Correlation between the Expression of Angiogenic Factors and Stem Cell Markers in Human Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
2.2. RNA Isolation and Reverse Transcription-PCR of Cancer Stem Cell Markers
2.3. SDS-PAGE and Western Blot Analysis of FZD6, HIF-1α and VEGFA Protein Expression
2.4. Tissue Microarray Construction
2.5. Histopathology and Immunohistochemistry
2.6. Statistical Analysis
3. Results
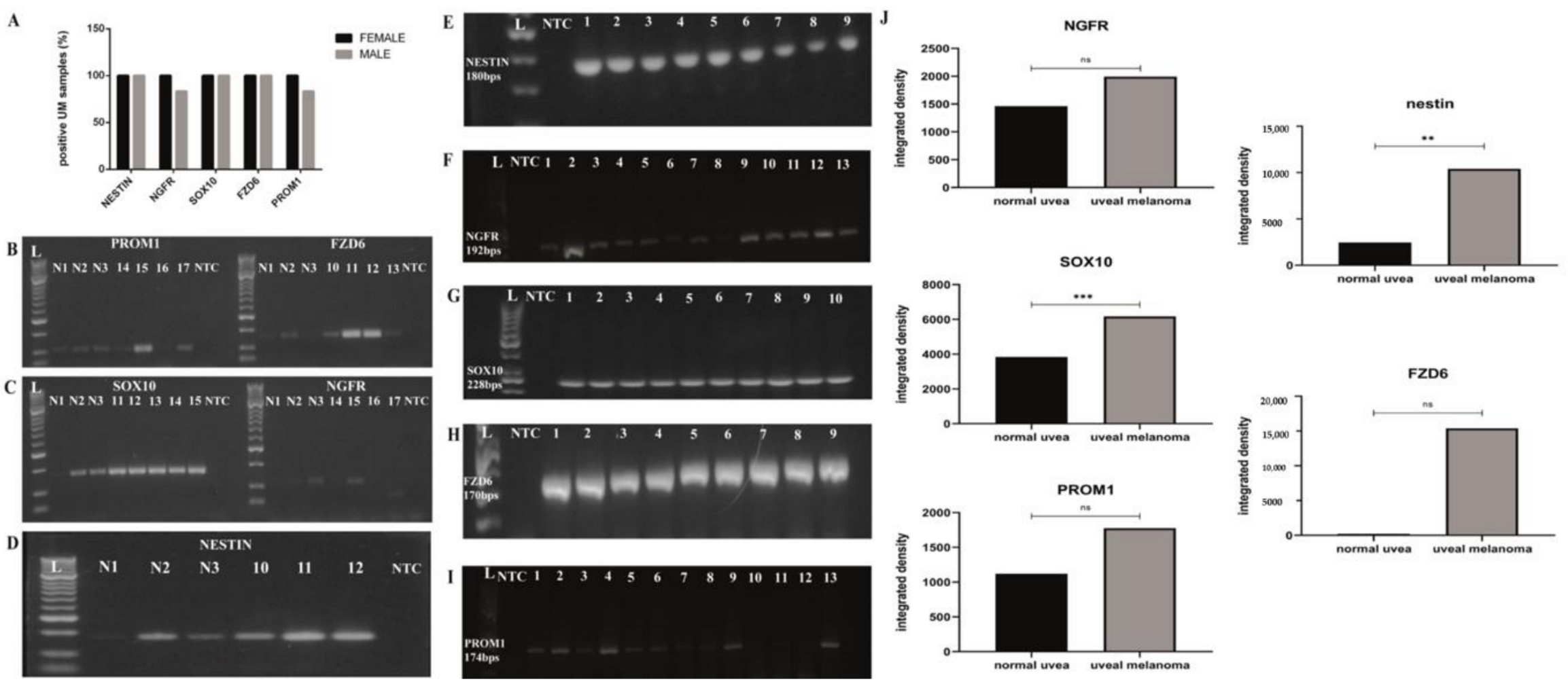
3.1. Investigation of the Gene Expression of Nestin, NGFR, SOX10, FZD6 and PROM1 Cancer Stem Cell Markers in 18 Human UM Specimens and in Three Normal Uvea Samples
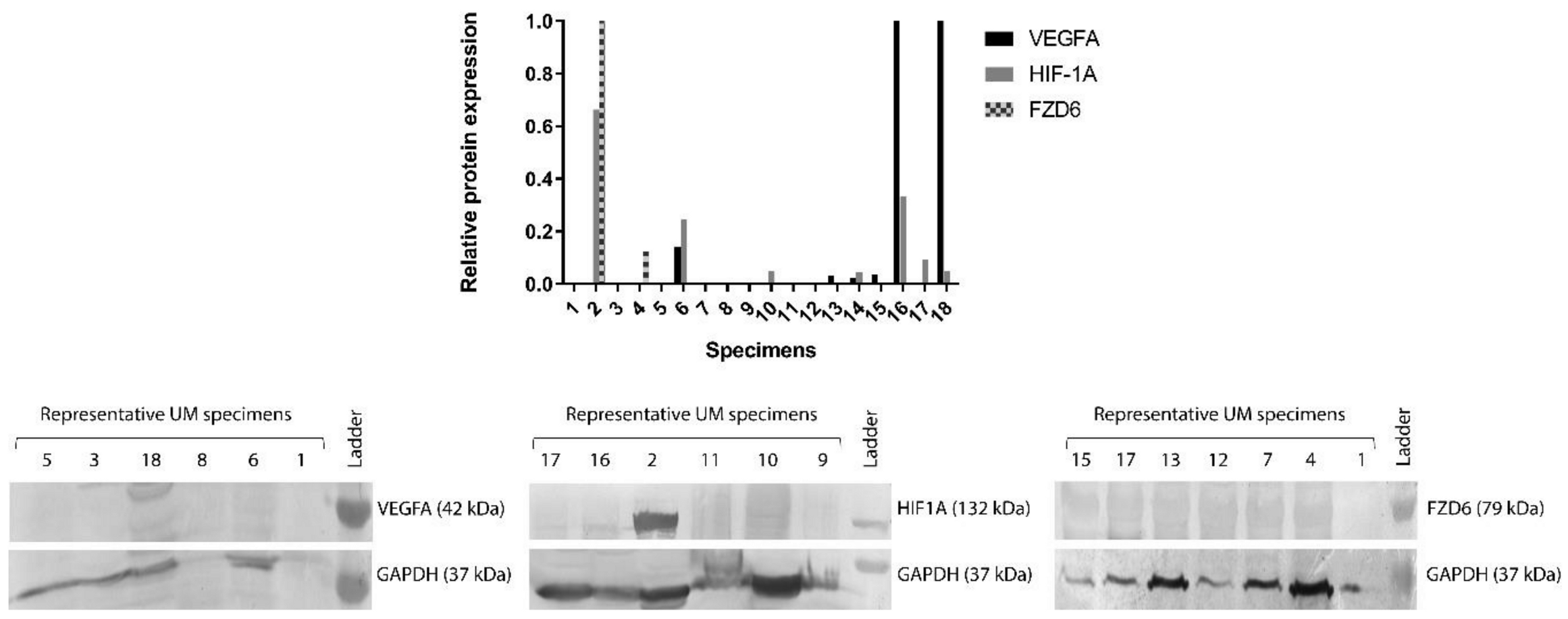
3.2. Protein Expression of FZD6, HIF-1α and VEGFA Genes in 18 Human Snap Frozen UM Specimens
3.3. Clinical, Pathological, and Molecular Biomarkers of UM Specimens in Tissue Microarray
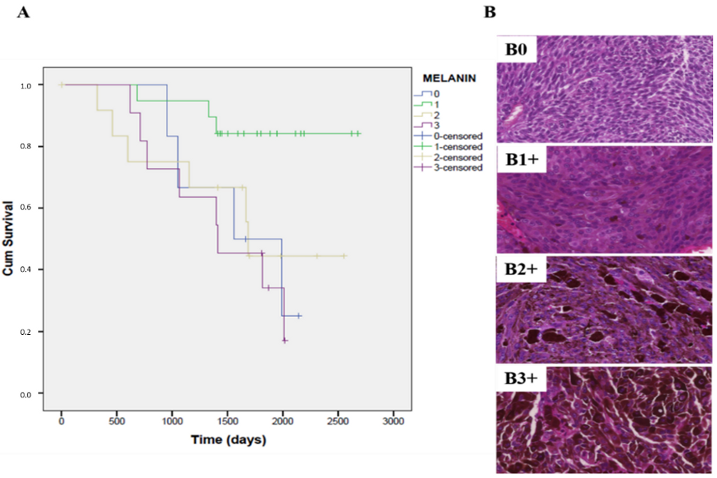
3.4. Overall Survival Could Depend on the Tumor Cell Type Constituting the UM Tumor
3.5. FZD6 Expression Is Related to VEGFA Expression and Has an Influence on the Survival Rate of the Patients with Primary UM
3.6. No Significant Correlation Was Detected between the Expression of Angiogenic Factors and Survival Rates in Primary UM Specimens
3.7. The Expression of Melanin Pigment Negatively Correlates with the Overall Survival Rate in Patients with Primary UM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Baggetto, L.G.; Gambrelle, J.; Dayan, G.; Labialle, S.; Barakat, S.; Michaud, M.; Grange, J.D.; Gayet, L. Major cytogenetic aberrations and typical multidrug resistance phenotype of uveal melanoma: Current views and new therapeutic prospects. Cancer Treat. Rev. 2005, 31, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Pons, F.; Plana, M.; Caminal, J.M.; Pera, J.; Fernandes, I.; Perez, J.; Garcia-Del-Muro, X.; Marcoval, J.; Penin, R.; Fabra, A.; et al. Metastatic uveal melanoma: Is there a role for conventional chemotherapy?—A single center study based on 58 patients. Melanoma Res. 2011, 21, 217–222. [Google Scholar] [CrossRef]
- Luke, J.J.; Triozzi, P.L.; McKenna, K.C.; Van Meir, E.G.; Gershenwald, J.E.; Bastian, B.C.; Gutkind, J.S.; Bowcock, A.M.; Streicher, H.Z.; Patel, P.M.; et al. Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res. 2015, 28, 135–147. [Google Scholar] [CrossRef]
- Castet, F.; Garcia-Mulero, S.; Sanz-Pamplona, R.; Cuellar, A.; Casanovas, O.; Caminal, J.M.; Piulats, J.M. Uveal Melanoma, Angiogenesis and Immunotherapy, Is There Any Hope? Cancers 2019, 11, 834. [Google Scholar] [CrossRef]
