Effect of Acetaminophen (APAP) on Physiological Indicators in Lactuca sativa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Plant Analyses
2.3. Data Analysis
3. Results and Discussion
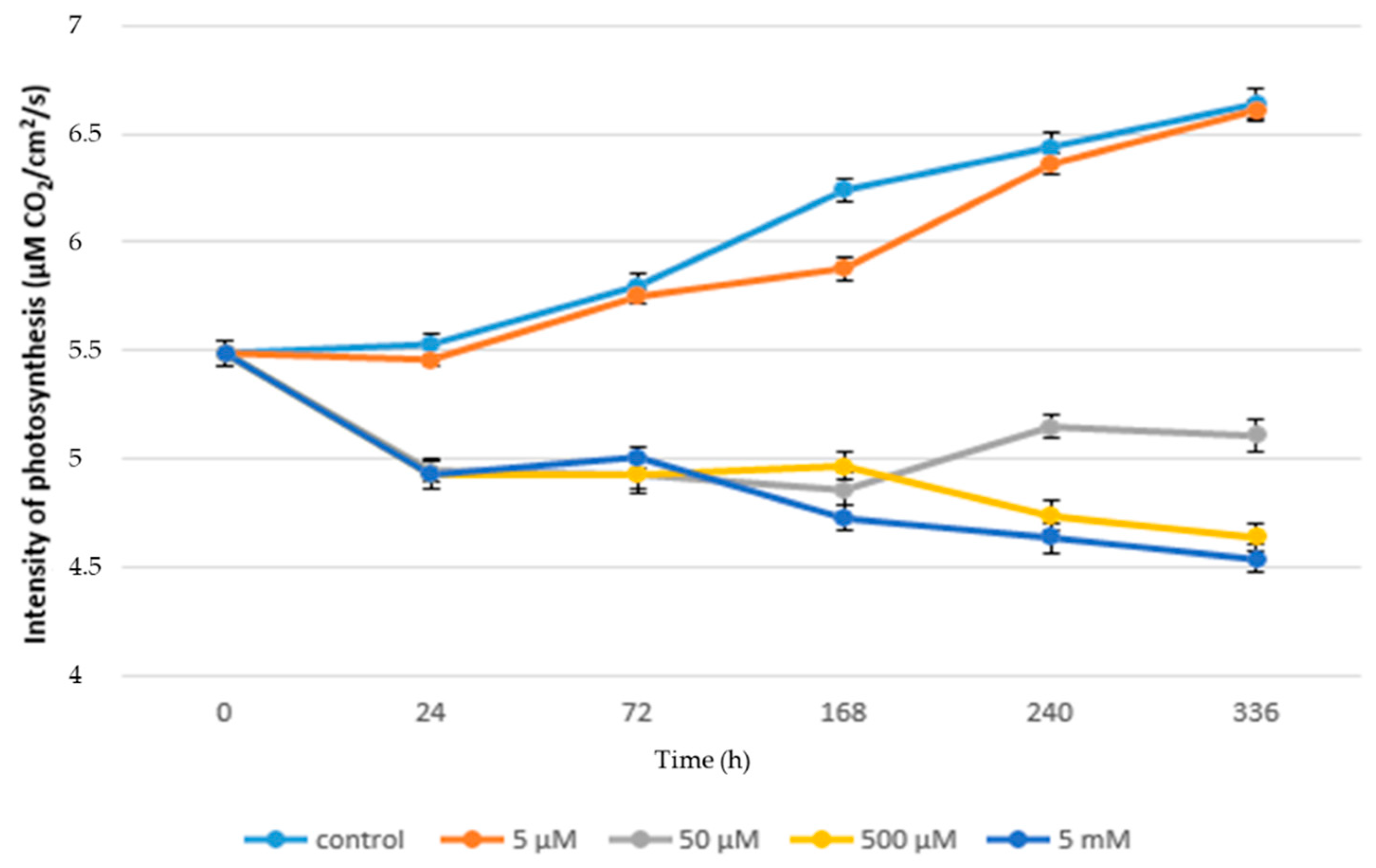
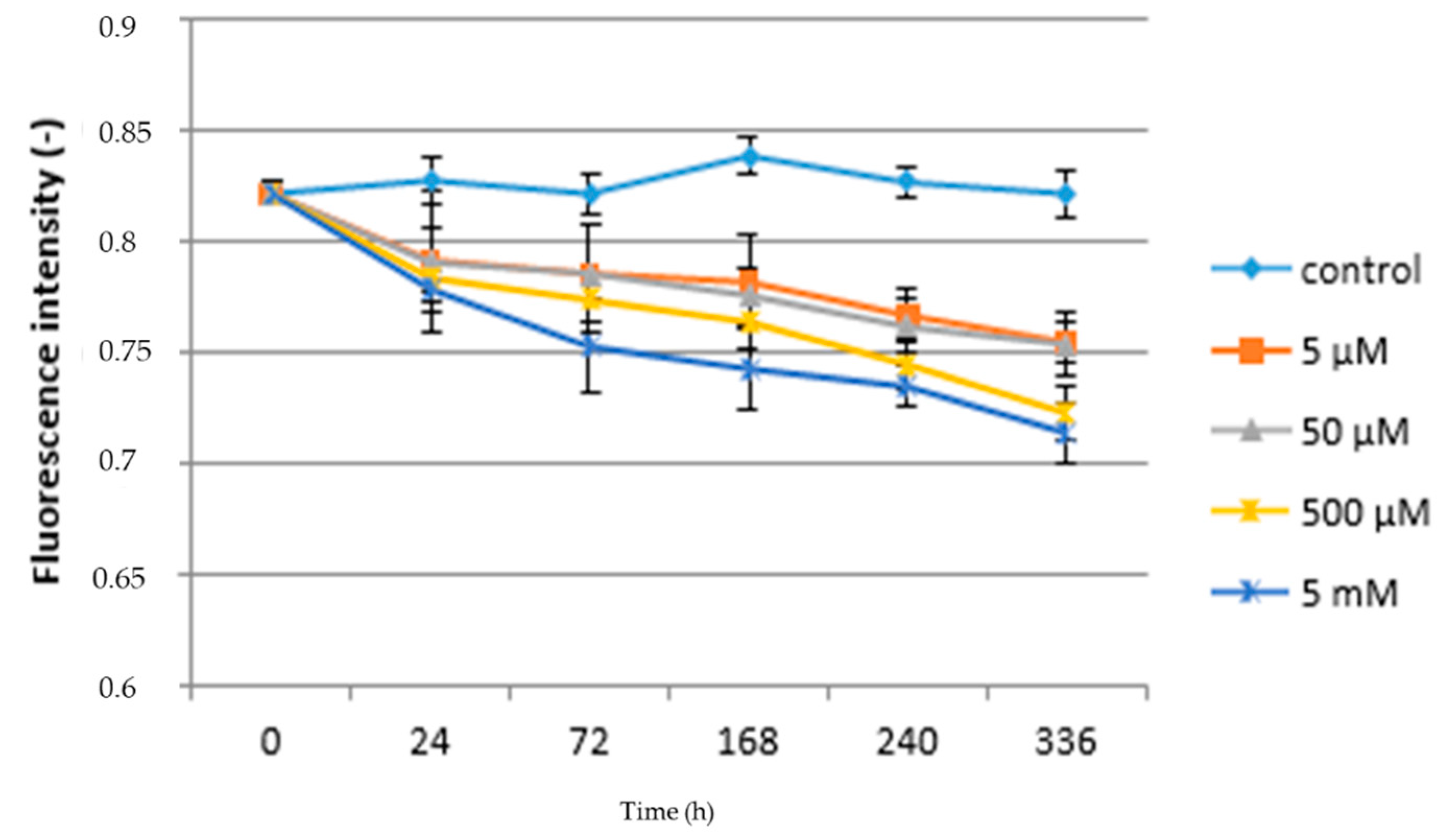
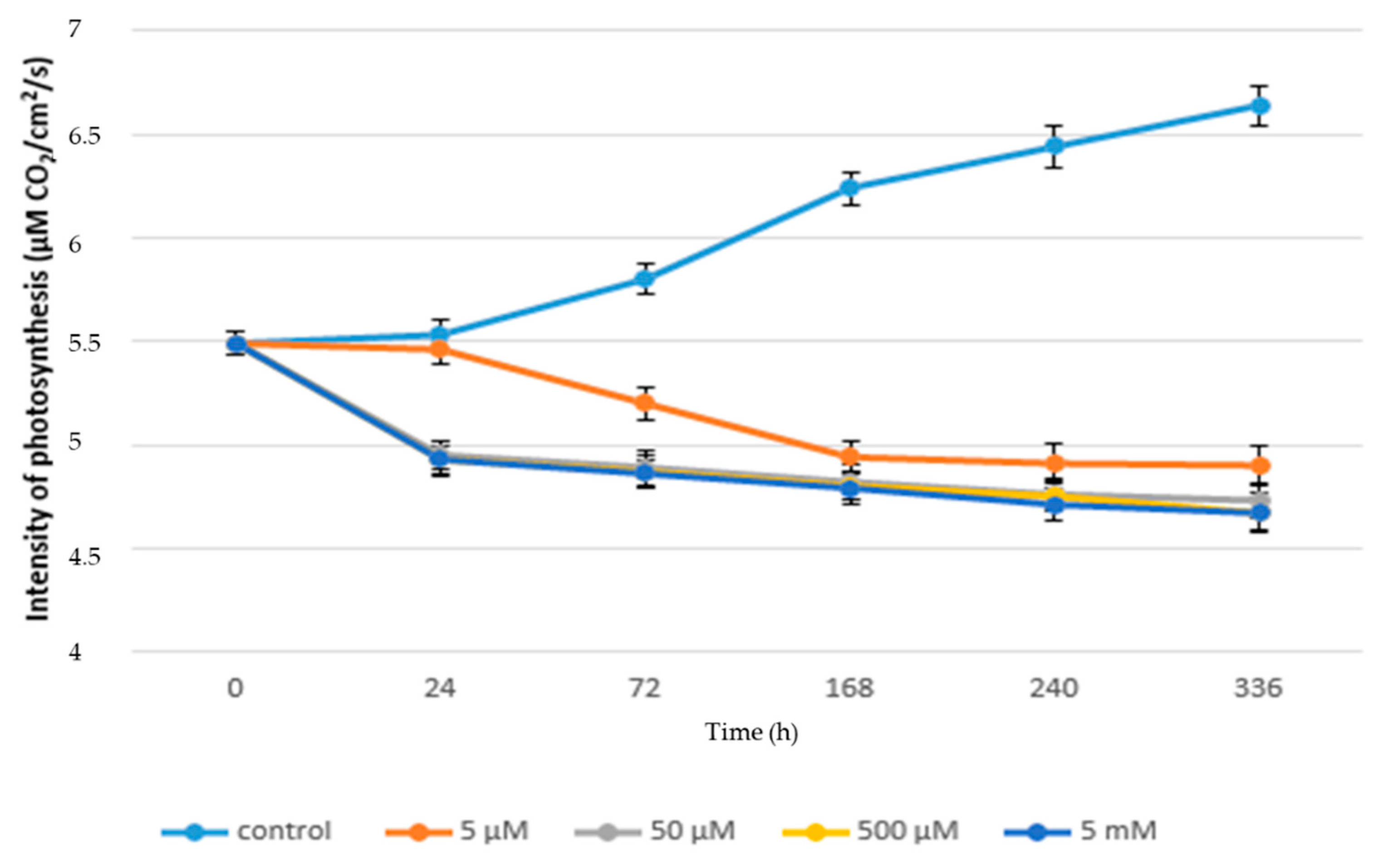
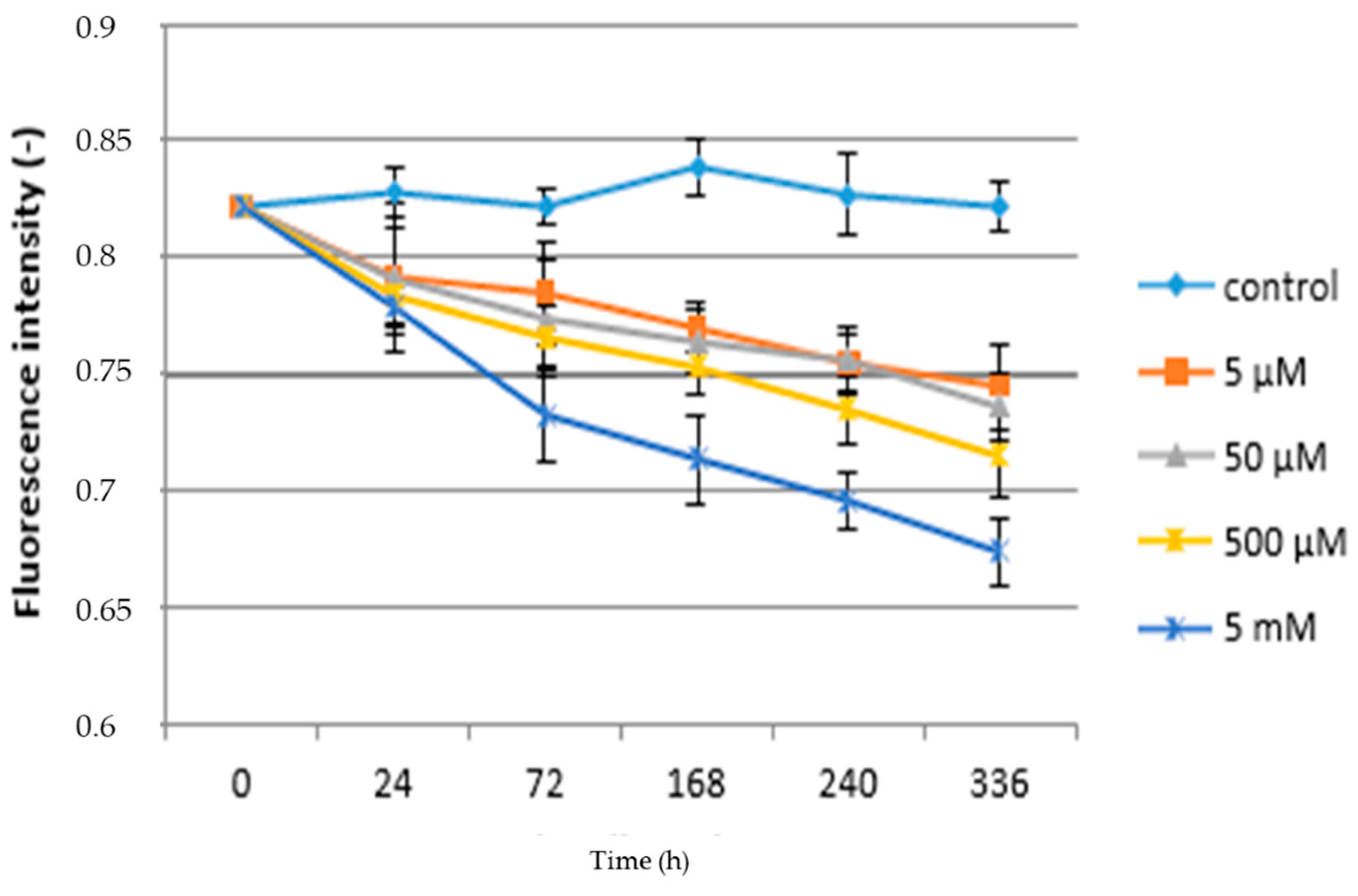
3.1. Acute Exposure to APAP
3.2. Chronic Exposure to APAP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carter, L.J.; Chefetz, B.; Abdeen, Z.; Boxall, A.B.A. Emerging investigator series: Towards a framework for establishing the impacts of pharmaceuticals in wastewater irrigation systems on agro-ecosystems and human health. Environ. Sci. Process. Impacts 2019, 21, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Choi, K.; Lee, S.; Park, S.; Kim, J.S.; Jo, E.H.; Choi, K.H.; Zhang, X.; Giesy, J.P. Effects of sulfathiazole, oxytetracycline and chlortetracycline onsteroidogenesis in the human adrenocarcinoma (H295R) cell line andfreshwater fish Oryzias latipes. J. Hazard. Mater. 2010, 182, 494–502. [Google Scholar] [PubMed]
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.; Thomas, K.V. Input of selected human pharmaceuticalmetabolites into the Norwegian aquatic environment. J. Environ. Monit. 2011, 13, 416–421. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Gheorghe, S.; Petre, J.; Lucaciu, I.; Stoica, C.; Nita-Lazar, M. Risk screening of pharmaceutical compounds in Romanian aquatic environment. Environ. Monit. Assess. 2016, 188, 1–16. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Bacillus thuringiensis B1(2015b) is a gram-positive bacteria able to degrade naproxen and ibuprofen. Water Air Soil Pollut. 2016, 227, 1–8. [Google Scholar] [CrossRef]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total. Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total. Environ. 2017, 303–320. