Abstract
The membrane binding by α-synuclein (αS), a presynaptic protein whose aggregation is strongly linked with Parkinson’s disease, influences its biological behavior under functional and pathological conditions. This interaction requires a conformational transition from a disordered-unbound to a partially helical membrane-bound state of the protein. In the present study, we used enhanced coarse-grained MD simulations to characterize the sequence and conformational determinants of the binding to synaptic-like vesicles by the N-terminal region of αS. This region is the membrane anchor and is of crucial importance for the properties of the physiological monomeric state of αS as well as for its aberrant aggregates. These results identify the key factors that play a role in the binding of αS with synaptic lipid bilayers in both membrane-tethered and membrane-locked conformational states.
1. Introduction
α-Synuclein (αS) is a 14 kDa protein that is highly abundant in the brain, where it localizes predominantly at the synaptic terminals [1,2,3]. The aggregation of αS is associated with the formation of Lewy Bodies in Parkinson’s disease (PD) and in other synucleinopathies [4,5,6,7,8]. Genetic links exist between αS and hereditary forms of early onset PD, including point mutations, duplications and triplications of the αS encoding gene [9,10,11]. Fibrillar aggregates of the non-amyloid-β component (NAC) region (residues 65–95) of αS are also found in the context of Alzheimer’s disease (AD), multiple system atrophy [12], dementia with Lewy bodies [13], and other synucleinopathies [7].
The functional role of αS, however, remains unclear [14]. The predominant localization of αS at the presynaptic terminals has indicated that it may be involved in various neuronal processes. The main body of evidence indicates that it has a role in regulating the trafficking of synaptic vesicles (SVs) [15,16,17,18], including a chaperone role in SNARE formation via an interaction with VAMP-2 at the surface of SVs [15,16,18]. Moreover, because of its ability to promote vesicle–vesicle interactions, clustering and fusion, which have been observed both in vitro [19,20] and in vivo [18], αS has been associated with the maintenance of SV pools [21,22] and with ER-to-golgi vesicle trafficking [17,23]. Additional putative roles include the mitigation of oxidative stress in mitochondria [24] and the interaction and regulation of ATP synthetase at the surface of mitochondrial membranes [25]. Taken together, the majority of the suggested functions of αS involve the crucial step of binding to cellular membranes, a process that is tightly regulated in vivo [26], and that affects the rate of αS aggregation [27,28,29] and the toxicity of its aggregates [22,30].
In the cytoplasm, αS is disordered and monomeric [31] and shows negligible secondary structure content throughout its sequence [32,33]. However, upon acetylation, the N-terminal residues adopt an increased α-helix character [32]. Upon binding with biological membranes, αS becomes highly enriched in α-helical structure [19,34,35], a transition that is promoted by a series of seven imperfect repeats located in the region 1–90 of the sequence that encode for lipophilic class A2 amphipathic α-helical segments [35]. These segments enable αS to adapt its binding properties to a large variety of amphipathic surfaces, ranging from detergent micelles to lipid bilayers as well as to the water–air interface [36]. NMR studies of αS bound to micelles have revealed a broken α-helix conformation formed by two helical segments in an antiparallel topological arrangement (residues 3–37 and 45–92) [34] whilst studies of the membrane-bound state reported mainly a single helix formed throughout the region 1–97 [37]. However, an emergent view suggests that a multitude of possible conformations are adopted by αS at the surface of membranes [19,34,38], including oligomeric states that promote self-assembly and aggregation [15,39].
Upon interaction with synaptic-like vesicles, three different regions of αS were found to exhibit distinctive structural and dynamical properties [40]. These regions include the N-terminal segment (residues 1–25), which is the primary membrane-anchoring region that adopts the conformation of a stable α-helix tightly bound to the surface of acidic lipid bilayers. A second region of the protein, spanning residues 26 to 97, was found to have an intermediate binding affinity for synaptic membranes and plays the role of “sensor” of the lipid properties that influences the overall membrane affinity of αS. Finally, the negatively charged C-terminal region (residues 98–140) was shown to remain mostly unbound from the membrane surface [40] and induces conformational changes in the protein upon calcium binding, thereby promoting strong co-localization with synaptic vesicles in synaptosomes [41].
