LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. RNA Extraction from Tissue
2.3. Statistical Analyses
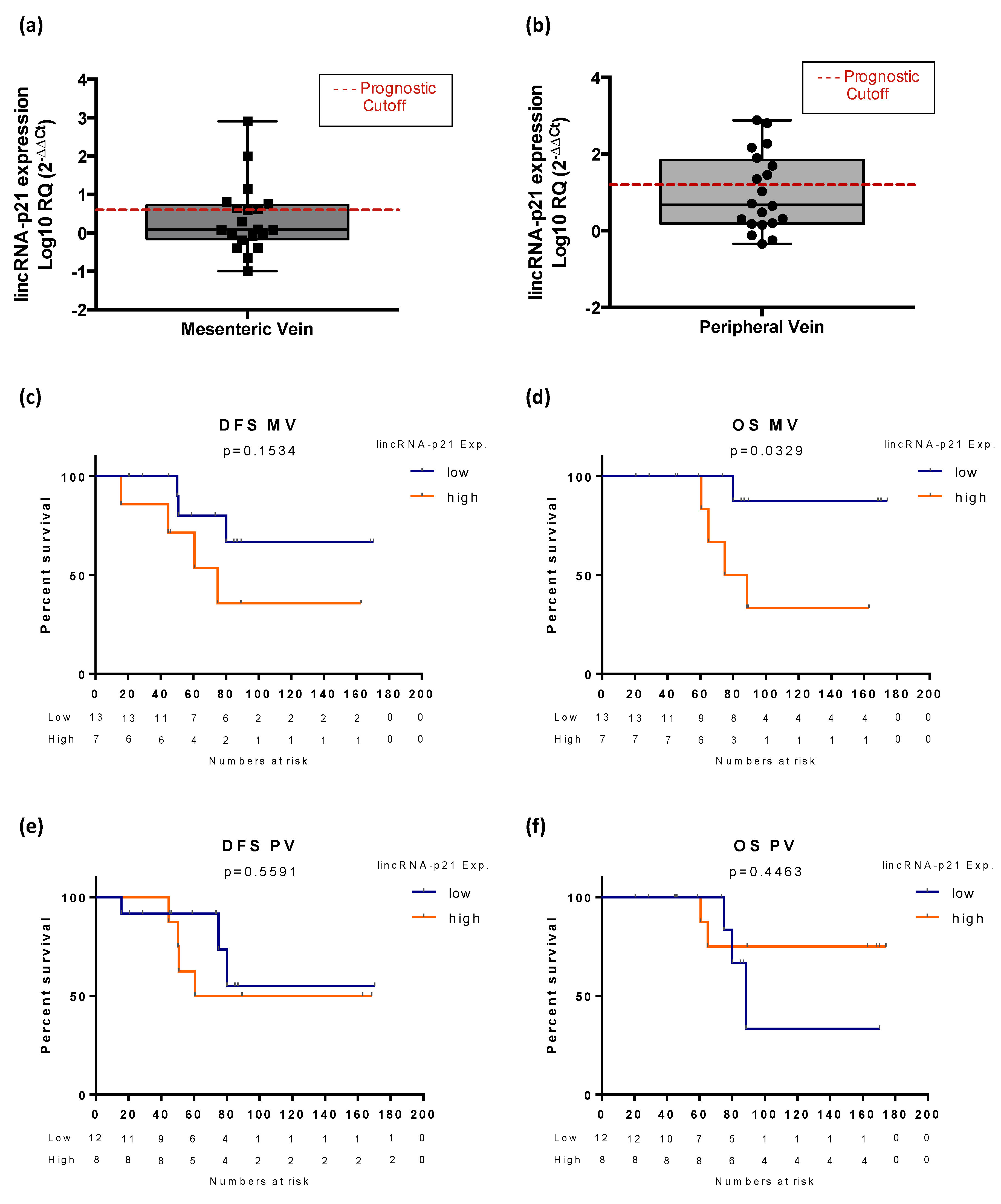
3. Results
3.1. Patients
3.2. LincRNA-p21 Expression Levels
3.3. LincRNA-p21 Expression and Survival
3.4. LincRNA-p21 is an Independent Prognosis Marker in CRC
3.5. LincRNA-p21 and Chemoradiotherapy in Rectal Cancer
3.6. Exploratory Analysis of LincRNA-p21 Expression in Plasma Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CC | Colon cancer |
| CI | Confidence intervals |
| CRC | Colorectal cancer |
| CT | Chemotherapy |
| CTR | Chemoradiotherapy |
| DFS | Disease-free survival |
| HR | Hazard ratios |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MV | Mesenteric vein |
| NT | Normal tissue |
| OS | Overall survival |
| PV | Peripheral vein |
| RC | Rectal cancer |
| TT | Tumor tissue |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Tamas, K.; Walenkamp, A.; de Vries, E.; van Vugt, M.; Beets-Tan, R.; van Etten, B.; de Groot, D.; Hospers, G. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat. Rev. 2015, 41, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Nordlinger, B.; Beretta, G.; Mosconi, S.; Mandalà, M.; Cervantes, A.; Arnold, D.; ESMO Guidelines Working Group. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi64–vi72. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Jia, M.; Jiang, L.; Wang, Y.D.; Huang, J.Z.; Yu, M.; Xue, H.Z. lincRNA-p21 inhibits invasion and metastasis of hepatocellular carcinoma through Notch signaling-induced epithelial–mesenchymal transition. Hepatol. Res. 2016, 46, 1137–1144. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef]
- Galamb, O.; Barták, B.K.; Kalmár, A.; Nagy, Z.B.; Szigeti, K.A.; Tulassay, Z.; Igaz, P.; Molnár, B. Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors. World J. Gastroenterol. 2019, 25, 5026. [Google Scholar] [CrossRef]
- Necsulea, A.; Soumillon, M.; Warnefors, M.; Liechti, A.; Daish, T.; Zeller, U.; Baker, J.C.; Grützner, F.; Kaessmann, H. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 2014, 505, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Washietl, S.; Kellis, M.; Garber, M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014, 24, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hu, Z.; Feng, Y.; Hu, X.; Yuan, J.; Zhao, S.D.; Zhang, Y.; Yang, L.; Shan, W.; He, Q. Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell 2015, 28, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Baassiri, A.; Nassar, F.; Mukherji, D.; Shamseddine, A.; Nasr, R.; Temraz, S. Exosomal non coding RNA in LIQUID biopsies as a promising biomarker for colorectal cancer. Int. J. Mol. Sci. 2020, 21, 1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Wang, X.; Song, Y.-X.; Zhao, J.-H.; Sun, J.-X.; Shi, J.-X.; Wu, Z.-H.; Wang, Z.-N. Circulating noncoding RNAs have a promising future acting as novel biomarkers for colorectal cancer. Dis. Markers 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef]
