Systematic Postoperative Assessment of a Minimally-Invasive Sheep Model for the Treatment of Osteochondral Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
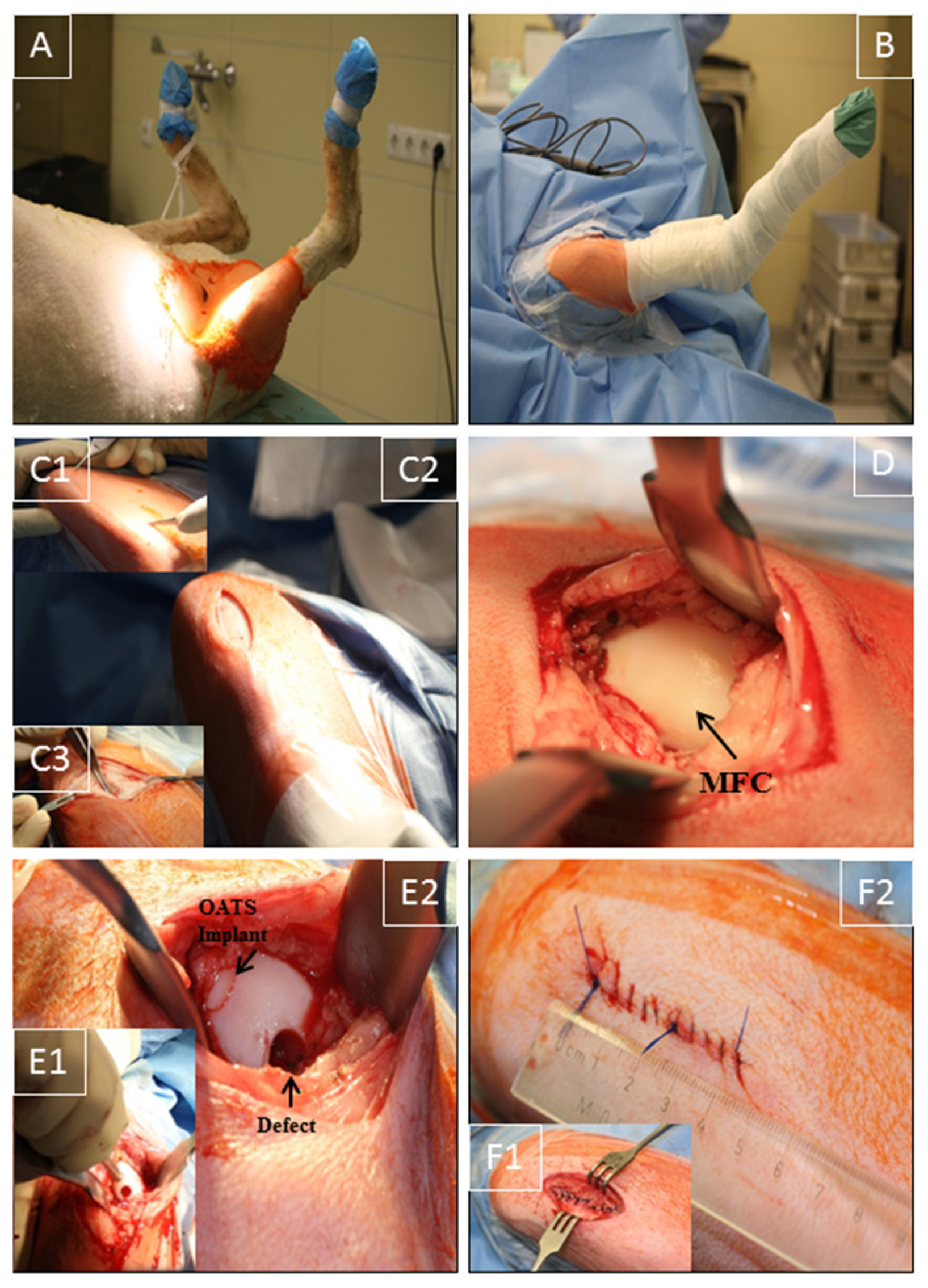
2.2. Anesthesia and Surgical Technique
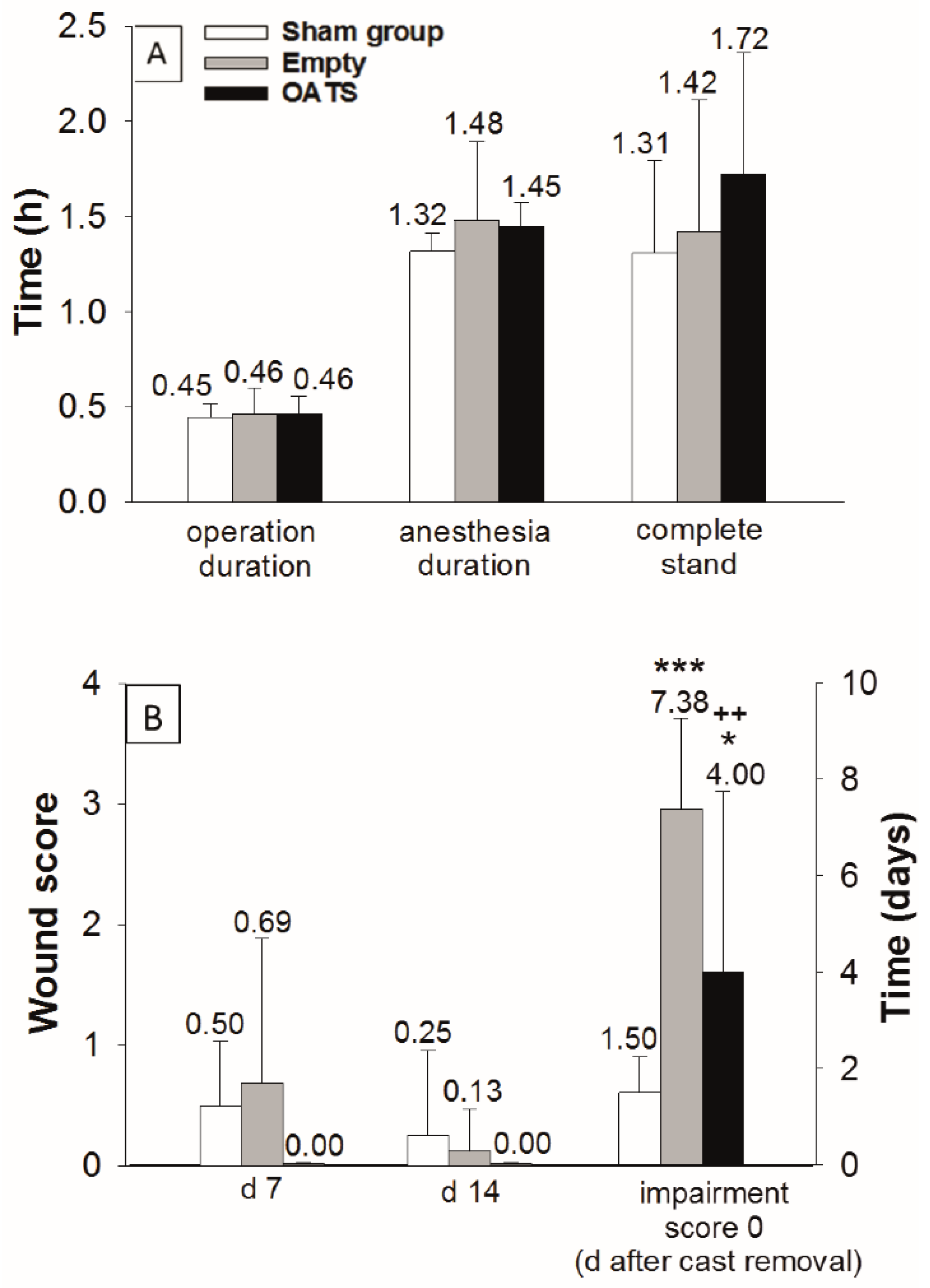
2.3. Assessment of Operation Duration and Postoperative Recovery
2.4. Radiographic Analysis
2.5. High-Resolution Micro-CT
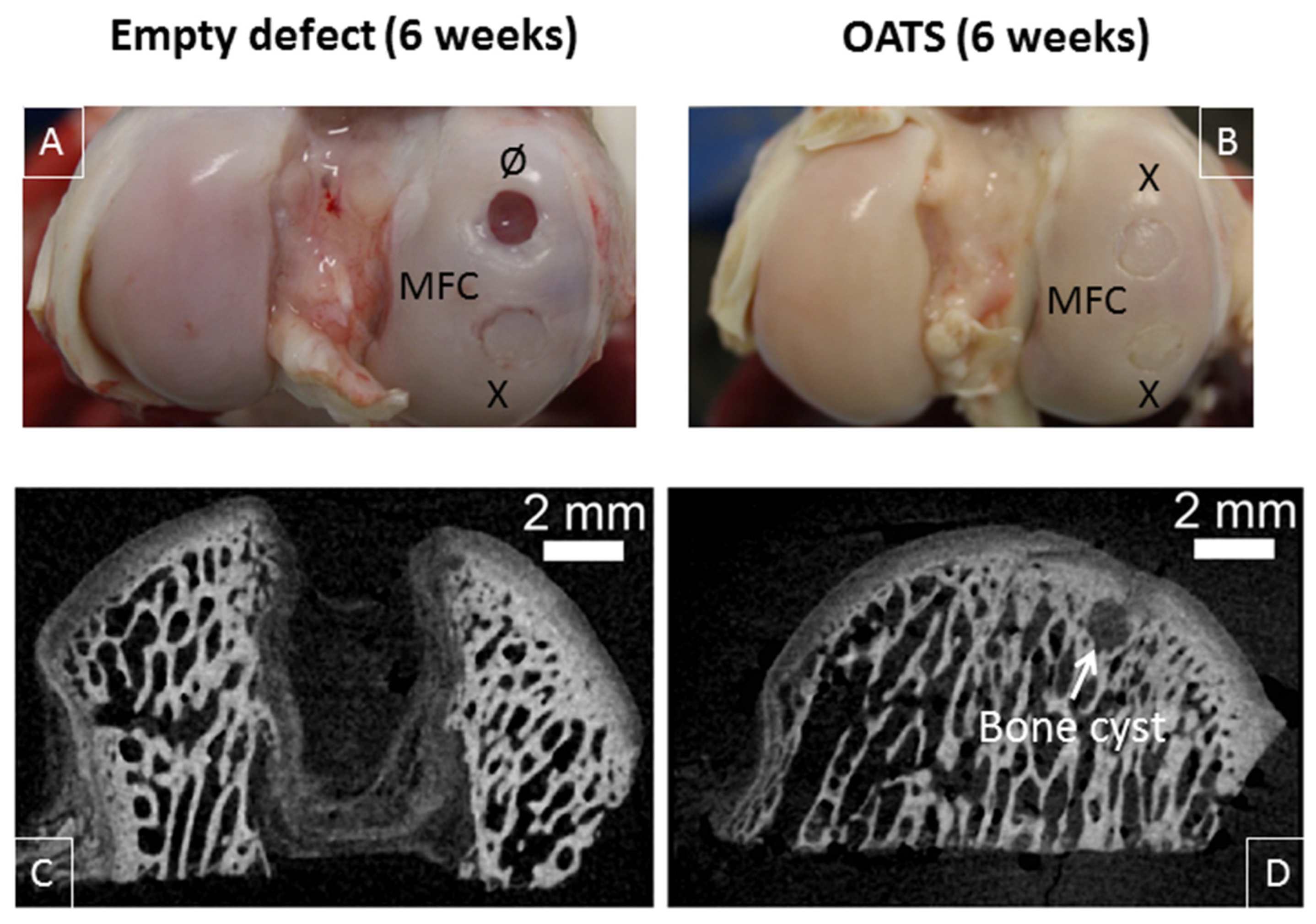
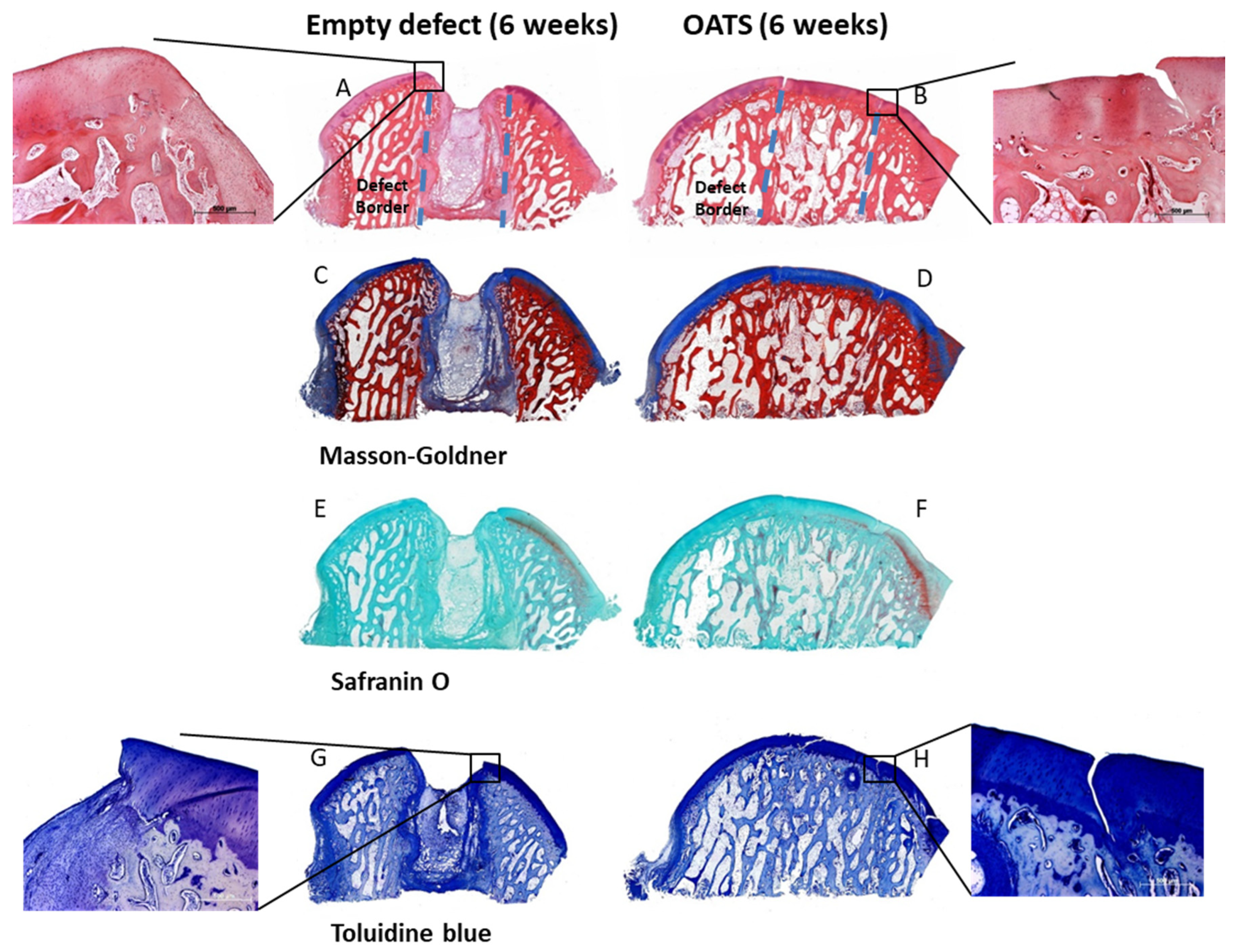
2.6. Gross Morphological Observation and Histological Evaluation
2.7. Statistical Analysis
3. Results
3.1. Operation Time and Postoperative Recovery
3.2. Radiography
3.3. Gross Morphological Observation and Micro-CT
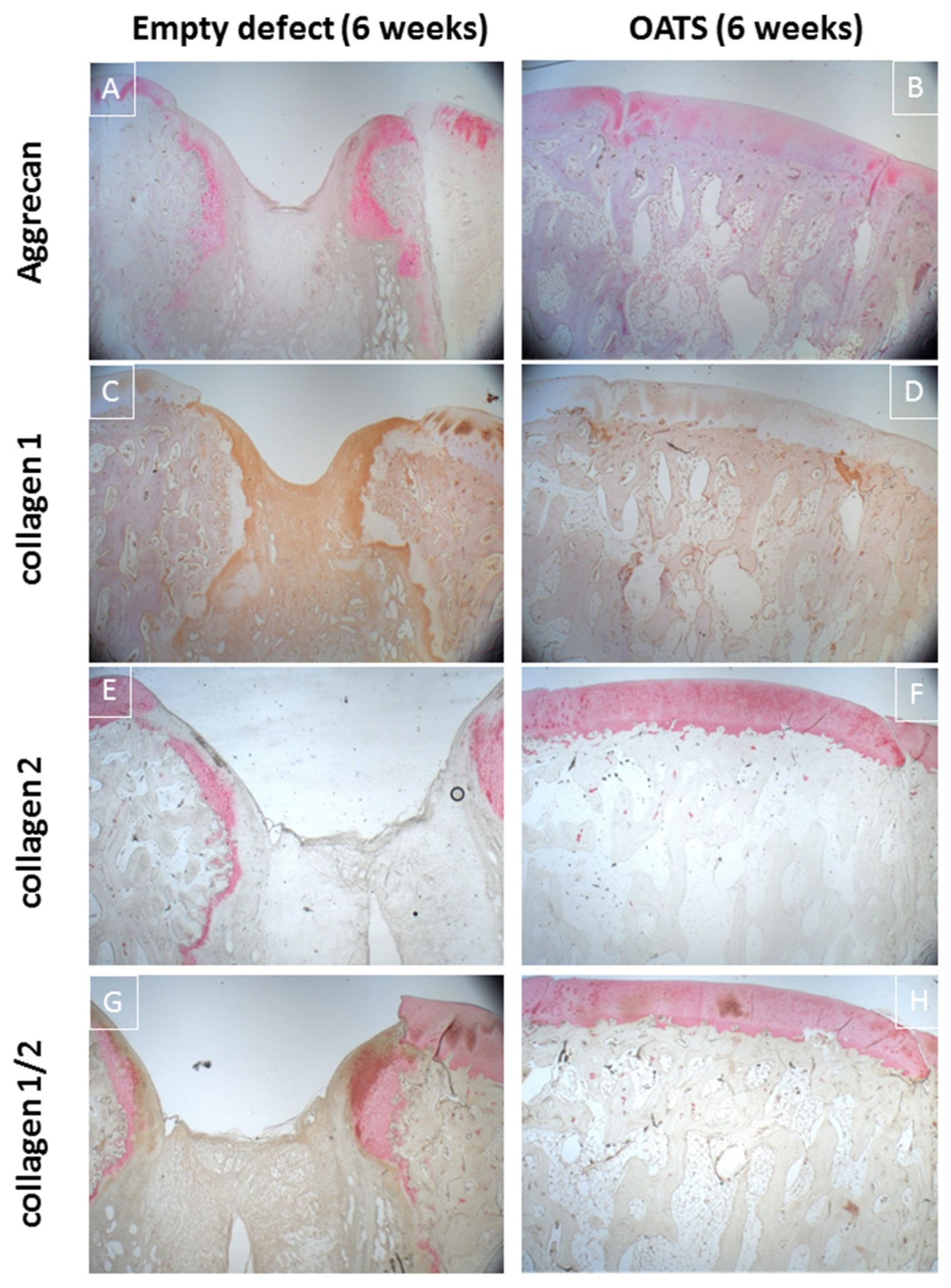
3.4. Histology
3.4.1. Empty Defects
3.4.2. Refilled Defects
4. Discussion
4.1. The Minimally-Invasive, Large Animal OC Defect Model
4.2. Comparison with Published Models
4.3. Comparison with the Sham Group
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| BSA | bovine serum albumin |
| β-TCP | β-Tricalcium phosphate |
| DAB | diaminobenzidine |
| HE | hematoxylin and eosin |
| HRP | horseradish peroxidase |
| Ig | immunoglobulin |
| IKDC | International Knee Documentation Commitee |
| MFC | medial femoral condyle |
| LFC | lateral femoral condyle |
| OATS | osteochondral autologous transplantation cylinder |
| OC | osteochondral |
| TBS | tris-buffered saline |
References
- Grassel, S.; Lorenz, J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: Use of mesenchymal stem cells. Curr. Rheumatol. Rep. 2014, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; Hoque, M.E.; Prasad, R.G.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J. Biomed. Mater. Res. A 2015, 103, 2460–2481. [Google Scholar] [CrossRef]
