Prevalence and Clinical Characteristics of Children and Adolescents with Metabolically Healthy Obesity: Role of Insulin Sensitivity
Abstract
1. Introduction
2. Methods
2.1. Study Procedures and Assays
2.2. Statistical Analysis
3. Results
3.1. Adipokines: Leptin and Adiponectin
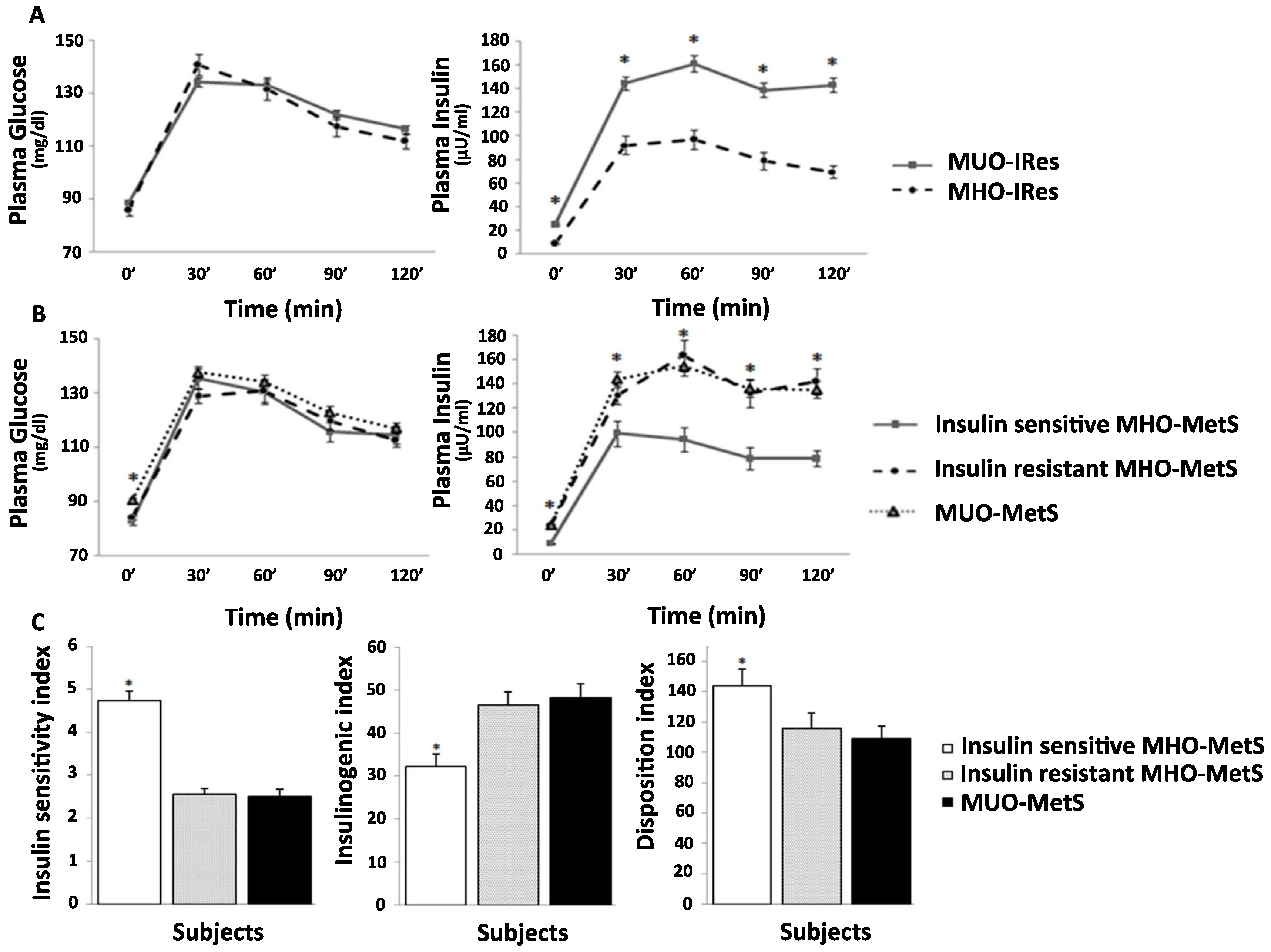
3.2. OGTT-Patterns and Derived Indices
3.3. Multivariate Logistic Regression Analyses
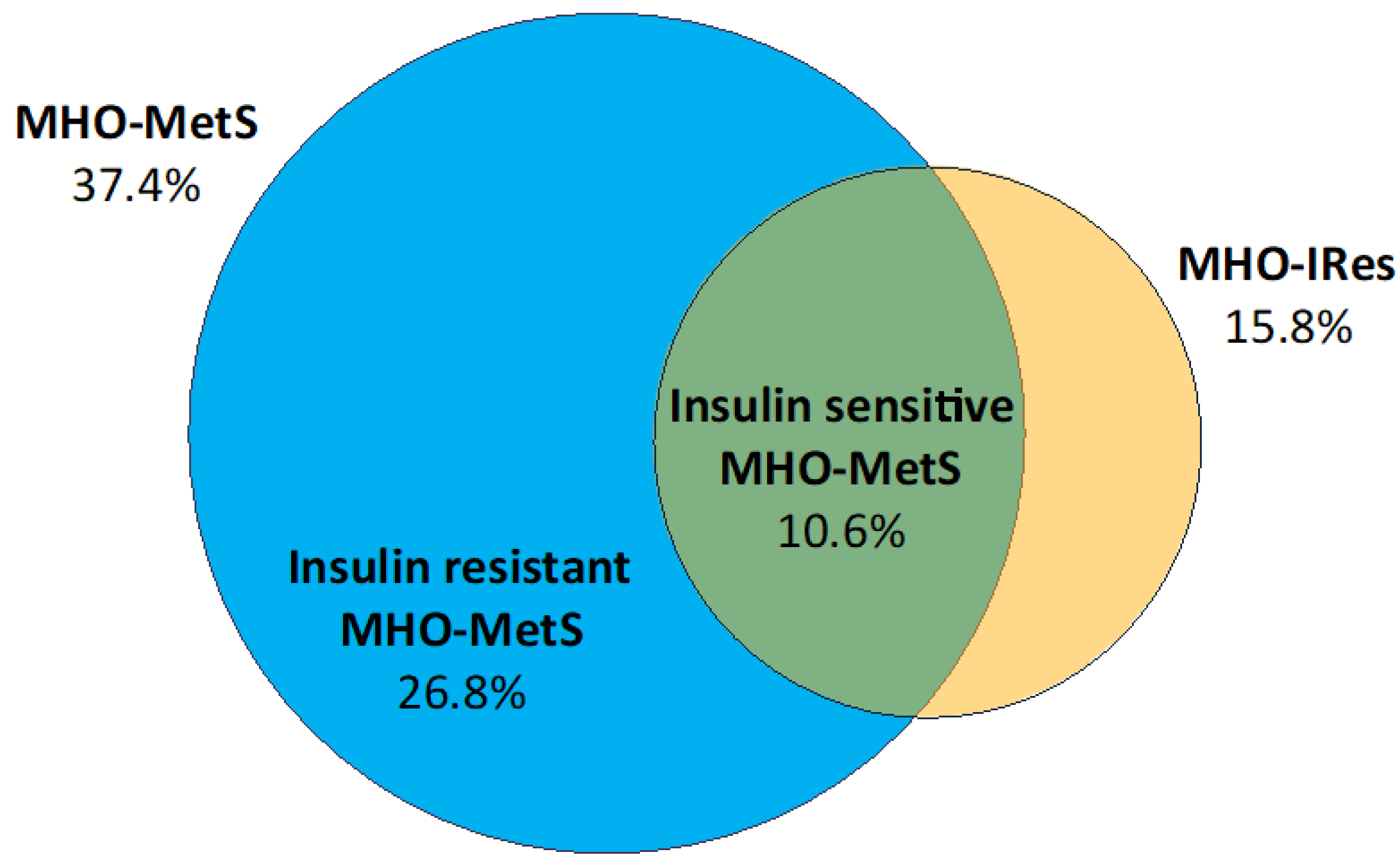
3.4. MHO-MetS Subgroups According to Insulin Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cali, A.M.; Caprio, S. Obesity in children and adolescents. J. Clin. Endocrinol. Metab. 2008, 93, S31–S36. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- De Onis, M.; Blossner, M.; Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Parrino, C.; Rossetti, P.; Baratta, R.; La Spina, N.; La Delfa, L.; Squatrito, S.; Vigneri, R.; Frittitta, L. Secular trends in the prevalence of overweight and obesity in Sicilian schoolchildren aged 11–13 years during the last decade. PLoS ONE 2012, 7, e34551. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, F.; Tumminia, A.; Roppolo, F.; Romeo, L.C.; La Spina, N.; Baratta, R.; Parrino, C.; Sciacca, L.; Vigneri, R.; Frittitta, L. Impact of unhealthy childhood and unfavorable parents’ characteristics on adiposity in schoolchildren. Diabetes Metab. Res. Rev. 2019, 35, e3199. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Olsen, L.W.; Sorensen, T.I. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 2007, 357, 2329–2337. [Google Scholar] [CrossRef]
- Freedman, D.S.; Mei, Z.; Srinivasan, S.R.; Berenson, G.S.; Dietz, W.H. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J. Pediatrics 2007, 150, 12–17. [Google Scholar] [CrossRef]
- Bokor, S.; Frelut, M.L.; Vania, A.; Hadjiathanasiou, C.G.; Anastasakou, M.; Malecka-Tendera, E.; Matusik, P.; Molnar, D. Prevalence of metabolic syndrome in European obese children. Int. J. Pediatric Obes. 2008, 3, 3–8. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef]
- Vukovic, R.; Santos, T.J.; Ybarra, M.; Atar, M. Children with Metabolically Healthy Obesity: A Review. Front. Endocrinol. 2019, 10, 865. [Google Scholar] [CrossRef]
- Bluher, S.; Schwarz, P. Metabolically healthy obesity from childhood to adulthood—Does weight status alone matter? Metab. Clin. Exp. 2014, 63, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Kivimaki, M.; Hamer, M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes. Rev. 2014, 15, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F. Metabolically healthy obesity: What’s in a name? Am. J. Clin. Nutr. 2019, 3, 533–539. [Google Scholar] [CrossRef]