- Tabernero, J. The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. 2007, 5, 203–220. [Google Scholar] [CrossRef]
- Brouwer, N.J.; Gezgin, G.; Wierenga, A.P.A.; Bronkhorst, I.H.G.; Marinkovic, M.; Luyten, G.P.M.; Versluis, M.; Kroes, W.G.M.; van der Velden, P.A.; Verdijk, R.M.; et al. Tumour Angiogenesis in Uveal Melanoma Is Related to Genetic Evolution. Cancers 2019, 11, 979. [Google Scholar] [CrossRef]
- Tura, A.; Pawlik, V.E.; Rudolf, M.; Ernesti, J.S.; Stutzer, J.N.; Grisanti, S.; Ranjbar, M. Uptake of Ranibizumab but Not Bevacizumab into Uveal Melanoma Cells Correlates with a Sustained Decline in VEGF-A Levels and Metastatic Activities. Cancers 2019, 11, 868. [Google Scholar] [CrossRef]
- Hu, K.; Babapoor-Farrokhran, S.; Rodrigues, M.; Deshpande, M.; Puchner, B.; Kashiwabuchi, F.; Hassan, S.J.; Asnaghi, L.; Handa, J.T.; Merbs, S.; et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget 2016, 7, 7816–7828. [Google Scholar] [CrossRef]
- Croce, M.; Ferrini, S.; Pfeffer, U.; Gangemi, R. Targeted Therapy of Uveal Melanoma: Recent Failures and New Perspectives. Cancers 2019, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Logan, P.; Burnier, J.; Burnier, M.N., Jr. Vascular endothelial growth factor expression and inhibition in uveal melanoma cell lines. Ecancermedicalscience 2013, 7, 336. [Google Scholar] [CrossRef]
- Jaszai, J.; Schmidt, M.H.H. Trends and Challenges in Tumor Anti-Angiogenic Therapies. Cells 2019, 8, 1102. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Malik, A.K.; Solar, G.P.; Sherman, D.; Liang, X.H.; Meng, G.; Hong, K.; Marsters, J.C.; Ferrara, N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 2002, 417, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W., Jr.; Miller, K.D. Angiogenesis and antiangiogenic therapy. Curr. Probl. Cancer 2002, 26, 1–60. [Google Scholar] [CrossRef]
- el Filali, M.; Ly, L.V.; Luyten, G.P.; Versluis, M.; Grossniklaus, H.E.; van der Velden, P.A.; Jager, M.J. Bevacizumab and intraocular tumors: An intriguing paradox. Mol. Vis. 2012, 18, 2454–2467. [Google Scholar]
- Yang, H.; Jager, M.J.; Grossniklaus, H.E. Bevacizumab suppression of establishment of micrometastases in experimental ocular melanoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2835–2842. [Google Scholar] [CrossRef]