- Curl, W.W.; Krome, J.; Gordon, E.S.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 1997, 13, 456–460. [Google Scholar] [CrossRef]
- Huber, R.; Hummert, C.; Gausmann, U.; Pohlers, D.; Koczan, D.; Guthke, R.; Kinne, R.W. Identification of intra-group, inter-individual, and gene-specific variances in mRNA expression profiles in the rheumatoid arthritis synovial membrane. Arthritis Res. Ther. 2008, 10, R98. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Kirsten, H.; Näkki, A.; Pohlers, D.; Thude, H.; Eidner, T.; Heinig, M.; Brand, K.; Ahnert, P.; Kinne, R.W. Association of Human FOS Promoter Variants with the Occurrence of Knee-Osteoarthritis in a Case Control Association Study. Int. J. Mol. Sci. 2019, 20, 1382. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, P.; Huber, R.; Weber, M.; Vlaic, S.; Haupl, T.; Koczan, D.; Guthke, R.; Kinne, R.W. Novel application of multi-stimuli network inference to synovial fibroblasts of rheumatoid arthritis patients. BMC Med. Genom. 2014, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Stuhlmüller, B.; Kunisch, E.; Kinne, R.W. Discrepancy between Jun/Fos proto-oncogene mRNA and protein expression in the rheumatoid arthritis synovial membrane. J. Multidiscip. Sci. J. 2020, 3, 181–195. [Google Scholar] [CrossRef]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng. Part. B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef]
- Ahern, B.J.; Parvizi, J.; Boston, R.; Schaer, T.P. Preclinical animal models in single site cartilage defect testing: A systematic review. Osteoarthr. Cartil. 2009, 17, 705–713. [Google Scholar] [CrossRef]
- EMEA. Reflection Paper on In-Vitro Cultured Chondrocyte Containing Products for Cartilage Repair of the Knee; EMEA: London, UK, 2009. [Google Scholar]
- Proffen, B.L.; McElfresh, M.; Fleming, B.C.; Murray, M.M. A comparative anatomical study of the human knee and six animal species. Knee 2012, 19, 493–499. [Google Scholar] [CrossRef]