- Zhao, M.; Lopez-Bermejo, A.; Caserta, C.A.; Medeiros, C.C.M.; Kollias, A.; Bassols, J.; Romeo, E.L.; Ramos, T.D.A.; Stergiou, G.S.; Yang, L.; et al. Metabolically Healthy Obesity and High Carotid Intima-Media Thickness in Children and Adolescents: International Childhood Vascular Structure Evaluation Consortium. Diabetes Care 2019, 42, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hinnouho, G.M.; Czernichow, S.; Dugravot, A.; Nabi, H.; Brunner, E.J.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. Eur. Heart J. 2015, 36, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Metabolically healthy obesity: Different prevalences using different criteria. Eur. J. Clin. Nutr. 2010, 64, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Vanlancker, T.; Schaubroeck, E.; Vyncke, K.; Cadenas-Sanchez, C.; Breidenassel, C.; Gonzalez-Gross, M.; Gottrand, F.; Moreno, L.A.; Beghin, L.; Molnar, D.; et al. Comparison of definitions for the metabolic syndrome in adolescents. The HELENA study. Eur. J. Pediatrics 2017, 176, 241–252. [Google Scholar] [CrossRef]
- Vukovic, R.; Mitrovic, K.; Milenkovic, T.; Todorovic, S.; Soldatovic, I.; Sipetic-Grujicic, S.; Zdravkovic, D. Insulin-sensitive obese children display a favorable metabolic profile. Eur. J. Pediatrics 2013, 172, 201–206. [Google Scholar] [CrossRef]
- Prince, R.L.; Kuk, J.L.; Ambler, K.A.; Dhaliwal, J.; Ball, G.D. Predictors of metabolically healthy obesity in children. Diabetes Care 2014, 37, 1462–1468. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G. Classification and clinical characterization of metabolically “healthy” obese children and adolescents. J. Pediatric Endocrinol. Metab. 2016, 29, 553–560. [Google Scholar] [CrossRef]
- Weghuber, D.; Zelzer, S.; Stelzer, I.; Paulmichl, K.; Kammerhofer, D.; Schnedl, W.; Molnar, D.; Mangge, H. High risk vs. “metabolically healthy” phenotype in juvenile obesity—neck subcutaneous adipose tissue and serum uric acid are clinically relevant. Exp. Clin. Endocrinol. Diabetes 2013, 121, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, J.; Cheng, H.; Wang, Y.; Gao, S.; Li, M.; Grant, S.F.; Li, C.; Mi, J.; Li, M. Identification of Genetic and Environmental Factors Predicting Metabolically Healthy Obesity in Children: Data From the BCAMS Study. J. Clin. Endocrinol. Metab. 2016, 101, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Camhi, S.M.; Waring, M.E.; Sisson, S.B.; Hayman, L.L.; Must, A. Physical activity and screen time in metabolically healthy obese phenotypes in adolescents and adults. J. Obes. 2013, 2013, 984613. [Google Scholar] [CrossRef] [PubMed]
- Damanhoury, S.; Newton, A.S.; Rashid, M.; Hartling, L.; Byrne, J.L.S.; Ball, G.D.C. Defining metabolically healthy obesity in children: A scoping review. Obes. Rev. 2018, 19, 1476–1491. [Google Scholar] [CrossRef]