The present study exploits an in silico research framework to study the details of the complex membrane binding process by αS, using enhanced molecular dynamics (MD) simulations based on coarse-grained force fields [42]. To this end, we adapted the Martini 3 force field to address specific questions about the binding modes of the 30 N-terminal residues of αS with synaptic membranes. We showed that this region has specific sequence properties that enable membrane binding in both helical conformation and in extended-tethered form. Deletion constructs known to alter the binding affinity of αS to membranes were indeed shown to affect the interaction of both conformational states in a correlated manner. The simulations also showed evidence for the role played by Phe 4 as a key promoter of the membrane interaction by αS. Overall, our study characterizes the nature of the balance between sequence and conformational properties in driving the interaction of this region with the membrane surface.
2. Materials and Methods
2.1. Simulation Setup
Simulations of the binding of the N-terminal region of αS to synaptic-like membranes were run using the GROMACS 4.6.7 platform [43] and a modified version of the Martini 3 force field [44]. In order to study the interaction of αS with synaptic-like membranes employed in previous experimental studies [40], we simulated a membrane composed of DOPE, DOPS and DOPC lipids in a 5:3:2 (w/w) ratio (167 lipid molecules in total) and the acetylated N-terminal region of αS (residues 1–30 designated as αS1–30, Table 1). The membrane was generated using the python script insane.py [45] and solvated with Martini water models and Cl− and Na+ ions to a salt concentration of 150 mM. Backbone termini at both ends of the protein were modeled as uncharged, including the acetylated first residue (M1) that was neutralized with the -nt flag and last residue (Ala 30) of αS1–30, which was also kept neutral. In addition to simulations of αS1–30, we run control simulations using the C-terminal region of the protein (αS111–140), which was modeled with an uncharged first residue (Gly 111) and a charged C-terminal residue (Ala 140). The conformations of αS1–30 or αS111–140 were generated using the martinize.py script starting from full atomic models of αS in helical or extended-disordered states. In each simulation, the peptide’s center of mass in the starting conformation was positioned at a distance of 4 nm from the lipid bilayer. The system was then equilibrated at different temperatures, using a series of 10 ns MD simulations in the NPT ensemble with an integration time step of 10 fs. Thermal equilibration was computed using the Velocity-rescale thermostat [46]. Fifteen equilibrations were run at the following temperatures: 310 K, 320 K, 330 K, 340 K, 350 K, 360 K, 370 K, 380 K, 390 K, 400 K, 410 K, 420 K, 430 K, 440 K, 450 K, with a coupling constant of 2 ps and by separately coupling water molecules, Na+ and Cl− ions, protein and the membrane. Pressure was equilibrated at 1 bar using a semi-isotropic Berendsen barostat [47], coupling the xy and z axis separately with a relaxation time of 12 ps and compressibility of 3 × 10−4 bar−1.

Table 1.
Simulation details.
Subsequently, sampling simulations were run using a timestep of 20 fs, for 15 independent samplings based on the same temperature range of the equilibration step (310 K to 450 K), and using the Velocity-rescale thermostat [46] and the Parrinello-Rahman barostat [48]. Electrostatic interactions were treated using the reaction field method with a Coulomb cut-off of 1.1 nm, whereas a cut off of 1.1 nm was used for van der Waals interactions. Bond lengths and sidechain angles were constrained using the Lincs algorithm [49]. Each simulation in the sampling phase was 4.8 μs with a total simulation sampling of 1368 μs. Details of the various simulations run in this study are reported in Table 1. Convergence of the simulations was checked by dividing the trajectories in three consecutive parts and by comparing the calculated observables in these independent segments.