- Osterhoff, G.; Loffler, S.; Steinke, H.; Feja, C.; Josten, C.; Hepp, P. Comparative anatomical measurements of osseous structures in the ovine and human knee. Knee 2011, 18, 98–103. [Google Scholar] [CrossRef] [PubMed]
- von Rechenberg, B.; Akens, M.K.; Nadler, D.; Bittmann, P.; Zlinszky, K.; Kutter, A.; Poole, A.R.; Auer, J.A. Changes in subchondral bone in cartilage resurfacing—An experimental study in sheep using different types of osteochondral grafts. Osteoarthr. Cartil. 2003, 11, 265–277. [Google Scholar] [CrossRef]
- Orth, P.; Goebel, L.; Wolfram, U.; Ong, M.F.; Graber, S.; Kohn, D.; Cucchiarini, M.; Ignatius, A.; Pape, D.; Madry, H. Effect of subchondral drilling on the microarchitecture of subchondral bone: Analysis in a large animal model at 6 months. Am. J. Sports Med. 2012, 40, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Russlies, M.; Behrens, P.; Ehlers, E.M.; Brohl, C.; Vindigni, C.; Spector, M.; Kurz, B. Periosteum stimulates subchondral bone densification in autologous chondrocyte transplantation in a sheep model. Cell Tissue Res. 2005, 319, 133–142. [Google Scholar] [CrossRef]
- Allen, M.J.; Houlton, J.E.; Adams, S.B.; Rushton, N. The surgical anatomy of the stifle joint in sheep. Vet. Surg. 1998, 27, 596–605. [Google Scholar] [CrossRef]
- Hepp, P.; Osterhoff, G.; Niederhagen, M.; Marquass, B.; Aigner, T.; Bader, A.; Josten, C.; Schulz, R. Perilesional changes of focal osteochondral defects in an ovine model and their relevance to human osteochondral injuries. J. Bone Jt. Surg Br. 2009, 91, 1110–1119. [Google Scholar] [CrossRef]