- Hall, J.; Messenger, Z.; Tam, H.; Phillips, S.; Recio, L.; Smart, R. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015, 6, e1700. [Google Scholar] [CrossRef]
- Ning, Y.; Yong, F.; Haibin, Z.; Hui, S.; Nan, Z.; Guangshun, Y. LincRNA-p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. Oncotarget 2015, 6, 28151. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Mei, Y.; Wu, M. Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Mol. Cell 2014, 53, 88–100. [Google Scholar] [CrossRef]
- Meng, S.-S.; Xu, X.-P.; Chang, W.; Lu, Z.-H.; Huang, L.-L.; Xu, J.-Y.; Liu, L.; Qiu, H.-B.; Yang, Y.; Guo, F.-M. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res. Ther. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Zhao, Q.; Zhu, Y.; Zhao, C.; Li, X.; Ma, Z.; Li, X.; Zhang, Y. LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/β-catenin signaling pathway. Oncol. Rep. 2014, 31, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Fesler, A.; Schee, K.; Fodstad, Ø.; Flatmark, K.; Ju, J. Clinical significance of long intergenic noncoding RNA-p21 in colorectal cancer. Clin. Colorectal Cancer 2013, 12, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Chaleshi, V.; Irani, S.; Alebouyeh, M.; Mirfakhraie, R.; Aghdaei, A.H. Association of lncRNA-p53 regulatory network (lincRNA-p21, lincRNA-ROR and MALAT1) and p53 with the clinicopathological features of colorectal primary lesions and tumors. Oncol. Lett. 2020, 19, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.J.; Navarro, A.; Viñolas, N.; Marrades, R.M.; Moises, J.; Cordeiro, A.; Saco, A.; Muñoz, C.; Fuster, D.; Molins, L. LincRNA-p21 impacts prognosis in resected non–small cell lung cancer patients through angiogenesis regulation. J. Thorac. Oncol. 2016, 11, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ruan, Y.; Wang, X.; Zhao, W.; Jiang, Q.; Jiang, C.; Zhao, Y.; Xu, Y.; Sun, F.; Zhu, Y. Long intragenic non-coding RNA linc RNA-p21 suppresses development of human prostate cancer. Cell Prolif. 2017, 50, e12318. [Google Scholar] [CrossRef]
- Peng, W.; Wu, J.; Feng, J. LincRNA-p21 predicts favorable clinical outcome and impairs tumorigenesis in diffuse large B cell lymphoma patients treated with R-CHOP chemotherapy. Clin. Exp. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Yang, W.; Yu, H.; Shen, Y.; Liu, Y.; Yang, Z.; Sun, T. MiR-146b-5p overexpression attenuates stemness and radioresistance of glioma stem cells by targeting HuR/lincRNA-p21/β-catenin pathway. Oncotarget 2016, 7, 41505. [Google Scholar] [CrossRef]