2.2. CG Implementation
We modified the standard Martini 3 CG force field to restrain the protein in two states, namely, α-helical or extended-disordered conformations. In the context of the interaction with the membrane surface, these conformations mimic the final bound state (α-helical) and the initial tethered state (extended-disordered) in which the protein is absorbed onto the membrane surface in a disordered manner. To restrict the conformational variability of αS to these conformations, we restrained the angle between three consecutive backbone particles i, j and k (θijk) in the Martini 3 force field using Gaussian function energy potential. The angle θijk between three particles is defined as
and the restraining Gaussian potential:
where is the force constant of the Gaussian potential, defines the minimum of the potential energy (adopting 96° and 124° in helical and extended-disordered conformations, respectively) and is a constant that determines the width of the Gaussian minimum. The value for was set to 14° for all simulations (both helical and extended-disordered conformations). Control simulations performed by using values of 114° generated consistent results (Figure S1). The angle restraining force in our potential is:
where denotes the atomic coordinate vector of each particle.
Our modified version of Martini 3 also employed the restraint of dihedral angles between four consecutive backbone particles i, j, k and l (φijkl), ranging from −180 to 180 degrees in value, and is defined as the four-quadrant inverse tangent:
The Gaussian potential is constructed as before:
where defines the minimum of the potential energy (set to 60° and 100° for helical and extended-disordered conformations, respectively). The dihedral potential is implemented with two additional values of , specifically set at , in order to avoid discontinuities at the periodic boundaries of the atan2 function. The value for was set to 14° for all simulations (both helical and extended-disordered conformations), and control simulations run with values of 114° resulted in consistent data (Figure S1). The dihedral restraining force in our potential is
3. Results
3.1. Conformational Dependency in the Membrane Binding of N-Terminal Region αS
In order to sample the binding modes of the N-terminal region (residues 1–30) of αS (αS1–30) with DOPE:DOPS:DOPC lipid bilayers, we performed a series of CG MD simulations using a modified version of the Martini 3 force field (see Methods). In particular, the conformations of the backbone were restrained to adopt two main basins. The first was an extended-disordered conformation describing the initial “tethered” state of the protein absorbed onto the membrane surface in an amorphous manner. The second was a helical conformation describing the final “locked” membrane bound state, which for this region is a stable α-helix anchoring the protein onto the membrane surface [36].
αS1–30 was modeled in the acetylated form of the protein and with an uncharged C-terminal residue 30. In addition to the protein and the DOPE:DOPS:DOPC lipid bilayer (167 lipid molecules), the systems included 8686 to 8981 water molecules (Table 1) and Na+ and Cl− ions at a concentration of 150 mM. In all the simulations, the starting configuration of the protein was positioned at 4 nm from the membrane (Figure 1a). Systems were equilibrated in the NPT ensemble for 10 ns until convergence of the area occupied by the lipids. Samplings of each system, duplicated to sample both the helical and the extended-disordered states, were carried out at 15 different temperatures (collectively ranging from 310 K to 450 K with a 10 K step increase). Each sampling was run for 4.8 μs, which collectively amounted to a total sampling of 1368 μs in this study.
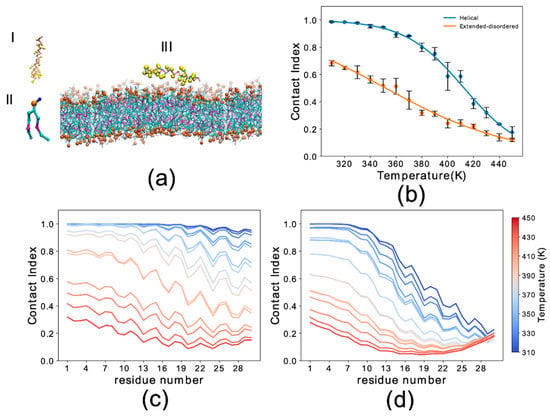
Figure 1.