- Mayr, H.O.; Klehm, J.; Schwan, S.; Hube, R.; Sudkamp, N.P.; Niemeyer, P.; Salzmann, G.; von Eisenhardt-Rothe, R.; Heilmann, A.; Bohner, M.; et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Biomechanical results. Acta Biomater. 2013, 9, 4845–4855. [Google Scholar] [CrossRef]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Sudkamp, N.P.; Hube, R.; Klehm, J.; Menzel, M.; von Eisenhart-Rothe, R.; Bohner, M.; Gorz, L.; et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 2013, 9, 7490–7505. [Google Scholar] [CrossRef]
- Orth, P.; Madry, H. A low morbidity surgical approach to the sheep femoral trochlea. BMC Musculoskelet. Disord. 2013, 14, 5. [Google Scholar] [CrossRef]
- Paul, J.; Sagstetter, A.; Kriner, M.; Imhoff, A.B.; Spang, J.; Hinterwimmer, S. Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J. Bone Jt. Surg. Am. Vol. 2009, 91, 1683–1688. [Google Scholar] [CrossRef]
- Reddy, S.; Pedowitz, D.I.; Parekh, S.G.; Sennett, B.J.; Okereke, E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am. J. Sports Med. 2007, 35, 80–85. [Google Scholar] [CrossRef]
- Zengerink, M.; Struijs, P.A.; Tol, J.L.; van Dijk, C.N. Treatment of osteochondral lesions of the talus: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. Esska 2010, 18, 238–246. [Google Scholar] [CrossRef]
- Ahmad, C.S.; Guiney, W.B.; Drinkwater, C.J. Evaluation of donor site intrinsic healing response in autologous osteochondral grafting of the knee. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2002, 18, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Nosewicz, T.L.; Reilingh, M.L.; van Dijk, C.N.; Duda, G.N.; Schell, H. Weightbearing ovine osteochondral defects heal with inadequate subchondral bone plate restoration: Implications regarding osteochondral autograft harvesting. Knee Surg. Sports Traumatol. Arthrosc. Off. J. Esska 2012, 20, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Bungartz, M.; Maenz, S.; Kunisch, E.; Horbert, V.; Xin, L.; Gunnella, F.