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen-toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen-related hepatotoxicity. Clin. Liver Dis. 2013, 17, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Nilsson, K.O. Enrichment of trace elements from sewage sludge fertilizer in soils and plants. Ambio 1972, 1, 176–179. [Google Scholar]
- Labrecque, M.; Teodorescu, T.I.; Daigle, S. Early performance and nutrition of two willow species in short-rotation intensive culture fertilized with wastewater sludge and impact on the soil characteristic. Can. J. For. Res. 1998, 28, 1621–1635. [Google Scholar]
- Coimbra, R.N.; Calisto, V.; Ferreira, C.I.A.; Esteves, V.I.; Otero, M. Removal of pharmaceuticals from municipal wastewater by adsorption onto pyrolyzed pulp mill sludge. Arab. J. Chem. 2019, 12, 3611–3620. [Google Scholar] [CrossRef]
- Shraim, A.; Diab, A.; AlSuhaimi, A.; Niazy, E.; Metwally, M.; Amad, M.; Sioud, S.; Dawoud, A. Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab. J. Chem. 2017, 10, S719–S729. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the united states-II) Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar]
- Freitas, R.; Coelho, D.; Pires, A.; Soares, A.M.V.M.; Figueira, E.; Nunes, B. Preliminary evaluation of Diopatra neapolitana regenerative capacity as a biomarker for paracetamol exposure. Environ. Sci. Pollut. Res. 2015, 22, 13382–13392. [Google Scholar] [CrossRef]
- Gómez-Oliván, L.M.; Neri-Cruz, N.; Galar-Martínez, M.; Vieyra-Reyes, P.; García-Medina, S.; Razo-Estrada, C.; Dublán-García, O.; Corral-Avitia, A.Y. Assessing the oxidative stress induced by paracetamol spiked in artificial sediment on Hyalella azteca. Water Air Soil Pollut. 2012, 223, 5097–5104. [Google Scholar] [CrossRef]
- Glick, B.R. Phytoremediation: Synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 2003, 21, 383–393. [Google Scholar] [CrossRef]