Simulation setup and contact index comparison between the helical and extended-disordered conformations. (a) Representative setup of the simulations. (I) αS1–30 molecule in the Martini 3 force field in helical conformation. Backbone and side chain particles are shown in pink and yellow, respectively. (II) Representative lipid molecule (DOPE). The phosphate group is shown in orange. (III) Lipid bilayer (DOPE, DOPS and DOPC in a 5:3:2 w/w ratio) and an αS1–30 molecule in helical conformation. (b) Membrane-binding melting curves showing the global contact index as a function of the temperature of the simulation. Blue and orange lines report the melting curves of αS1–30 binding to DOPE:DOPS:DOPC lipid bilayer, with the protein in helical and extended-disordered conformations, respectively. Error bars report the standard deviation between three segments of the simulation. (c,d) Residue specific contact indexes in temperatures ranging from 310 K (dark blue) to 450 K (dark red), with a step increment of 10 K. Contact indexes for αS1–30 binding to DOPE:DOPS:DOPC lipid bilayer in helical (c) and extended-disordered (d) conformations.
We first analyzed the trajectories to obtain the probability of membrane interaction by each residue of the protein using the membrane contact index, which is calculated from the minimum distance between Cα atoms of the protein residues and phosphate atoms of the membrane. Membrane contacts were attributed based on a threshold of 1 nm. Residue-specific contact indexes were calculated for each frame of the sampling and averaged across the whole trajectory. The average of the residue contact indexes along the sequence provided the global contact index, which was plotted as a function of temperature to plot melting curves of the interaction with the membrane (Figure 1b). In addition to providing information on the membrane binding affinity, melting curves were also used to estimate the convergence of the simulations by comparing the data from three independent trajectory segments (Figure S2).
The results indicated that the backbone conformation has a significant influence on the binding properties of αS1–30. In particular the helical conformation strongly favors the membrane-binding of this region of αS, resulting in an increase of 60 K in the melting temperature when compared to the extended-disordered conformation (Figure 1b). At the lowest temperatures the global contact index reached a value of 1 for the helical state, indicating complete binding of αS1–30 at 310 K. In contrast, the maximum global contact index for the extended-disordered conformation was only 0.69. Another difference in the binding modes of the two conformations is that membrane contacts at 310 K were uniform throughout the sequence for the helical conformation (Figure 1c), with contact indexes showing a sequence periodicity recalling the nature of the α-helix structure. By contrast, in the extended-disordered conformation, strong membrane contacts were only observed in the initial seven residues (Figure 1d), with the rest of the sequence showing a progressive decrease in binding. As the temperature increases from 310 K (dark blue) to 450 K (dark red), the probability of contacts is reduced non-uniformly across the sequence in both types of conformations, with the initial 11 and 7 residues establishing the strongest contacts overall in the case of helical and extended disordered conformations, respectively.
3.2. Sequence Dependencies in the Membrane Affinity of αS1–30
Experimental studies indicated a key role of residue Phe 4 in the membrane binding properties of αS [31]. Here, we simulated F4A αS1–30 to study the effect of this mutation with our in silico membrane-binding assay. Our results showed that F4A induces a dramatic reduction in the membrane affinity of αS1–30 (Figure 2). The impairment of the membrane binding by this mutation was observed for both helical (Figure 2a) and extended-disordered states (Figure 2b), with a reduction in the melting temperature of 31 K and 42 K, respectively. The data therefore indicate that F4 has a role in both stabilizing the final helical bound state of the protein at the surface of DOPE:DOPS:DOPC lipid bilayers as well as the initial tethering process.
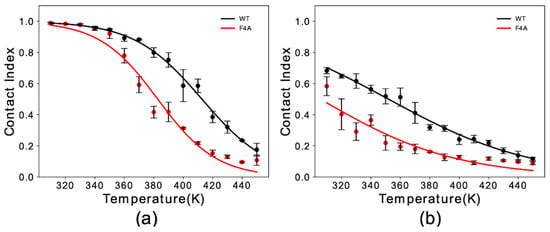
Figure 2.
Membrane binding melting curves of binding to DOPE:DOPS:DOPC lipid bilayers by WT and F4A αS1–30. The curves illustrate the dependency of the global contact index as a function of the temperature of the simulation. Data for WT and F4A αS1–30 are shown in black and red, respectively. Panels (a,b) report data for αS1–30 in helical and extended conformation, respectively. Error bars report the standard deviation between three segments of the simulation.