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. First-time systematic postoperative clinical assessment of a minimally invasive approach for lumbar ventrolateral vertebroplasty in the large animal model sheep. Spine J. 2016, 16, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Bungartz, M.; Kunisch, E.; Maenz, S.; Horbert, V.; Xin, L.; Gunnella, F.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. GDF5 significantly augments the bone formation induced by an injectable, PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2017, 17, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.R. Minimally invasive total knee arthroplasty: Surgical technique. Am. J. Orthop. 2006, 35 (Suppl. 7), 7–11. [Google Scholar] [PubMed]
- Bonutti, P.M.; Zywiel, M.G.; McGrath, M.S.; Mont, M.A. Surgical techniques for minimally invasive exposures for total knee arthroplasty. Instr. Course Lect. 2010, 59, 83–91. [Google Scholar]
- Neely, C.D.; Thomson, D.U.; Kerr, C.A.; Reinhardt, C.D. Effects of three dehorning techniques on behavior and wound healing in feedlot cattle. J. Anim. Sci. 2014, 92, 2225–2229. [Google Scholar] [CrossRef]
- Otto, K.; Steiner, K.; Zailskas, F.; Wippermann, B. Comparison of the postoperative analgesic effects of buprenorphine and piritramide following experimental orthopaedic surgery in sheep. J. Exp. Anim. Sci. 2000, 41, 133–143. [Google Scholar] [CrossRef]
- Welsh, E.M.; Gettinby, G.; Nolan, A.M. Comparison of a visual analogue scale and a numerical rating scale for assessment of lameness, using sheep as a model. Am. J. Vet. Res. 1993, 54, 976–983. [Google Scholar] [PubMed]