- Cadenas-Sanchez, C.; Ruiz, J.R.; Labayen, I.; Huybrechts, I.; Manios, Y.; Gonzalez-Gross, M.; Breidenassel, C.; Kafatos, A.; De Henauw, S.; Vanhelst, J.; et al. Prevalence of Metabolically Healthy but Overweight/Obese Phenotype and Its Association With Sedentary Time, Physical Activity, and Fitness. J. Adolesc. Health 2017, 61, 107–114. [Google Scholar] [CrossRef]
- Senechal, M.; Wicklow, B.; Wittmeier, K.; Hay, J.; MacIntosh, A.C.; Eskicioglu, P.; Venugopal, N.; McGavock, J.M. Cardiorespiratory fitness and adiposity in metabolically healthy overweight and obese youth. Pediatrics 2013, 132, e85–e92. [Google Scholar] [CrossRef]
- Schulze, M.B. Metabolic health in normal-weight and obese individuals. Diabetologia 2019, 62, 558–566. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Parrino, C.; Vinciguerra, F.; La Spina, N.; Romeo, L.; Tumminia, A.; Baratta, R.; Squatrito, S.; Vigneri, R.; Frittitta, L. Influence of early-life and parental factors on childhood overweight and obesity. J. Endocrinol. Investig. 2016, 39, 1315–1321. [Google Scholar] [CrossRef]
- Jiang, Y.; Dou, Y.L.; Xiong, F.; Zhang, L.; Zhu, G.H.; Wu, T.; Zhang, Y.; Yan, W.L. Waist-to-height ratio remains an accurate and practical way of identifying cardiometabolic risks in children and adolescents. Acta Paediatr. 2018, 9, 1629–1634. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Baratta, R.; Rossetti, P.; Prudente, S.; Barbetti, F.; Sudano, D.; Nigro, A.; Farina, M.G.; Pellegrini, F.; Trischitta, V.; Frittitta, L. Role of the ENPP1 K121Q polymorphism in glucose homeostasis. Diabetes 2008, 57, 3360–3364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdul-Ghani, M.A.; Williams, K.; DeFronzo, R.A.; Stern, M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007, 30, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Ardern, C.I. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009, 32, 2297–2299. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Lattuada, G.; Piemonti, L.; Garancini, M.P.; Ragogna, F.; Villa, M.; Mannino, S.; Crosignani, P.; Bosi, E.; Luzi, L.; et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: The Cremona Study. Diabetes Care 2011, 34, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Durward, C.M.; Hartman, T.J.; Nickols-Richardson, S.M. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J. Obes. 2012, 2012, 460321. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Huang, K.C.; Lin, R.C.; Kormas, N.; Lee, L.T.; Chen, C.Y.; Gill, T.P.; Caterson, I.D. Plasma leptin is associated with insulin resistance independent of age, body mass index, fat mass, lipids, and pubertal development in nondiabetic adolescents. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 470–475. [Google Scholar] [CrossRef]