- Greenberg, B.; Huang, X.; Gurska, Y.; Gerhardt, K.; Wang, W.; Lampi, M.; Zhang, C.; Khalid, A.; Isherwood, D.; Chang, P.; et al. Successful field tests of a multi-process phytoremediation system for decontamination of persistent petroleum and organic contaminants. In Proceedings of the 29th Arctic and Marine Oilspill Program (AMOP) Technical Seminar, Vancouver, BC, Canada, 6–8 June 2006; Environment Canada: Ottawa, ON, Canada, 2006; p. 1122. [Google Scholar]
- Huang, X.D.; El-Alawi, Y.; Gurska, J.; Glick, B.R.; Greenberg, B.M. A multi-process phytoremediation system for decontamination of per-sistent total petroleum hydrocarbons (TPHs) from soils. Micro-Chem. J. 2005, 81, 139–147. [Google Scholar]
- An, J.; Zhou, Q.X.; Sun, F.H.; Zhang, L. Eco-toxicological effects of paracetamol on seed germination and seedling development of wheat (Triticum aestivum L.). J. Hazard. Mater. 2009, 169, 751–757. [Google Scholar] [PubMed]
- Zezulka, Š.; Kummerová, M.; Babula, P.; Hájková, M.; Oravec, M. Sensitivity of physiological and biochemical endpoints in early ontogenic stages of crops under diclofenac and paracetamol treatments. Environ. Sci. Pollut. Res. 2019, 26, 3965–3979. [Google Scholar] [PubMed]
- Huber, C.; Bartha, B.; Harpaintner, R.; Schröder, P. Metabolism of acetaminophen (paracetamol) in plants-two independent pathways result in the formation of a glutathione and a glucose conjugate. Environ. Sci. Pollut. Res. 2009, 16, 206–213. [Google Scholar] [CrossRef]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.; Lazzeri, S.; Torzillo, G. Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars (Olea europaea L.) tolerant to drought stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Dias, M.C.; Bruggemann, W. Limitations of photosynthesis in Phaseolus vulgaris under drought stress: Gas exchange, chlorophyll fluorescence and Calvin cycle enzymes. Photosynthesis 2010, 48, 96–102. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Sayyad-Amin, P.; Jahansooz, M.-R.; Borzouei, A.; Ajili, F. Changes in photosynthetic pigments and chlorophyll-a fluorescence attributes of sweet-forage and grain sorghum cultivars under salt stress. J. Biol. Phys. 2016, 42, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Marcińska, I.; Dziurka, K.; Waligórski, P.; Janowiak, F.; Skrzypek, E.; Warchoł, M.; Juzoń, K.; Kapłoniak, K.; Czyczyło-Mysza, I. Exogenous polyamines only indirectly induce stress tolerance in wheat growing in hydroponic culture under polyethylene glycol-induced osmotic stress. Life 2020, 10, 151. [Google Scholar] [CrossRef]
- Jain, N.; Singh, G.P.; Pandey, R.; Ramya, P.; Singh, P.K.; Nivedita; Prabhu, K.V. Chlorophyll fluorescence kinetics and response of wheat (Triticum aestivum L.) under high temperature stress. Indian J. Exp. Biol. 2018, 56, 194–201. [Google Scholar]
- Borowiak, K.; Gąsecka, M.; Mleczek, M.; Dąbrowski, J.; Chadzinikolau, T.; Magdziak, Z.; Goliński, P.; Rutkowski, P.; Kozubik, T. Photosynthetic activity in relation to chlorophylls, carbohydrates, phenolics and growth of a hybrid Salix purpurea × triandra × viminalis 2 at various Zn concentrations. Acta Physiol. Plant. 2015, 37, 37. [Google Scholar] [CrossRef]
- Martins, M.; Sousa, B.; Lopes, J.; Soares, C.; Machado, J.; Carvalho, S.; Fidalgo, F.; Teixeira, J. Diclofenac shifts the role of root glutamine synthetase and glutamate dehydrogenase for maintaining nitrogen assimilation and proline production at the expense of shoot carbon reserves in Solanum lycopersicum L. Environ. Sci. Pollut. Res. 2020, 27, 29130–29142. [Google Scholar] [CrossRef]
- Hájková, M.; Kummerová, M.; Zezulka, Š.; Babula, P.; Váczi, P. Diclofenac as an environmental threat: Impact on the photosynthetic processes of Lemna minor chloroplasts. Chemosphere 2019, 224, 892–899. [Google Scholar] [CrossRef]