- Orth, P.; Meyer, H.L.; Goebel, L.; Eldracher, M.; Ong, M.F.; Cucchiarini, M.; Madry, H. Improved repair of chondral and osteochondral defects in the ovine trochlea compared with the medial condyle. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Smith, M.M.; Cake, M.A.; Read, R.A.; Murphy, M.J.; Barry, F.P. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S80–S92. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.; Bhamra, J.S.; Gikas, P.D.; Skinner, J.A.; Carrington, R.; Briggs, T.W. Repair of osteochondral defects in joints—How to achieve success. Injury 2013, 44 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef]
- Raub, C.B.; Hsu, S.C.; Chan, E.F.; Shirazi, R.; Chen, A.C.; Chnari, E.; Semler, E.J.; Sah, R.L. Microstructural remodeling of articular cartilage following defect repair by osteochondral autograft transfer. Osteoarthr. Cartil. OarsOsteoarthr. Res. Soc. 2013, 21, 860–868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, D.W.; Lalor, P.A.; Aberman, H.M.; Simon, T.M. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J. Bone Jt. Surg. Am. Vol. 2001, 83, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Zscharnack, M.; Hepp, P.; Richter, R.; Aigner, T.; Schulz, R.; Somerson, J.; Josten, C.; Bader, A.; Marquass, B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am. J. Sports Med. 2010, 38, 1857–1869. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 116–124. [Google Scholar] [CrossRef]
- Vizesi, F.; Oliver, R.; Smitham, P.; Gothelf, T.; Yu, Y.; Walsh, W.R. Influence of surgical preparation on the in-vivo response of osteochondral defects. Proc. Inst. Mech. Eng. H 2007, 221, 489–498. [Google Scholar] [CrossRef]
- Shettko, D.L.; Trostle, S.S. Diagnosis and surgical repair of patellar luxations in a flock of sheep. J. Am. Vet. Med. Assoc. 2000, 216, 564–566. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63 (Suppl. 11), S208–S228. [Google Scholar]
- Ruediger, T.; Horbert, V.; Reuther, A.; Kalla, P.K.; Burgkart, R.H.; Walther, M.; Kinne, R.W.; Mika, J. Thickness of the stifle joint articular cartilage in different large animal models of cartilage repair and regeneration. Cartilage 2020, in press. [Google Scholar] [CrossRef] [PubMed]