Structural studies by solid-state NMR [50] and in vitro binding assays [51] have evidenced the crucial role of the first 11 residues of the αS sequence in stabilizing the membrane binding of the protein. Constructs of αS with progressive truncations of the N-terminal residues were found to have altered membrane-binding affinities, with the deletion of residues 2–11 generating the strongest impairment in the binding [51]. We assessed in silico the membrane interaction of truncated versions of αS1–30, both in the helical and extended-disordered states.
Global contact indexes were calculated by using a window of 15 residues starting at the first amino acid of each construct. The resulting melting curves (Figure S3) were further condensed into a temperature-averaged global contact index (TAGCI) to provide a direct comparison between the membrane affinities of different αS constructs using a single value. TAGCI values of helical (Figure 3a) and extended conformations (Figure 3b) of the αS constructs featuring different levels of N-terminal truncation were in excellent agreement with experimental data [51]. The results indicate that the helical conformation maintains a higher membrane-binding affinity across all the constructs with respect to the extended-disordered state. Some sequence-specific patterns in the binding affinities of the truncated constructs were also observed. In particular, the deletion of residues Asp 2 and Val 3 from the sequence (Del-2 and Del-2-3) induced stronger membrane binding, as also observed experimentally [51]. This observation is likely associated with the removal of a negatively charged residue from the sequence. This effect was more prominent for the extended-disordered conformations, suggesting a negative regulation by Asp 2 of the tethering of αS to acidic membranes. Larger truncations generated a decrease in the binding affinity of αS in both helical and extended-disordered conformations, with the strongest effects found in the construct Del-2-11, which is in line with the experimental data [51]. Taken together, the data in the literature [50,51] and the present simulations highlight the key role of the first 11 residues of αS in the membrane binding of αS. In addition, the correlated patterns in helical (Figure 3a) and extended-disordered (Figure 3b) conformations indicate that the effects of the truncations are conformationally independent, thereby highlighting the intrinsic role of the sequence properties of αS1–30 in the membrane binding. The intrinsic role of the N-terminal sequence is further corroborated by control simulations of the region spanning the last 30 residues of αS (αS111–140), indicating a significant difference in the binding properties of αS1–30 and αS111–140. In particular, αS111–140 showed essentially no membrane affinity and a negligible conformational effect when comparing helical versus extended-disordered conformations (Figure 4).
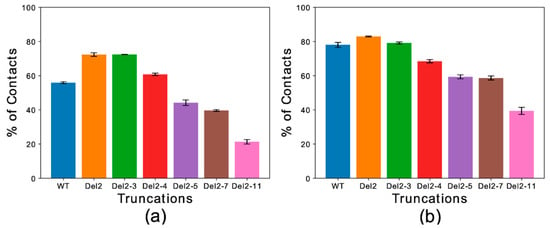
Figure 3.
Temperature-averaged global contact index (TAGCI). (a,b) The membrane interactions of different deletion constructs αS1–30 were simulated to mimic the conditions of previous experimental studies [51]. Helical (a) and extended-disordered (b) conformations were simulated across 15 temperatures. The TAGCI reports the average contact indexes by the first 15 residues of each construct and further averaged across the 15 temperatures employed in this study. The construct nomenclature reports the residues that have been deleted from the WT sequence.

Figure 4.
Binding properties of αS111–140 to DOPE:DOPS:DOPC lipid bilayers. (a,b) Membrane-binding melting curves of αS111–140 calculated from plotting the global contact index as a function of the temperature of the simulation. Helical and extended-disordered conformations are shown in panels (a) and (b), respectively. Error bars report the standard deviation between three segments of the simulations. (c,d) Residue specific contact indexes in the range of temperatures going from 310 K (dark blue) to 450 K (dark red) at step increment of 10 K. Plots for αS111–140 binding to DOPE:DOPS:DOPC lipid bilayer in helical (c) and extended-disordered (d) conformations are shown.
