Head Neck Pathol. 2011, 5, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Pietrobon, G.; Fazio, E.; Abousiam, M.; Awny, S.; Bruschini, R.; Accorona, R. Anatomically-based transoral surgical approach to early-stage oral tongue squamous cell carcinoma. Head Neck 2020, 42, 1105–1109. [Google Scholar] [CrossRef]
- Beunk, L.; Brown, K.; Nagtegaal, I.; Friedl, P.; Wolf, K. Cancer invasion into musculature: Mechanics, molecules and implications. Semin. Cell Dev. Biol. 2019, 93, 36–45. [Google Scholar] [CrossRef]
- Ansarin, M.; Bruschini, R.; Navach, V.; Giugliano, G.; Calabrese, L.; Chiesa, F.; Medina, J.E.; Kowalski, L.P.; Shah, J.P. Classification of GLOSSECTOMIES: Proposal for tongue cancer resections. Head Neck 2019, 41, 821–827. [Google Scholar] [CrossRef]
- Sonntag, C.F. The comparative anatomy of the tongues of the mammalia. XII. Summary, classification, and phylogeny. Proc. Zool. Soc. Lond. 1925, 95, 701–762. [Google Scholar] [CrossRef]
- Saito, H.; Itoh, I. Three-dimensional architecture of the intrinsic tongue muscles, particularly the longitudinal muscle, by the chemical-maceration method. Anat. Sci. Int. 2003, 78, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y. Structural arrangement of the intrinsic muscles of the tongue and their relationships with the extrinsic muscles. Surg. Radiol. Anat. 2018, 40, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sanders, I.; Mu, L. A three-dimensional atlas of human tongue muscles. Anat. Rec. 2013, 296, 1102–1114. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Z.; Shang, D.; Cheng, J.; Yuan, H.; Wu, Y.; Song, X.; Jiang, H. α-Smooth muscle actin-positive myofibroblasts, in association with epithelial-mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J. Oral. Pathol. Med. 2014, 43, 335–343. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Pearson, A.T.; Kutys, M.L.; Choi, C.K.; Wozniak, M.A.; Baker, B.M.; Chen, C.S. Extracellular matrix alignment dictates the organization of focal adhesions and directs uniaxial cell migration. APL Bioeng. 2018, 2, 046107. [Google Scholar] [CrossRef] [PubMed]
- Van Helvert, S.; Storm, C.; Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018, 20, 8–20. [Google Scholar] [CrossRef]
- Calabrese, L.; Giugliano, G.; Bruschini, R.; Ansarin, M.; Navach, V.; Grosso, E.; Gibelli, B.; Ostuni, A.; Chiesa, F. Compartmental surgery in tongue tumours: Description of a new surgical technique. Acta Otorhinolaryngol. Ital. 2009, 29, 259–264. [Google Scholar]
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewicz, A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef]
- Krause, M.; Wolf, K. Cancer cell migration in 3D tissue: Negotiating space by proteolysis and nuclear deformability. Cell Adh. Migr. 2015, 9, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of atomic force microscopy in cancer research. J. Nanobiotechnology 2018, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Friedl, P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011, 21, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Paul, C.D.; Stahl, R.; Vanmeerbeeck, G.; Reumers, V.; Liu, C.; Konstantopoulos, K.; Lagae, L. Time-lapse lens-free imaging of cell migration in diverse physical microenvironments. Lab Chip 2016, 16, 3304–3316. [Google Scholar] [CrossRef]