- Copolovici, L.; Timis, D.; Taschina, M.; Copolovici, D.; Cioca, G.; Bungau, S. Diclofenac influence on photosynthetic parameters and volatile organic compounds emision from Phaseolus vulgaris L. plants. Rev. Chim. 2017, 68, 2076–2078. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Alkimin, G.; Daniel, D.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Francini, A.; Huber, C.; Sebastiani, L.; Schröder, P. Poplar and diclofenac pollution: A focus on physiology, oxidative stress and uptake in plant organs. Sci. Total Environ. 2018, 636, 944–952. [Google Scholar] [CrossRef]
- Kummerová, M.; Zezulka, Š.; Babula, P.; Tříska, J. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Taschina, M.; Copolovici, D.M.; Bungau, S.G.; Andreea, L.P.; Copolovici, L.; Iovan, C. The influence of residual acetaminophen on Phaseolus vulgaris L. Secondary metabolites. Farmacia 2017, 65, 709–713. [Google Scholar]
- Renberg, L.; Johansson, A.I.; Shutova, T.; Stenlund, H.; Aksmann, A.; Raven, J.A.; Gardeström, P.; Moritz, T.; Samuelsson, G. A Metabolomic approach to study major metabolite changes during acclimation to limiting CO2 in Chlamydomonas reinhardtii. Plant. Physiol. 2010, 154, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar]
- Guo, Y.; Tan, J. Recent advances in the application of chlorophyllafluorescence from photosystem II. Photochem. Photobiol. 2014, 91, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant. Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of photosynthesis to different concentrations of heavy metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. Regulation of photosynthetic electron transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef]
- Galle, A.; Flexas, J. Gas-exchange and chlorophyll fluorescence measurements in grapevine leaves in the field. In Methodologies and Results in Grapevine Research; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2010; pp. 107–121. [Google Scholar]