- Hussain, R.N.; Heimann, H.; Damato, B. Neoadjuvant intravitreal ranibizumab treatment in high-risk ocular melanoma patients: A two-stage single-centre phase II single-arm study. Melanoma Res. 2020, 30, 102–106. [Google Scholar] [CrossRef]
- Lima, B.R.; Schoenfield, L.R.; Singh, A.D. The impact of intravitreal bevacizumab therapy on choroidal melanoma. Am. J. Ophthalmol. 2011, 151, 323–328.e322. [Google Scholar] [CrossRef]
- Francis, J.H.; Kim, J.; Lin, A.; Folberg, R.; Iyer, S.; Abramson, D.H. Growth of Uveal Melanoma following Intravitreal Bevacizumab. Ocul. Oncol. Pathol. 2017, 3, 117–121. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Frankel, P.; Margolin, K.A.; Christensen, S.; Ruel, C.; Shipe-Spotloe, J.; Gandara, D.R.; Chen, A.; Kirkwood, J.M. Aflibercept (VEGF Trap) in inoperable stage III or stage iv melanoma of cutaneous or uveal origin. Clin. Cancer Res. 2011, 17, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.U.; Shields, C.L.; Bianciotto, C.G.; Iturralde, J.; Al-Dahmash, S.A.; Say, E.A.T.; Badal, J.; Mashayekhi, A.; Shields, J.A. Intravitreal bevacizumab at 4-month intervals for prevention of macular edema after plaque radiotherapy of uveal melanoma. Ophthalmology 2014, 121, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Moon, J.; Margolin, K.A.; Weber, J.S.; Lao, C.D.; Othus, M.; Aparicio, A.M.; Ribas, A.; Sondak, V.K. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS ONE 2012, 7, e48787. [Google Scholar] [CrossRef] [PubMed]
- Piperno-Neumann, S.; Diallo, A.; Etienne-Grimaldi, M.C.; Bidard, F.C.; Rodrigues, M.; Plancher, C.; Mariani, P.; Cassoux, N.; Decaudin, D.; Asselain, B.; et al. Phase II Trial of Bevacizumab in Combination With Temozolomide as First-Line Treatment in Patients With Metastatic Uveal Melanoma. Oncologist 2016, 21, 281–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herbst, R.S.; Johnson, D.H.; Mininberg, E.; Carbone, D.P.; Henderson, T.; Kim, E.S.; Blumenschein, G., Jr.; Lee, J.J.; Liu, D.D.; Truong, M.T.; et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 2544–2555. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M. Epidermal growth factor receptor in tumor angiogenesis. Hematol. Oncol. Clin. N. Am. 2004, 18, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Paez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Vinals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Storkebaum, E.; Carmeliet, P. VEGF: A critical player in neurodegeneration. J. Clin. Investig. 2004, 113, 14–18. [Google Scholar] [CrossRef]