- Ding, W.; Cheng, H.; Chen, F.; Yan, Y.; Zhang, M.; Zhao, X.; Hou, D.; Mi, J. Adipokines are Associated with Hypertension in Metabolically Healthy Obese (MHO) Children and Adolescents: A Prospective Population-Based Cohort Study. J. Epidemiol. 2018, 28, 19–26. [Google Scholar] [CrossRef]
- Fu, J.; Li, Y.; Esangbedo, I.C.; Li, G.; Feng, D.; Li, L.; Xu, L.; Han, L.; Li, M.; Li, C.; et al. Circulating Osteonectin and Adipokine Profiles in Relation to Metabolically Healthy Obesity in Chinese Children: Findings from BCAMS. J. Am. Heart Assoc. 2018, 7, e009169. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Yeckel, C.W.; Weiss, R.; Dziura, J.; Taksali, S.E.; Dufour, S.; Burgert, T.S.; Tamborlane, W.V.; Caprio, S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J. Clin. Endocrinol. Metab. 2004, 89, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Levy-Marchal, C.; Arslanian, S.; Cutfield, W.; Sinaiko, A.; Druet, C.; Marcovecchio, M.L.; Chiarelli, F. Insulin resistance in children: Consensus, perspective, and future directions. J. Clin. Endocrinol. Metab. 2010, 95, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Ten, S.; Maclaren, N. Insulin resistance syndrome in children. J. Clin. Endocrinol. Metab. 2004, 89, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Allison, G.; Abbasi, F.; Lamendola, C.; Reaven, G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metab. Clin. Exp. 2004, 53, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Jacobs, D.R., Jr.; Steinberger, J.; Hong, C.P.; Prineas, R.; Luepker, R.; Sinaiko, A.R. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes 1999, 48, 2039–2044. [Google Scholar] [CrossRef]
- Goran, M.I.; Gower, B.A. Longitudinal study on pubertal insulin resistance. Diabetes 2001, 50, 2444–2450. [Google Scholar] [CrossRef]
- Sinaiko, A.R.; Steinberger, J.; Moran, A.; Hong, C.P.; Prineas, R.J.; Jacobs, D.R., Jr. Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides, and high-density lipoprotein cholesterol at age 19. Hypertension 2006, 48, 730–736. [Google Scholar] [CrossRef]
- Robins, S.J.; Lyass, A.; Zachariah, J.P.; Massaro, J.M.; Vasan, R.S. Insulin resistance and the relationship of a dyslipidemia to coronary heart disease: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1208–1214. [Google Scholar] [CrossRef]
- Bacha, F.; Saad, R.; Gungor, N.; Arslanian, S.A. Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care 2006, 29, 1599–1604. [Google Scholar] [CrossRef]