4. Discussion
The interaction of αS with cellular membranes is central to defining the properties of its biological form [52]. Binding to synaptic membranes is a common step in the majority of the putative functions of αS [36,53] as well as a fundamental property of αS aggregates forming under pathological conditions. It has been shown that membrane binding influences the kinetics of αS aggregation, with both acceleration and inhibition being observed under different experimental conditions [28,54]. Membrane binding also modulates the properties of αS oligomers, whose toxicity is promoted by the binding and disruption of synaptic membranes [55]. Under physiological conditions, αS establishes a crucial interaction with SVs [22,36] that has been implicated in a number of its putative functions [15,16,18,20,22]. Several experimental studies have demonstrated that the N-terminal anchor of αS is the primary binding region for the interaction with the membrane component of SVs [19,40,51]. Here, we investigated this process by means of a simulation platform that enables analysis of the sequence and conformational dependencies of the binding of αS1–30 at a residue-specific resolution, with lipid bilayers mimicking the membrane component of SVs. Our results show that the interaction of αS1–30 with DOPE:DOPS:DOPC lipid bilayers is largely dependent on the conformation adopted by the protein, with the helical state having stronger binding affinity than the extended-disordered conformation. Our data also provide evidence that the sequence properties of αS1–30 crucially influence its binding modes to synaptic membranes. Indeed, simulation of the C-terminal sequence, αS111–140, resulted in negligible membrane interactions for both helical and extended-disordered conformations. Moreover, the simulations of αS1–30 constructs with progressive N-terminal truncations revealed trends in membrane affinities that are independent from the protein conformations. This result indicates that while the helical conformation generally promotes stronger membrane interactions, the sequence of αS1–30 is the key driver for the overall membrane binding by αS1–30. A key residue of this sequence is Phe 4, which was shown to anchor αS1–30 to the membrane in both types of conformations, suggesting that this residue is fundamental for both membrane-tethering and membrane-locking steps.
In conclusion, taken together, our data reveal the importance of the sequence properties of the N-terminal region of αS in promoting strong and adaptable binding to synaptic membranes. This region of αS has specificity to tether on the surface of acidic membranes in a disordered extended conformation, while the amphipathic α-helical conformation promotes the locking to the membrane surface. The mechanism of membrane interaction of this region is fundamental for the biological behavior of αS because it induces strong binding affinity without requiring a transmembrane helix. This topological property promotes a degree of reversibility in the membrane interaction by αS, a crucial characteristic that enables αS to bind with multiple membranes at the synaptic termini. Our data suggest that such binding reversibility is at least in part associated with the ability of αS1–30 to interact with lipid bilayers in both helical and extended-disordered conformations. However, this binding versatility, which we postulate has functional relevance, can also be utilized by αS oligomers in aberrant processes; indeed, one of the key steps for the toxicity of these aggregates is the membrane anchoring by the N-terminal region [55]. The adaptability of binding by the N-terminal region in both membrane-locked and membrane-tethered states can therefore be exploited by oligomeric species to promote binding promiscuity to different synaptic membranes and organelles, generating downstream effects leading to cellular toxicity.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-1729/10/6/98/s1, Figure S1: Effects of the variation in the σ of the restraining potential, Figure S2: Simulations convergence, Figure S3: Membrane interactions by different deletion constructs αS1–30.
Author Contributions
Conceptualization, A.D.S.; methodology, C.N.-P., M.S.-H. and A.D.S.; software, C.N.-P. and M.S.-H.; formal analysis, C.N.-P.; investigation, C.N.-P., M.S.-H. and A.D.S.; data curation, C.N.-P. and A.D.S.; writing—original draft preparation, C.N.-P. and A.D.S.; writing—review and editing, All; supervision, M.S.-H. and A.D.S.; funding acquisition, A.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
European Research Council (ERC) Consolidator Grant (CoG) “BioDisOrder” (819644) and the UK Engineering and Physical Sciences Research Council (1961334).