- Djirackor, L.; Shakir, D.; Kalirai, H.; Petrovski, G.; Coupland, S.E. Nestin expression in primary and metastatic uveal melanoma—Possible biomarker for high-risk uveal melanoma. Acta Ophthalmol. 2018, 96, 503–509. [Google Scholar] [CrossRef]
- Kalirai, H.; Damato, B.E.; Coupland, S.E. Uveal melanoma cell lines contain stem-like cells that self-renew, produce differentiated progeny, and survive chemotherapy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8458–8466. [Google Scholar] [CrossRef]
- Onken, M.D.; Ehlers, J.P.; Worley, L.A.; Makita, J.; Yokota, Y.; Harbour, J.W. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006, 66, 4602–4609. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tiede, B.; Massague, J.; Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2007, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E. Looking ahead in cancer stem cell research. Nat. Biotechnol. 2009, 27, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Worley, L.A.; Onken, M.D.; Harbour, J.W. Prognostic biomarkers in uveal melanoma: Evidence for a stem cell-like phenotype associated with metastasis. Melanoma Res. 2008, 18, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Fusi, A.; Reichelt, U.; Busse, A.; Ochsenreither, S.; Rietz, A.; Maisel, M.; Keilholz, U. Expression of the stem cell markers nestin and CD133 on circulating melanoma cells. J. Investig. Dermatol. 2011, 131, 487–494. [Google Scholar] [CrossRef]
- Lai, C.Y.; Schwartz, B.E.; Hsu, M.Y. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012, 72, 5111–5118. [Google Scholar] [CrossRef]
- Thill, M.; Berna, M.J.; Grierson, R.; Reinhart, I.; Voelkel, T.; Piechaczek, C.; Galambos, P.; Jager, M.J.; Richard, G.; Lange, C.; et al. Expression of CD133 and other putative stem cell markers in uveal melanoma. Melanoma Res. 2011, 21, 405–416. [Google Scholar] [CrossRef]
- Girouard, S.D.; Murphy, G.F. Melanoma stem cells: Not rare, but well done. Lab. Investig. A J. Tech. Methods Pathol. 2011, 91, 647–664. [Google Scholar] [CrossRef]
- Schatton, T.; Frank, M.H. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008, 21, 39–55. [Google Scholar] [CrossRef]
- Onken, M.D.; Lin, A.Y.; Worley, L.A.; Folberg, R.; Harbour, J.W. Association between microarray gene expression signature and extravascular matrix patterns in primary uveal melanomas. Am. J. Ophthalmol. 2005, 140, 748–749. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed]