- Khan, U.I.; Wang, D.; Thurston, R.C.; Sowers, M.; Sutton-Tyrrell, K.; Matthews, K.A.; Barinas-Mitchell, E.; Wildman, R.P. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: The Study of Women’s Health Across the Nation (SWAN). Atherosclerosis 2011, 217, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Novo, G.; Manno, G.; Russo, R.; Buccheri, D.; Dell’Oglio, S.; Morreale, P.; Evola, G.; Vitale, G.; Novo, S. Impact of insulin resistance on cardiac and vascular function. Int. J. Cardiol. 2016, 221, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Mellor, K.M.; Bell, J.R.; Ritchie, R.H.; Delbridge, L.M. Myocardial insulin resistance, metabolic stress and autophagy in diabetes. Clin. Exp. Pharmacol. Physiol. 2013, 40, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Domenighetti, A.A.; Danes, V.R.; Curl, C.L.; Favaloro, J.M.; Proietto, J.; Delbridge, L.M. Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca(2+) and proton flux dysregulation. J. Mol. Cell. Cardiol. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Oreto, L.; Pepe, G.; Calabro, M.P.; Longobardo, L.; Morabito, L.; Pajno, G.B.; Alibrandi, A.; Aversa, T.; Wasniewska, M. Precocious Preclinical Cardiovascular Sonographic Markers in Metabolically Healthy and Unhealthy Childhood Obesity. Front. Endocrinol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Peters, R.K.; Kjos, S.L.; Marroquin, A.; Goico, J.; Ochoa, C.; Tan, S.; Berkowitz, K.; Hodis, H.N.; et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002, 51, 2796–2803. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Prigeon, R.L.; Faulenbach, M.V.; Tong, J.; Carr, D.B.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Fujimoto, W.Y.; Kahn, S.E. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009, 32, 335–341. [Google Scholar] [CrossRef]
| Method Used to Identify MHO Individuals | Reference | Years | Country | MHO Prevalence |
|---|---|---|---|---|
| • ≤1 of the following: TG ≥ 110 mg/dL, HDL-C < 40 mg/dL, SBP/DBP ≥ 90th percentile for age and gender and height, and FPG ≥ 100 mg/dL | [23] | 2013 | USA | 68% |
| • Homeostasis model assessment–insulin resistance (HOMA-IR) ≤ 2.75 | [18] | 2013 | Serbia | 25% |
| • Absence of age- and gender-specific cut-off points for FG, TG, HDL- C, SBP and DBP | [26] | 2013 | Canada | 25% |
| • Absence of: TG ≥ 150 mg/dL, HDL-C < 40 mg/dL (males) and <50 (females), SBP ≥ 130 mmHg or DBP ≥ 85 mmHg, FPG ≥ 100 mg/dL | [21] | 2013 | Austria | 16% |
| • A: HOMA-IR < 3.16 • B: Absence of: SBP or DBP ≥ 90th percentile for age, gender and height; TG ≥ 25 mmol/L; HDL-C ≥ 1.02 mmol/L; FPG ≥ 5.6 mmol/L. • C: A + B | [19] | 2014 | Canada | A: 21.5% B: 31.5% C: 11.6% |
| • A: Absence of: HDL-C < 40 mg/dL (males) and <50 mg/dL (females), TG ≥ 150 mg/dL; SBP ≥ 130 mmHg or DBP ≥ 85 mmHg; FPG ≥ 100 mg/dL • B: HOMA-IR < 3.16 • C: A + B | [20] | 2016 | Belgium | A: 18.6% B: 19.2% C: 6.4% |
| • A: HOMA-IR ≤ 2.3 • B: Absence of: SBP or DBP ≥ 90th percentile for age, gender and height, TG ≥ 1.24 mmol/L; HDL-C ≤ 1.03 mmol/L, FPG ≥ 5.6 mmol/L | [22] | 2016 | China | A: 27.1% B: 37.2% |