Acknowledgments
We thank Joseph Barritt for critical discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jakes, R.; Spillantini, M.G.; Goedert, M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994, 345, 27–32. [Google Scholar] [CrossRef]
- Clayton, D.F.; George, J.M. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998, 21, 249–254. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; Brien, P.O.; Trojanowski, J.Q.; Lee, V.M. Pathological a-synuclein transmission initiates parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.; Eliezer, D. Biophysics of parkinsons disease: Structure and aggregation of α-synuclein. Curr. Protein Pept. Sci. 2009, 10, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.; Kuhn, W.; Müller, T.; Woitalla, D.; Graeber, M.; Kösel, S.; Przuntek, H.; Epplen, J.T.; Schöls, L.; Riess, O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998, 18, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. α-Synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar] [CrossRef]
- Zarranz, J.J.; Alegre, J.; Gómez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atarés, B.; et al. The new mutation, E46K, of α-Synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004, 55, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998, 251, 205–208. [Google Scholar] [CrossRef]
- Galvin, J.E.; Uryu, K.; Lee, V.M.Y.; Trojanowski, J.Q. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains α-, β-, and γ-synuclein. Proc. Natl. Acad. Sci. USA 1999, 96, 13450–13455. [Google Scholar] [CrossRef] [PubMed]
- Burré, J. The synaptic function of α-synuclein. J. Parkinsons. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Südhof, T.C. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA 2014, 111, E4274–E4283. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. A-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 1663, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Bevis, B.J.; Shorter, J.; Strathearn, K.E.; Hamamichi, S.; Su, L.J.; Caldwell, K.A.; Caldwell, G.A.; Rochet, J.-C.; McCaffery, J.M.; et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 145–150. [Google Scholar] [CrossRef]
- Diao, J.; Burré, J.; Vivona, S.; Cipriano, D.J.; Sharma, M.; Kyoung, M.; Südhof, T.C.; Brunger, A.T. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife 2013, 2013, 1–17. [Google Scholar] [CrossRef]
- Bodner, C.R.; Dobson, C.M.; Bax, A. Multiple tight phospholipid-binding modes of α-Synuclein revealed by solution NMR spectroscopy. J. Mol. Biol. 2009, 390, 775–790. [Google Scholar] [CrossRef]
- Fusco, G.; Pape, T.; Stephens, A.D.; Mahou, P.; Costa, A.R.; Kaminski, C.F.; Kaminski Schierle, G.S.; Vendruscolo, M.; Veglia, G.; Dobson, C.M.; et al. Structural basis of synaptic vesicle assembly promoted by α-synuclein. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of α-Synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Auluck, P.K.; Caraveo, G.; Lindquist, S. α-Synuclein: Membrane interactions and toxicity in Parkinson’s disease. Annu. Rev. Cell Dev. Biol. 2010, 26, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.A. α-Synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 2006, 313, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Menges, S.; Minakaki, G.; Schaefer, P.M.; Meixner, H.; Prots, I.; Schlötzer-Schrehardt, U.; Friedland, K.; Winner, B.; Outeiro, T.F.; Winklhofer, K.F.; et al. Alpha-synuclein prevents the formation of spherical mitochondria and apoptosis under oxidative stress. Sci. Rep. 2017, 7, 1–25. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Ninkina, N.N.; Gandhi, S.; Buchman, V.L.; Abramov, A.Y. Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J. Neurosci. 2016, 36, 10510–10521. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, C.; Lee, S.J. Membrane-bound α-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 2002, 277, 671–678. [Google Scholar] [CrossRef]
- Galvagnion, C.; Buell, A.K.; Meisl, G.; Michaels, T.C.T.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015, 11, 229–234. [Google Scholar] [CrossRef]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 2001, 276, 41958–41962. [Google Scholar] [CrossRef]