- Alamodi, A.A.; Eshaq, A.M.; Hassan, S.Y.; Al Hmada, Y.; El Jamal, S.M.; Fothan, A.M.; Arain, O.M.; Hassan, S.L.; Haikel, Y.; Megahed, M.; et al. Cancer stem cell as therapeutic target for melanoma treatment. Histol. Histopathol. 2016, 31, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Valyi-Nagy, K.; Kormos, B.; Ali, M.; Shukla, D.; Valyi-Nagy, T. Stem cell marker CD271 is expressed by vasculogenic mimicry-forming uveal melanoma cells in three-dimensional cultures. Mol. Vis. 2012, 18, 588–592. [Google Scholar] [PubMed]
- Civenni, G.; Walter, A.; Kobert, N.; Mihic-Probst, D.; Zipser, M.; Belloni, B.; Seifert, B.; Moch, H.; Dummer, R.; van den Broek, M.; et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011, 71, 3098–3109. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.M.; Kahn, M. Pharmacologic Manipulation of Wnt Signaling and Cancer Stem Cells. Methods Mol. Biol. 2017, 1613, 463–478. [Google Scholar] [CrossRef]
- Kahn, M. Wnt Signaling in Stem Cells and Cancer Stem Cells: A Tale of Two Coactivators. Prog. Mol. Biol. Transl. Sci. 2018, 153, 209–244. [Google Scholar] [CrossRef]
- Katoh, M. WNT/PCP signaling pathway and human cancer (review). Oncol. Rep. 2005, 14, 1583–1588. [Google Scholar] [CrossRef]
- Lee, K.H.; Li, M.; Michalowski, A.M.; Zhang, X.; Liao, H.; Chen, L.; Xu, Y.; Wu, X.; Huang, J. A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 69–74. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, J.; Jin, B.; Pan, J. Class III-specific HDAC inhibitor Tenovin-6 induces apoptosis, suppresses migration and eliminates cancer stem cells in uveal melanoma. Sci. Rep. 2016, 6, 22622. [Google Scholar] [CrossRef]



| Primers | Sequence | PCR Product Size |
|---|---|---|
| Nestin | Forward: 5′-AGAACTCCCGGCTGCAAA-3′ | 180 bps |
| Reverse: 5′-GCACAGGTGTCTCAAGGGTAG-3′ | ||
| NGFR | Forward: 5′-TGCCAGGACAAGCAGAAC-3′ | 192 bps |
| Reverse: 5′-GGGTGTGGACCGTGTAATC-3′ | ||
| SOX10 | Forward: 5′-CGTCAGCCAGGTGCTCAG-3′ | 228 bps |
| Reverse: 5′-CGCTTGTCACTTTCGTTCAG-3′ | ||
| PROM1 | Forward: 5′-GCACTTACGGCACTCTTCAC-3′ | 174 bps |
| Reverse: 5′-TTCCACAAGCAGCAAAATCC-3′ | ||
| FZD6 | Forward: 5′-TGAGCAAGTGAACAGGATTACC-3′ | 170 bps |
| Reverse: 5′-CCCAGAAGACAGCAGAGATG-3′ | ||
| β-actin | Forward: 5′-GGCATCCTCACCCTGAAGTA-3′ | 200 bps |
| Reverse: 5′-GGGGTGTTGAAGGTCTCAAA-3′ |
| Clinical and Pathological Features | n = 18 |
|---|---|
| Average age (range) | 63.1 (30–84) |
| Female (patient) | 6 |
| Male (patient) | 12 |
| Epitheloid tumor cell type (patient) | 7 |