- Sarkar, S.; Egelhoff, T.; Baskaran, H. Insights into the Roles of Non-Muscle Myosin Iia in Human Keratinocyte Migration. Cell Mol. Bioeng. 2009, 2, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Brandwein-Gensler, M.; Teixeira, M.S.; Lewis, C.M.; Lee, B.; Rolnitzky, L.; Hille, J.J.; Genden, E.; Urken, M.L.; Wang, B.Y. Oral squamous cell carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am. J. Surg. Pathol. 2005, 29, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.; Bailey, B.M. Squamous carcinoma of the tongue: Review. Br. J. Oral. Maxillofac. Surg. 1999, 37, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Woolgar, J.A. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral. Oncol. 2006, 42, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, H.; Kleinsasser, O. Growth and spread of squamous cell carcinoma of the floor of the mouth. Eur. Arch. Oto Rhino Laryngol. 1993, 250, 358–361. [Google Scholar] [CrossRef]
- Shaheen, O.H. Carcinoma of the floor of the mouth. J. Laryngol. Otol. 1985, 99, 881–886. [Google Scholar] [CrossRef]
- Calabrese, L.; Bruschini, R.; Giugliano, G.; Ostuni, A.; Maffini, F.; Massaro, M.A.; Santoro, L.; Navach, V.; Preda, L.; Alterio, D.; et al. Compartmental tongue surgery: Long term oncologic results in the treatment of tongue cancer. Oral. Oncol. 2011, 47, 174–179. [Google Scholar] [CrossRef]
- Carta, F.; Quartu, D.; Mariani, C.; Tatti, M.; Marrosu, V.; Gioia, E.; Gerosa, C.; Zanda, J.S.A.; Chuchueva, N.; Figus, A.; et al. Compartmental Surgery with Microvascular Free Flap Reconstruction in Patients with T1-T4 Squamous Cell Carcinoma of the Tongue: Analysis of Risk Factors, and Prognostic Value of the 8th Edition AJCC TNM Staging System. Front. Oncol. 2020, 10, 984. [Google Scholar] [CrossRef]
- Rosa, I.; Taverna, C.; Novelli, L.; Marini, M.; Ibba-Manneschi, L.; Manetti, M. Telocytes constitute a widespread interstitial meshwork in the lamina propria and underlying striated muscle of human tongue. Sci. Rep. 2019, 9, 5858. [Google Scholar] [CrossRef]
- Liao, C.T.; Lee, L.Y.; Hsueh, C.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Hsieh, C.H.; Ng, S.H.; Lin, C.H.; Tsao, C.K.; et al. Clinical Outcomes in pT4 Tongue Carcinoma are Worse than in pT3 Disease: How Extrinsic Muscle Invasion Should be Considered? Ann. Surg. Oncol. 2017, 24, 2570–2579. [Google Scholar] [CrossRef]
- Calabrese, L.; Ostuni, A.; Ansarin, M.; Giugliano, G.; Maffini, F.; Alterio, D.; Rocca, M.C.; Petralia, G.; Bruschini, R.; Chiesa, F.; et al. Future challenges in head and neck cancer: From the bench to the bedside? Crit. Rev. Oncol. Hematol. 2012, 84 (Suppl. 1), e90–e96. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Castellana, S.; Carella, M.; Palumbo, O.; Tiberio, C.; Fusilli, C.; Capocefalo, D.; Biagini, T.; Mazza, T.; Lo Muzio, L. A primary tumor gene expression signature identifies a crucial role played by tumor stroma myofibroblasts in lymph node involvement in oral squamous cell carcinoma. Oncotarget 2017, 8, 104913–104927. [Google Scholar] [CrossRef]
- Shaikh, I.; Ansari, A.; Ayachit, G.; Gandhi, M.; Sharma, P.; Bhairappanavar, S.; Joshi, C.G.; Das, J. Differential gene expression analysis of HNSCC tumors deciphered tobacco dependent and independent molecular signatures. Oncotarget 2019, 10, 6168–6183. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Carcedo, C.; Hooper, S.; Chaudhry, S.I.; Williamson, P.; Harrington, K.; Leitinger, B.; Sahai, E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat. Cell Biol. 2011, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de la Forest Divonne, S.; et al. Fibronectin-guided migration of carcinoma collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef] [PubMed]