| • Absence of sex- and age-specific metabolic syndrome cut-off points for FG, TG, HDL- C, SBP and DBP | [25] | 2017 | Europe | 80% |
| • A: Absence of: SBP or DBP ≥ 90th percentile for age, gender and height, TG ≥ 110 mg/dL, HDL-C < 40 mg/dL and FPG ≥ 110 mg/dL • B: Absence of: elevated SBP or DBP (≥120/80 mmHg), TG ≥ 150 mg/dL, HDL-C < 40 mg/dL, FPG ≥ 100 mg/dL | [14] | 2019 | Brazil, China, Greece, Italy Spain | A: 4.5% B: 8.2% |
| •A: HOMA-IR < 2.5 (MHO-IRes) • B: Absence of: FPG ≥ 100 mg/dL, TG ≥ 95th percentile, HDL-C ≤ 5th percentile, SBP or DBP ≥ 95th percentile (MHO-MetS) • C: A + B (insulin sensitive MHO-MetS) | Present study | 2020 | Italy | A: 15.8% B: 37.4% C: 10.6% |
| MHO-IRes | MHO-MetS | Insulin Sensitive MHO-MetS | Insulin Resistant MHO-MetS |
|---|---|---|---|
| HOMA index < 2.5 | FPG < 100 mg/dL | FPG < 100 mg/dL | FPG < 100 mg/dL |
| TG ≤ 95th percentile | TG ≤ 95th percentile | TG ≤ 95th percentile | |
| HDL-C ≥ 5th percentile | HDL-C ≥ 5th percentile | HDL-C ≥ 5th percentile | |
| SBP or DBP ≤ 95th percentile | SBP or DBP ≤ 95th percentile | SBP or DBP ≤ 95th percentile | |
| HOMA index < 2.5 | HOMA index ≥ 2.5 |
| Characteristics | Overall n = 246 | MUO-IRes n = 207 | MHO-IRes n = 39 | p | MUO-MetS n = 154 | MHO-MetS n = 92 | p |
|---|---|---|---|---|---|---|---|
| Age (years) | 12.8 ± 2.8 | 12.8 ± 2.8 | 12.9 ± 3.1 | 0.71 | 12.8 ± 2.7 | 12.8 ± 3.1 | 0.89 |
| Males (n, %) | 126 (51.2) | 102 (49.3) | 25 (64.1) | 0.08 | 80 (51.9) | 46 (50) | 0.77 |
| Pubertal–Tanner stages 2–5 (n, %) | 127 (51.6) | 111 (53.6) | 16 (41.0) | 0.20 | 80 (51.9) | 47 (51.1) | 0.75 |
| Tanner stage 3 (n, %) | 30 (12.2) | 26 (12.6) | 4 (10.2) | 0.69 | 21 (13.6) | 9 (9.8) | 0.37 |
| Tanner stage 5 (n, %) | 70 (28.4) | 59 (28.5) | 11 (28.2) | 0.87 | 41 (26.6) | 29 (31.5) | 0.41 |
| Early obesity onset (<5 years) (n, %) | 126 (51.2) | 103 (49.8) | 22 (56.4) | 0.67 | 80 (51.9) | 46 (50) | 0.94 |
| BMI z-score | 2.5 ± 0.5 | 2.6 ± 0.5 | 2.2 ± 0.3 | <0.01 | 2.5 ± 0.5 | 2.4 ± 0.5 | 0.09 |
| WC (cm) | 106.1 ± 13.6 | 107.5 ± 13.4 | 97.9 ± 11.3 | <0.01 | 107.1 ± 13.8 | 104.3 ± 13.0 | 0.06 |
| WtHR | 0.68 ± 0.07 | 0.68 ± 0.07 | 0.64 ± 0.05 | <0.01 | 0.68 ± 0.07 | 0.67 ± 0.06 | 0.16 |
| SBP (mmHg) | 111.6 ± 13.1 | 112.3 ± 13.4 | 107.8 ± 10.5 | 0.02 | 115.1 ± 13.5 | 105.9 ± 10.0 | <0.01 |
| DBP (mmHg) | 69.7 ± 8.8 | 70.1 ± 9.1 | 67.2 ± 7.1 | 0.05 | 72.3 ± 9.0 | 65.3 ± 6.5 | <0.01 |
| Total cholesterol (mg/dL) | 156.7 ± 28.5 | 156.0 ± 28.9 | 159.9 ± 26.0 | 0.44 | 157.5 ± 30.9 | 155.2 ± 23.9 | 0.52 |
| HDL-C (mg/dL) | 40.0 ± 10.0 | 39.5 ± 10.0 | 43.3 ± 8.8 | 0.01 | 36.7 ± 10.7 | 42.2 ± 8.0 | <0.01 |