| Spindle tumor cell type (patient) | 8 |
| Mixed tumor cell type (patient) | 3 |
| Median tumor diameter (range) | 9.22 mm (2.5–23) |
| Median tumor thickness (range) | 7.19 mm (1–16) |
| Patients with sclera infiltration | 9 |
| Patients without sclera infiltration | 9 |
| Patients with nervus opticus infiltration | 2 |
| Patients without nervus opticus infiltration | 16 |
| Median follow-up time (years, range) | 3.71 (1.70–6.37) |
| Ruthenium applicator (patient) | 5 |
| Clinical and Pathological Features | n = 52 |
|---|---|
| Average age (range) | 59.55 (35–83) |
| Female (patient nr.) | 22 |
| Male (patient nr.) | 30 |
| Epitheloid tumor cell type (patient nr.) | 16 |
| Spindle tumor cell type (patient nr.) | 24 |
| Mixed tumor cell type (patient nr.) | 12 |
| Median tumor diameter (range) | 12.68 mm (2.5–23) |
| Median tumor width (range) | 9.56 mm (5–14) |
| Median tumor thickness (range) | 6.47 mm (1–16) |
| Patients with sclera infiltration | 26 |
| Patients without sclera infiltration | 26 |
| Patients with nervus opticus infiltration | 3 |
| Patients without nervus opticus infiltration | 49 |
| Median follow-up time (years, range) | 4.43 (1.1–7.33) |
| Ruthenium applicator (patient) | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodor, K.; Sipos, É.; Dobos, N.; Nagy, J.; Steiber, Z.; Méhes, G.; Dull, K.; Székvölgyi, L.; Schally, A.V.; Halmos, G. Correlation between the Expression of Angiogenic Factors and Stem Cell Markers in Human Uveal Melanoma. Life 2020, 10, 310. https://doi.org/10.3390/life10120310
Fodor K, Sipos É, Dobos N, Nagy J, Steiber Z, Méhes G, Dull K, Székvölgyi L, Schally AV, Halmos G. Correlation between the Expression of Angiogenic Factors and Stem Cell Markers in Human Uveal Melanoma. Life. 2020; 10(12):310. https://doi.org/10.3390/life10120310
Chicago/Turabian StyleFodor, Klára, Éva Sipos, Nikoletta Dobos, János Nagy, Zita Steiber, Gábor Méhes, Kata Dull, Lóránt Székvölgyi, Andrew V. Schally, and Gábor Halmos. 2020. "Correlation between the Expression of Angiogenic Factors and Stem Cell Markers in Human Uveal Melanoma" Life 10, no. 12: 310. https://doi.org/10.3390/life10120310
APA StyleFodor, K., Sipos, É., Dobos, N., Nagy, J., Steiber, Z., Méhes, G., Dull, K., Székvölgyi, L., Schally, A. V., & Halmos, G. (2020). Correlation between the Expression of Angiogenic Factors and Stem Cell Markers in Human Uveal Melanoma. Life, 10(12), 310. https://doi.org/10.3390/life10120310