- Saenz-de-Santa-Maria, I.; Celada, L.; Chiara, M.D. The Leader Position of Mesenchymal Cells Expressing N-Cadherin in the Collective Migration of Epithelial Cancer. Cells 2020, 9. [Google Scholar] [CrossRef]
- Pereira, E.R.; Kedrin, D.; Seano, G.; Gautier, O.; Meijer, E.F.J.; Jones, D.; Chin, S.M.; Kitahara, S.; Bouta, E.M.; Chang, J.; et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018, 359, 1403–1407. [Google Scholar] [CrossRef]
- Hamada, M.; Ebihara, Y.; Nagata, K.; Yano, M.; Kogashiwa, Y.; Nakahira, M.; Sugasawa, M.; Nagatsuka, H.; Yasuda, M. Podoplanin is an efficient predictor of neck lymph node metastasis in tongue squamous cell carcinoma with low tumor budding grade. Oncol. Lett. 2020, 19, 2602–2608. [Google Scholar] [CrossRef]
- Tagliabue, M.; Gandini, S.; Maffini, F.; Navach, V.; Bruschini, R.; Giugliano, G.; Lombardi, F.; Chiocca, S.; Rebecchi, E.; Sica, E.; et al. The role of the T-N tract in advanced stage tongue cancer. Head Neck 2019, 41, 2756–2767. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Kapeleris, J.; Kimberley, R.; Mattarollo, S.R.; Thompson, E.W.; Thiery, J.P.; Kenny, L.; O’Byrne, K.; Punyadeera, C. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018, 7, 5910–5919. [Google Scholar] [CrossRef]
- Aceto, N. Bring along your friends: Homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed. J. 2020, 43, 18–23. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Schmidt, H.; Perry, C.; Whitfield, B.; Kenny, L.; Nelson, C.; Warkiani, M.E.; Punyadeera, C. A Collective Route to Head and Neck Cancer Metastasis. Sci. Rep. 2018, 8, 746. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liao, B.; Jiang, X.; Dong, Z.; Hu, S.; Liu, Y.; Xiao, M. Prognosis Value of Platelet Counts, Albumin and Neutrophil-Lymphocyte Ratio of Locoregional Recurrence in Patients with Operable Head and Neck Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Werner-Klein, M.; Scheitler, S.; Hoffmann, M.; Hodak, I.; Dietz, K.; Lehnert, P.; Naimer, V.; Polzer, B.; Treitschke, S.; Werno, C.; et al. Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nat. Commun. 2018, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, L.; Bizzoca, M.E.; Grigolato, R.; Maffini, F.A.; Tagliabue, M.; Negro, R.; Leuci, S.; Mignogna, M.D.; Lo Muzio, L. From Bench to Bedside in Tongue Muscle Cancer Invasion and Back again: Gross Anatomy, Microanatomy, Surgical Treatments and Basic Research. Life 2020, 10, 197. https://doi.org/10.3390/life10090197
Calabrese L, Bizzoca ME, Grigolato R, Maffini FA, Tagliabue M, Negro R, Leuci S, Mignogna MD, Lo Muzio L. From Bench to Bedside in Tongue Muscle Cancer Invasion and Back again: Gross Anatomy, Microanatomy, Surgical Treatments and Basic Research. Life. 2020; 10(9):197. https://doi.org/10.3390/life10090197
Chicago/Turabian StyleCalabrese, Luca, Maria Eleonora Bizzoca, Roberto Grigolato, Fausto Antonio Maffini, Marta Tagliabue, Rosa Negro, Stefania Leuci, Michele Davide Mignogna, and Lorenzo Lo Muzio. 2020. "From Bench to Bedside in Tongue Muscle Cancer Invasion and Back again: Gross Anatomy, Microanatomy, Surgical Treatments and Basic Research" Life 10, no. 9: 197. https://doi.org/10.3390/life10090197
APA StyleCalabrese, L., Bizzoca, M. E., Grigolato, R., Maffini, F. A., Tagliabue, M., Negro, R., Leuci, S., Mignogna, M. D., & Lo Muzio, L. (2020). From Bench to Bedside in Tongue Muscle Cancer Invasion and Back again: Gross Anatomy, Microanatomy, Surgical Treatments and Basic Research. Life, 10(9), 197. https://doi.org/10.3390/life10090197