| Triglycerides (mg/dL) | 92.3 ± 45.8 | 94.5 ± 46.8 | 80.5 ± 38.0 | 0.06 | 97.8 ± 49.6 | 83.1 ± 36.8 | 0.01 |
| LDL-C (mg/dL) | 98.2 ± 24.5 | 97.7 ± 24.8 | 100.5 ± 22.6 | 0.46 | 99.3 ± 26.3 | 96.3 ± 20.9 | 0.34 |
| FPG (mg/dL) | 88.1 ± 11.9 | 88.5 ± 12.0 | 85.7 ± 11.5 | 0.18 | 90.5 ± 13.1 | 83.9 ± 8.0 | <0.01 |
| Fasting plasma insulin (µU/mL) | 22.3 ± 10.9 | 24.9 ± 10.0 | 8.9 ± 2.2 | <0.01 | 24.1 ± 10.8 | 19.4 ± 10.4 | <0.01 |
| 2 h OGTT plasma glucose (mg/dL) | 115.6 ± 21.5 | 116.3 ± 22.3 | 111.8 ± 16.1 | 0.23 | 116.9 ± 22.7 | 113.2 ± 18.8 | 0.20 |
| 2 h OGTT plasma insulin (µU/mL) | 131.2 ± 85.0 | 142.7 ± 86.8 | 69.5 ± 33.1 | <0.01 | 135.2 ± 86.3 | 124.3 ± 82.6 | 0.30 |
| IFG, IGT, T2D (n, %) | 58 (23.6) | 51 (24.6) | 7 (17.9) | 0.37 | 50 (32.5) | 8 (8.7) | <0.01 |
| HOMA-IR | 4.9 ± 2.4 | 5.4 ± 2.3 | 1.9 ± 0.5 | <0.01 | 5.3 ± 2.5 | 4.1 ± 2.3 | <0.01 |
| ISI | 2.8 ± 1.4 | 2.4 ± 1.0 | 4.9 ± 1.5 | <0.01 | 2.5 ± 1.3 | 3.2 ± 1.5 | <0.01 |
| IGI | 46.1 ± 25.5 | 49.0 ± 25.7 | 30.3 ± 17.9 | <0.01 | 48.2 ± 26.6 | 42.5 ± 23.5 | 0.09 |
| DI | 114.8 ± 70.9 | 110.0 ± 68.1 | 139.9 ± 81.0 | 0.01 | 109.2 ± 67.6 | 124.0 ± 75.6 | 0.11 |
| Leptin (ng/mL) | 31.8 ± 21.2 | 33.9 ± 20.4 | 20.1 ± 13.9 | <0.01 | 34.0 ± 19.0 | 27.9 ± 21.4 | 0.01 |
| Adiponectin (µg/mL) | 12.2 ± 5.6 | 12.0 ± 5.5 | 13.4 ± 5.7 | 0.14 | 12.1 ± 5.5 | 12.4 ± 5.7 | 0.61 |
| MHO-IRes | MHO-MetS | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| BMI z-score | 0.15 (0.02–1.10) | 0.06 | 0.65 (0.21–2.00) | 0.45 |
| WC | 1.04 (0.96–1.11) | 0.31 | 0.99 (0.96–1.02) | 0.66 |
| SBP | 1.02 (0.96–1.08) | 0.64 | 0.96 (0.93–0.99) | 0.03 |
| DBP | 0.96 (0.86–1.05) | 0.36 | 0.85 (0.81–0.90) | <0.01 |
| HDL-C | 1.02 (0.96–1.08) | 0.56 | 1.07 (1.03–1.12) | <0.01 |
| Triglycerides | 1.00 (0.99–1.02) | 0.40 | 0.99 (0.98–1.01) | 0.47 |
| FPG | 0.99 (0.94–1.05) | 0.85 | 0.88 (0.85–0.92) | <0.01 |
| ISI | 7.49 (3.80–14.84) | <0.01 | 1.38 (1.09–1.74) | <0.01 |
| IGI | 0.98 (0.95–1.01) | 0.25 | 0.99 (0.98–1.01) | 0.38 |
| DI | 0.99 (0.98–1.01) | 0.20 | 1.00 (0.99–1.01) | 0.65 |
| Leptin | 0.96 (0.92–0.99) | 0.04 | 0.98 (0.97–1.01) | 0.26 |
| Adiponectin | 0.96 (0.87–1.05) | 0.39 | 0.99 (0.93–1.06) | 0.81 |
| MUO-MetS n = 154 | MHO-MetS n = 92 | p | ||
|---|---|---|---|---|
| Insulin Resistant MHO-MetS n = 66 | Insulin Sensitive MHO-MetS n = 26 | |||
| Age (yrs) | 12.8 ± 2.7 | 12.8 ± 2.9 | 12.9 ± 3.4 | 0.81 |
| Males (n, %) | 80 (51.9) | 30 (45.4) | 16 (61.5) | 0.30 |
| Pubertal–Tanner stages 2–5 (n, %) | 80 (51.9) | 36 (54.5) | 14 (53.8) | 0.80 |
| Tanner stage 3 (n, %) | 21 (13.6) | 6 (9.1) | 3 (11.5) | 0.72 |
| Tanner stage 5 (n, %) | 41 (26.6) | 22 (33.3) | 7 (26.9) | 0.55 |
| Early obesity onset (<5 years) (n, %) | 80 (51.9) | 30 (45.4) | 16 (61.5) | 0.80 |