- Galvagnion, C.; Maltsev, A.; Meisl, G.; Müller, M.B.D.; Challa, P.K.; Kirkegaard, J.B.; Cohen, S.I.A.; Cascella, R.; Chen, S.W.; Limboker, R.; et al. Correction for Perni et al., A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity. Proc. Natl. Acad. Sci. USA 2017, 114, E2543. [Google Scholar] [CrossRef]
- Chen, S.W.; Drakulic, S.; Deas, E.; Ouberai, M.; Aprile, F.A.; Arranz, R.; Ness, S.; Roodveldt, C.; Guilliams, T.; De-Genst, E.J.; et al. Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation. Proc. Natl. Acad. Sci. USA 2015, 112, E1994–E2003. [Google Scholar] [CrossRef]
- Theillet, F.X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; Van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.S.; Ying, J.; Bax, A. Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties. Biochemistry 2012, 51, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Sanz-Hernandez, M.; Ruggeri, F.S.; Vendruscolo, M.; Dobson, C.M.; De Simone, A. Molecular determinants of the interaction of EGCG with ordered and disordered proteins. Biopolymers 2018, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, T.S.; Bax, A.; Cole, N.B.; Nussbaum, R.L. Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 2005, 280, 9595–9603. [Google Scholar] [CrossRef]
- Eliezer, D.; Kutluay, E.; Bussell, R.; Browne, G. Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001, 307, 1061–1073. [Google Scholar] [CrossRef]
- Fusco, G.; Sanz-Hernandez, M.; De Simone, A. Order and disorder in the physiological membrane binding of α-synuclein. Curr. Opin. Struct. Biol. 2018, 48, 49–57. [Google Scholar] [CrossRef]
- Trexler, A.J.; Rhoades, E. α-Synuclein binds large unilamellar vesicles as an extended helix. Biochemistry 2009, 48, 2304–2306. [Google Scholar] [CrossRef]
- Jao, C.C.; Hegde, B.G.; Chen, J.; Haworth, I.S.; Langen, R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc. Natl. Acad. Sci. USA 2008, 105, 19666–19671. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J. Neurosci. 2015, 35, 5221–5232. [Google Scholar] [CrossRef]
- Fusco, G.; De Simone, A.; Gopinath, T.; Vostrikov, V.; Vendruscolo, M.; Dobson, C.M.; Veglia, G. Direct observation of the three regions in α-synuclein that determine its membrane-bound behaviour. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Lautenschläger, J.; Stephens, A.D.; Fusco, G.; Ströhl, F.; Curry, N.; Zacharopoulou, M.; Michel, C.H.; Laine, R.; Nespovitaya, N.; Fantham, M.; et al. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bond, P.J.; Holyoake, J.; Ivetac, A.; Khalid, S.; Sansom, M.S.P. Coarse-grained molecular dynamics simulations of membrane proteins and peptides. J. Struct. Biol. 2007, 157, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Bruininks, B.M.H.; Souza, P.C.T.; Marrink, S.J. A practical view of the martini force field. Methods Mol. Biol. 2019, 2022, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.A.; Ingólfsson, H.I.; Böckmann, R.A.; Tieleman, D.P.; Marrink, S.J. Computational lipidomics with insane: A versatile tool for generating custom membranes for molecular simulations. J. Chem. Theory Comput. 2015, 11, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Fusco, G.; De Simone, A.; Arosio, P.; Vendruscolo, M.; Veglia, G.; Dobson, C.M. Structural ensembles of membrane-bound α-Synuclein reveal the molecular determinants of synaptic vesicle affinity. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Vamvaca, K.; Volles, M.J.; Lansbury, P.T. The first N-terminal amino acids of α-Synuclein are essential for α-Helical structure formation in vitro and membrane binding in yeast. J. Mol. Biol. 2009, 389, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.W.; Leong, J.T.; Chow, E.D.; Kampmann, M.; DeGrado, W.F. Deep mutational scanning reveals the structural basis for α-synuclein activity. Nat. Chem. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Snead, D.; Eliezer, D. Alpha-Synuclein function and dysfunction on cellular membranes. Exp. Neurobiol. 2014, 23, 292–313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fink, A.L. Lipid binding inhibits α-synuclein fibril formation. J. Biol. Chem. 2003, 278, 16873–16877. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by a-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).