| Mayr HO [18] | Bernstein A [19] | Orth P [20] | Present Study | |
|---|---|---|---|---|
| Sheep (gender; age) | Merino (female; 2–4 years) | Merino (female; 2–4 years) | Merino (female; 2–4 years) | Merino (female; 2–6 years) |
| Position | Supine; leaning ˜30° to caudal | Supine; leaning ˜30° to caudal | Supine position | Supine position |
| Invasiveness | Mini-arthrotomy (3 cm cut) | Mini-arthrotomy (3 cm cut) | Mini-arthrotomy (4–5 cm cut) | Mini-arthrotomy (3–4 cm skin cut; 2–3 cm deep incision) |
| Stifle joint | Bilateral | Bilateral | Bilateral | Right side |
| Defect location | Medial femoral condyle (1 defect; center of load-bearing area) | Medial femoral condyle (1 defect; center of load-bearing area) | None; (exposure; lateral + medial trochlear facet; medial + lateral femoral condyle; menisci) | Medial femoral condyle (2 defects; anterior + central part of load-bearing area) |
| Critical-size OC defect(s) | Diameter: 7 mm; Depth: 25 mm | Diameter: 7 mm; Depth: 25 mm | n.a. | Diameter: 7 mm; Depth: 10 mm |
| Specific tools | Drill (with fluid cooling) | Drill (with fluid cooling) | - | OATS punch, (Arthrex, Munich, Germany) |
| Implant (size; modification) | ß-TCP implant (diameter:7 mm; depth: 25 mm; seeded with autologous chondrocytes); empty control | ß-TCP implant (diameter: 7 mm; depth: 25 mm; seeded with autologous chondrocytes); empty control | none | OATS cylinder (diameter: 6 mm; depth: 10 mm); empty control |
| Operation/ anesthesia duration | Not determined | Not determined | Approximately 20 min/ Not determined (no defect/implant) | Approximately 0.5 h/ approximately 1.4 h |