- Kitajima, M.; Butler, W. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA)-Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant. Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynt. Res. 2004, 79, 209. [Google Scholar]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll afluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar]
- Faseela, P.; Sinisha, A.K.; Brestic, M.; Puthur, J. Special issue in honour of Prof. Reto J. Strasser-Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthesis 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Dubey, R.; Mishra, S. Heavy metal toxicity induced alterations in photosynthetic metabolism in plants. In Biological Approaches to Sustainable Soil Systems; Informa UK Limited: London, UK, 2005; Volume 20051262, pp. 845–863. [Google Scholar]
- Aggarwal, A.; Sharma, I.; Tripathi, B.N.; Munjal, A.K.; Baunthiyal, M.; Sharma, V. Metal Toxicity and Photosynthesis in Photosynthesis: Overviews on Recent Progress and Future Perspectives; IK International Publishing House (Pvt) Limited: New Delhi, India, 2012. [Google Scholar]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Guangyu, S. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Koyama, K.; Takemoto, S. Morning reduction of photosynthetic capacity before midday depression. Sci. Rep. 2014, 4, 4389. [Google Scholar] [CrossRef]
- Lin, M.-Z.; Jin, M.-F. Soil Cu contamination destroys the photosynthetic systems and hampers the growth of green vegetables. Photosynthesis 2018, 56, 1336–1345. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Wang, J.; Hu, X.; Wang, L.; Miao, Z. Study on the law of nitrogen transfer and conversionand use of fertilizer nitrogen in paddy fields under water-saving irrigation mode. Water 2019, 11, 218. [Google Scholar]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wang, Z.; Niu, J. Effects of water stress on fluorescence parameters and photosynthetic characteristics of drip irrigation in rice. Water 2020, 12, 289. [Google Scholar] [CrossRef]