- Monzo, M.; Santasusagna, S.; Moreno, I.; Martinez, F.; Hernáez, R.; Muñoz, C.; Castellano, J.J.; Moreno, J.; Navarro, A. Exosomal microRNAs isolated from plasma of mesenteric veins linked to liver metastases in resected patients with colon cancer. Oncotarget 2017, 8, 30859. [Google Scholar] [CrossRef]
- Santasusagna, S.; Moreno, I.; Navarro, A.; Rodenas, F.M.; Hernandez, R.; Castellano, J.J.; Munoz, C.; Monzo, M. Prognostic impact of miR-200 family members in plasma and exosomes from tumor-draining versus peripheral veins of colon cancer patients. Oncology 2018, 95, 309–318. [Google Scholar] [CrossRef]
- Castellano, J.J.; Marrades, R.M.; Molins, L.; Viñolas, N.; Moises, J.; Canals, J.; Han, B.; Li, Y.; Martinez, D.; Monzó, M. Extracellular vesicle lincRNA-p21 expression in tumor-draining pulmonary vein defines prognosis in NSCLC and modulates endothelial cell behavior. Cancers 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, M.; Chen, B.; Calin, G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018, 7 (Suppl. 2), S243. [Google Scholar] [CrossRef] [PubMed]
- Sole, C.; Arnaiz, E.; Manterola, L.; Otaegui, D.; Lawrie, C.H. The Circulating Transcriptome as a Source of Cancer Liquid Biopsy Biomarkers. Semin. Cancer Biol. 2019, 58, 100–108. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Value | All CRC Patients (n = 177) | Colon Cancer (n = 96) | Rectal Cancer (n = 81) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | DFS | OS | N (%) | DFS | OS | N (%) | DFS | OS | ||
| Sex | Male | 103 (58.2) | 0.644 | 0.311 | 52 (54.2) | 0.473 | 0.928 | 51 (63) | 0.081 | 0.111 |
| Female | 74 (41.8) | 44 (45.8) | 30 (37) | |||||||
| Age (years) | ≤65 | 59 (33.3) | 0.002 | 0.001 | 31 (32.3) | 0.02 | 0.012 | 28 (34.6) | 0.024 | 0.002 |
| >65 | 118 (66.7) | 65 (67.7) | 53 (65.4) | |||||||
| ECOG PS * | 0 | 8 (4.5) | 0.041 | 0.017 | 6 (6.3) | 0.017 | 0.013 | 2 (2.5) | 0.405 | 0.292 |
| 1 | 148 (84.2) | 79 (82.3) | 70 (86.4) | |||||||
| 2 | 20 (11.3) | 11 (11.5) | 9 (11.1) | |||||||
| T | 1 | 11 (6.2) | 0.467 | 0.266 | 3 (3.1) | 0.373 | 0.446 | 8 (9.9) | 0.761 | 0.391 |
| 2 | 36 (20.3) | 10 (10.4) | 26 (32.1) | |||||||
| 3 | 112 (63.3) | 68 (70.8) | 44 (54.3) | |||||||
| 4 | 18 (10.2) | 15 (15.6) | 3 (3.7) | |||||||
| N | 0 | 120 (67.8) | 0.233 | 0.418 | 63 (65.6) | 0.385 | 0.344 | 57 (70.4) | 0.659 | 0.858 |
| 1 | 40 (22.6) | 25 (26) | 15 (18.5) | |||||||
| 2 | 17 (9.6) | 8 (8.3) | 9 (11.1) | |||||||
| Stage | I | 37 (20.9) | 0.314 | 0.554 | 10 (10.4) | 0.258 | 0.353 | 27 (33.4) | 0.763 | 0.994 |
| II | 83 (46.9) | 53 (55.2) | 30 (37) | |||||||