| BMI z-score | 2.5 ± 0.5 | 2.5 ± 0.4 | 2.2 ± 0.3 *° | 0.02 |
| WC (cm) | 107.1 ± 13.8 | 106.4 ± 12.8 | 99.0 ± 12.2 *° | 0.01 |
| WtHR | 0.68 ± 0.07 | 0.68 ± 0.06 | 0.65 ± 0.05 *° | 0.01 |
| SBP (mmHg) | 115.1 ± 13.5 | 105.4 ± 10.4 * | 107.0 ± 8.7 * | <0.01 |
| DBP (mmHg) | 72.3 ± 9.0 | 64.8 ± 6.8 * | 66.4 ± 5.7 * | <0.01 |
| Total cholesterol (mg/dL) | 157.5 ± 30.9 | 153.6 ± 21.8 | 159.1 ± 28.6 | 0.56 |
| HDL-C (mg/dL) | 36.7 ± 10.7 | 41.8 ± 8.5 * | 43.4 ± 6.7 * | 0.01 |
| Triglycerides (mg/dL) | 97.8 ± 49.6 | 85.1 ± 36.3 | 78.2 ± 38.6 * | 0.04 |
| LDL-C (mg/dL) | 99.3 ± 26.3 | 94.8 ± 19.9 | 100.1 ± 23.3 | 0.42 |
| FPG (mg/dL) | 90.5 ± 13.1 | 84.4 ± 8.0 * | 82.8 ± 8.0 * | <0.01 |
| Fasting plasma insulin (µU/mL) | 24.1 ± 10.8 | 23.6 ± 9.2 | 8.7 ± 2.4 *° | <0.01 |
| 2 h OGTT plasma glucose (mg/dL) | 116.9 ± 22.7 | 112.7 ± 19.7 | 114.5 ± 16.6 | 0.32 |
| 2 h OGTT plasma insulin (µU/mL) | 135.2 ± 86.3 | 141.5 ± 89.1 | 78.6 ± 32.7 *° | <0.01 |
| IFG, IGT, T2D (n, %) | 50 (32.5) | 7 (10.6) * | 1 (3.8) * | <0.01 |
| HOMA-IR | 5.3 ± 2.5 | 4.9 ± 2.1 | 1.8 ± 0.5 *° | <0.01 |
| ISI | 2.5 ± 1.3 | 2.6 ± 1.0 | 4.7 ± 1.2 *° | <0.01 |
| IGI | 48.2 ± 26.6 | 46.5 ± 25.0 | 32.2 ± 14.8 *° | <0.01 |
| DI | 109.2 ± 67.6 | 116.1 ± 80.8 | 143.8 ± 56.7 * | 0.02 |
| Leptin (ng/mL) | 34.0 ± 19.0 | 31.9 ± 22.3 | 17.9 ± 15.5 *° | <0.01 |
| Adiponectin (µg/mL) | 12.1 ± 5.5 | 12.0 ± 5.5 | 13.4 ± 6.0 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinciguerra, F.; Tumminia, A.; Baratta, R.; Ferro, A.; Alaimo, S.; Hagnäs, M.; Graziano, M.; Vigneri, R.; Frittitta, L. Prevalence and Clinical Characteristics of Children and Adolescents with Metabolically Healthy Obesity: Role of Insulin Sensitivity. Life 2020, 10, 127. https://doi.org/10.3390/life10080127
Vinciguerra F, Tumminia A, Baratta R, Ferro A, Alaimo S, Hagnäs M, Graziano M, Vigneri R, Frittitta L. Prevalence and Clinical Characteristics of Children and Adolescents with Metabolically Healthy Obesity: Role of Insulin Sensitivity. Life. 2020; 10(8):127. https://doi.org/10.3390/life10080127
Chicago/Turabian StyleVinciguerra, Federica, Andrea Tumminia, Roberto Baratta, Alfredo Ferro, Salvatore Alaimo, Maria Hagnäs, Marco Graziano, Riccardo Vigneri, and Lucia Frittitta. 2020. "Prevalence and Clinical Characteristics of Children and Adolescents with Metabolically Healthy Obesity: Role of Insulin Sensitivity" Life 10, no. 8: 127. https://doi.org/10.3390/life10080127
APA StyleVinciguerra, F., Tumminia, A., Baratta, R., Ferro, A., Alaimo, S., Hagnäs, M., Graziano, M., Vigneri, R., & Frittitta, L. (2020). Prevalence and Clinical Characteristics of Children and Adolescents with Metabolically Healthy Obesity: Role of Insulin Sensitivity. Life, 10(8), 127. https://doi.org/10.3390/life10080127