- Ralph, P.J.; Burchett, M. Impact of petrochemicals on the photosynthesis of Halophila ovalis using chlorophyll fluorescence. Mar. Pollut. Bull. 1998, 36, 429–436. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Elefiherion, E.P.; Karataglis, S. Ecophysiological and ultrastructural effects of copper in Thlaspi ochroleucum (Cruciferae). Can. J. Bot. 1992, 70, 947–957. [Google Scholar]
- Li, H.; Zhang, G.C.; Xie, H.C.; Li, K.; Zhang, S.Y. The effects of the phenol concentrations on photosynthetic parameters of Salix babylonica L. Photosynthesis 2015, 53, 430–435. [Google Scholar] [CrossRef]
- Borjigidai, A.; Hikosaka, K.; Hirose, T.; Hasegawa, T.; Okada, M.; Kobayashi, K. Seasonal changes in temperature dependence of photosynthetic rate in rice under a free-air CO2 enrichment. Ann. Bot. 2006, 97, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hejnák, V. The effect of growth regulators on photosynthesis and water regime of sugar beet during water stress. Listy Cukrov. Řepař. 2010, 126, 27–30. [Google Scholar]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 279–319. [Google Scholar] [CrossRef]
| Control | Control |
|---|---|
| 5 µM acute | 5 µM chronic |
| 50 µM acute | 50 µM chronic |
| 500 µM acute | 500 µM chronic |
| 5 mM acute | 5 mM chronic |
| Suma of Chlorophyll | Carotenoids | ||
|---|---|---|---|
| 5 µM | acute | 3.498 ± 0.334 | 0.643 ± 0.067 |
| chronic | 3.543 ± 0.317 | 0.669 ± 0.063 | |
| 50 µM | acute | 3.439 ± 0.343 * | 0.667 ± 0.063 |
| chronic | 3.849 ± 0.308 | 0.739 ± 0.057 * | |
| 500 µM | acute | 3.665 ± 0.320 | 0.692 ± 0.061 |
| chronic | 4.455 ± 0.320 * | 0.824 ± 0.059 * | |
| 5 mM | acute | 3.779 ± 0.375 | 0.706 ± 0.067 * |