| III | 57 (32.2) | 33 (34.4) | 24 (29.6) | |||||||
| Previous polyp | Yes | 40 (22.6) | 0.311 | 0.179 | 26 (27.1) | 0.142 | 0.03 | 14 (17.3) | 0.915 | 0.896 |
| No | 137 (77.4) | 70 (72.9) | 67 (82.7) | |||||||
| Mucin secretion | Yes | 33 (18.6) | 0.534 | 0.129 | 27 (28.1) | 0.691 | 0.292 | 75 (92.6) | 0.152 | 0.054 |
| No | 144 (81.4) | 69 (71.9) | 6 (7.4) | |||||||
| Relapse | Yes | 33 (18.6) | --- | --- | 17 (17.7) | --- | --- | 16 (19.8) | --- | --- |
| No | 144 (81.4) | 79 (82.3) | 65 (80.2) | |||||||
| DFS | OS | ||||
|---|---|---|---|---|---|
| Factor | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| CRC patients | Advanced age | 1.043 (1.016–1.071) | 0.02 | 1.075 (1.042–1.110) | p < 0.001 |
| Stage III | 1.379 (0.687–2.769) | 0.367 | 1.435 (0.676–3.046) | 0.348 | |
| No adjuvant treatment | 0.778 (0.339–1.785) | 0.554 | 0.658 (0.258–1.678) | 0.380 | |
| ECOG PS (0) | 0.274 (0.056–1.341) | 0.110 | 0.408 (0.081–2.046) | 0.276 | |
| High lincRNA-p21 | 1.747 (1.074–2.841) | 0.025 | 1.884 (1.104–3.217) | 0.020 | |
| RC Patients | Advanced age | 1.032 (0.996–1.069) | 0.087 | 1.064 (1.017–1.113) | 0.007 |
| Male | 0.570 (0.290–1.119) | 0.102 | - | - | |
| Mucin secretion | - | - | 2.468 (0.833–7.317) | 0.103 | |
| Stage III | 0.769 (0.148–4.013) | 0.756 | 0.606 (0.095–3.861) | 0.596 | |
| No adjuvant treatment | 0.924 (0.409–2.090) | 0.850 | 0.996 (0.423–2.345) | 0.992 | |
| High lincRNA-p21 | 1.986 (1.013–3.895) | 0.046 | 1.318(0.562–3.091) | 0.562 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Castellano, J.J.; Moreno, I.; Martínez-Rodenas, F.; Hernandez, R.; Canals, J.; Diaz, T.; Han, B.; Muñoz, C.; Biete, A.; et al. LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients. Life 2020, 10, 172. https://doi.org/10.3390/life10090172
Li Y, Castellano JJ, Moreno I, Martínez-Rodenas F, Hernandez R, Canals J, Diaz T, Han B, Muñoz C, Biete A, et al. LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients. Life. 2020; 10(9):172. https://doi.org/10.3390/life10090172
Chicago/Turabian StyleLi, Yan, Joan J. Castellano, Isabel Moreno, Francisco Martínez-Rodenas, Raquel Hernandez, Jordi Canals, Tania Diaz, Bing Han, Carmen Muñoz, Albert Biete, and et al. 2020. "LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients" Life 10, no. 9: 172. https://doi.org/10.3390/life10090172
APA StyleLi, Y., Castellano, J. J., Moreno, I., Martínez-Rodenas, F., Hernandez, R., Canals, J., Diaz, T., Han, B., Muñoz, C., Biete, A., Monzo, M., & Navarro, A. (2020). LincRNA-p21 Levels Relates to Survival and Post-Operative Radiotherapy Benefit in Rectal Cancer Patients. Life, 10(9), 172. https://doi.org/10.3390/life10090172