| Wound score; days 7 and 14 | Not determined (2 superficial; 1 deep infection) | Not determined | Not determined (no deep wound infections or empyema) | 0.4 and 0.1 (of max. 4) |
| Time to complete stand | Not determined (normal walking at 42 d) | Not determined | 1–2 h | 1.5 h |
| Immobilization | None (full weight-bearing + range of motion) | None (full weight-bearing + range of motion) | None (full weight-bearing + range of motion) | 1 week; (fiber glass cast) |
| Follow-up time (weeks) | 6, 12, 26, 52 | 6, 12, 26, 52 | 26 (6 months) | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, L.; Mika, J.; Horbert, V.; Bischoff, S.; Schubert, H.; Borowski, J.; Maenz, S.; Huber, R.; Sachse, A.; Illerhaus, B.; et al. Systematic Postoperative Assessment of a Minimally-Invasive Sheep Model for the Treatment of Osteochondral Defects. Life 2020, 10, 332. https://doi.org/10.3390/life10120332
Xin L, Mika J, Horbert V, Bischoff S, Schubert H, Borowski J, Maenz S, Huber R, Sachse A, Illerhaus B, et al. Systematic Postoperative Assessment of a Minimally-Invasive Sheep Model for the Treatment of Osteochondral Defects. Life. 2020; 10(12):332. https://doi.org/10.3390/life10120332
Chicago/Turabian StyleXin, Long, Joerg Mika, Victoria Horbert, Sabine Bischoff, Harald Schubert, Juliane Borowski, Stefan Maenz, René Huber, Andre Sachse, Bernhard Illerhaus, and et al. 2020. "Systematic Postoperative Assessment of a Minimally-Invasive Sheep Model for the Treatment of Osteochondral Defects" Life 10, no. 12: 332. https://doi.org/10.3390/life10120332
APA StyleXin, L., Mika, J., Horbert, V., Bischoff, S., Schubert, H., Borowski, J., Maenz, S., Huber, R., Sachse, A., Illerhaus, B., & Kinne, R. W. (2020). Systematic Postoperative Assessment of a Minimally-Invasive Sheep Model for the Treatment of Osteochondral Defects. Life, 10(12), 332. https://doi.org/10.3390/life10120332