| chronic | 4.057 ± 0.338 * | 0.790 ± 0.061 * | |
| Control | acute | 3.495 ± 0.367 * | 0.581 ± 0.073 * |
| chronic | 3.495 ± 0.367 * | 0.581 ± 0.073 * | |
| Fv/Fm | Fv/F0 | Fm/F0 | ||
|---|---|---|---|---|
| 5 µM | acute | 0.788 ± 0.025 * | 4.508 ± 0.542 * | 5.747 ± 0.670 * |
| chronic | 0.783 ± 0.030 * | 4.079 ± 0.599 * | 5.208 ± 0.667 * | |
| 50 µM | acute | 0,786 ± 0.029 * | 4.182 ± 0.599 * | 5.197 ± 0.649 * |
| chronic | 0.779 ± 0.033 * | 3.970 ± 0.684 * | 5.397 ± 0.748 * | |
| 500 µM | acute | 0.776 ± 0.036 * | 4.369 ± 0.558 * | 5.208 ± 0.661 * |
| chronic | 0.769 ± 0.039 * | 4.060 ± 0.686 * | 5.553 ± 0.649 * | |
| 5 mM | acute | 0.765 ± 0.041 * | 4.179 ± 0.588 * | 5.458 ± 0.665 * |
| chronic | 0.745 ± 0.057 * | 3.921 ± 0.655 * | 5.266 ± 0.676 * | |
| control | acute | 0.826 ± 0.015 | 4.814 ± 0.452 | 5.815 ± 0.452 |
| chronic | 0.826 ± 0.015 | 4.814 ± 0.452 | 5.815 ± 0.452 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudrna, J.; Hnilicka, F.; Kubes, J.; Vachova, P.; Hnilickova, H.; Kuklova, M. Effect of Acetaminophen (APAP) on Physiological Indicators in Lactuca sativa. Life 2020, 10, 303. https://doi.org/10.3390/life10110303
Kudrna J, Hnilicka F, Kubes J, Vachova P, Hnilickova H, Kuklova M. Effect of Acetaminophen (APAP) on Physiological Indicators in Lactuca sativa. Life. 2020; 10(11):303. https://doi.org/10.3390/life10110303
Chicago/Turabian StyleKudrna, Jiri, Frantisek Hnilicka, Jan Kubes, Pavla Vachova, Helena Hnilickova, and Margita Kuklova. 2020. "Effect of Acetaminophen (APAP) on Physiological Indicators in Lactuca sativa" Life 10, no. 11: 303. https://doi.org/10.3390/life10110303
APA StyleKudrna, J., Hnilicka, F., Kubes, J., Vachova, P., Hnilickova, H., & Kuklova, M. (2020). Effect of Acetaminophen (APAP) on Physiological Indicators in Lactuca sativa. Life, 10(11), 303. https://doi.org/10.3390/life10110303







