Six ‘Must-Have’ Minerals for Life’s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite
Abstract
… molecular physics is the true basis of biology. [1]
… physics approximates biology because there is no such thing as an organism at thermodynamic equilibrium. [2]
1. Introduction
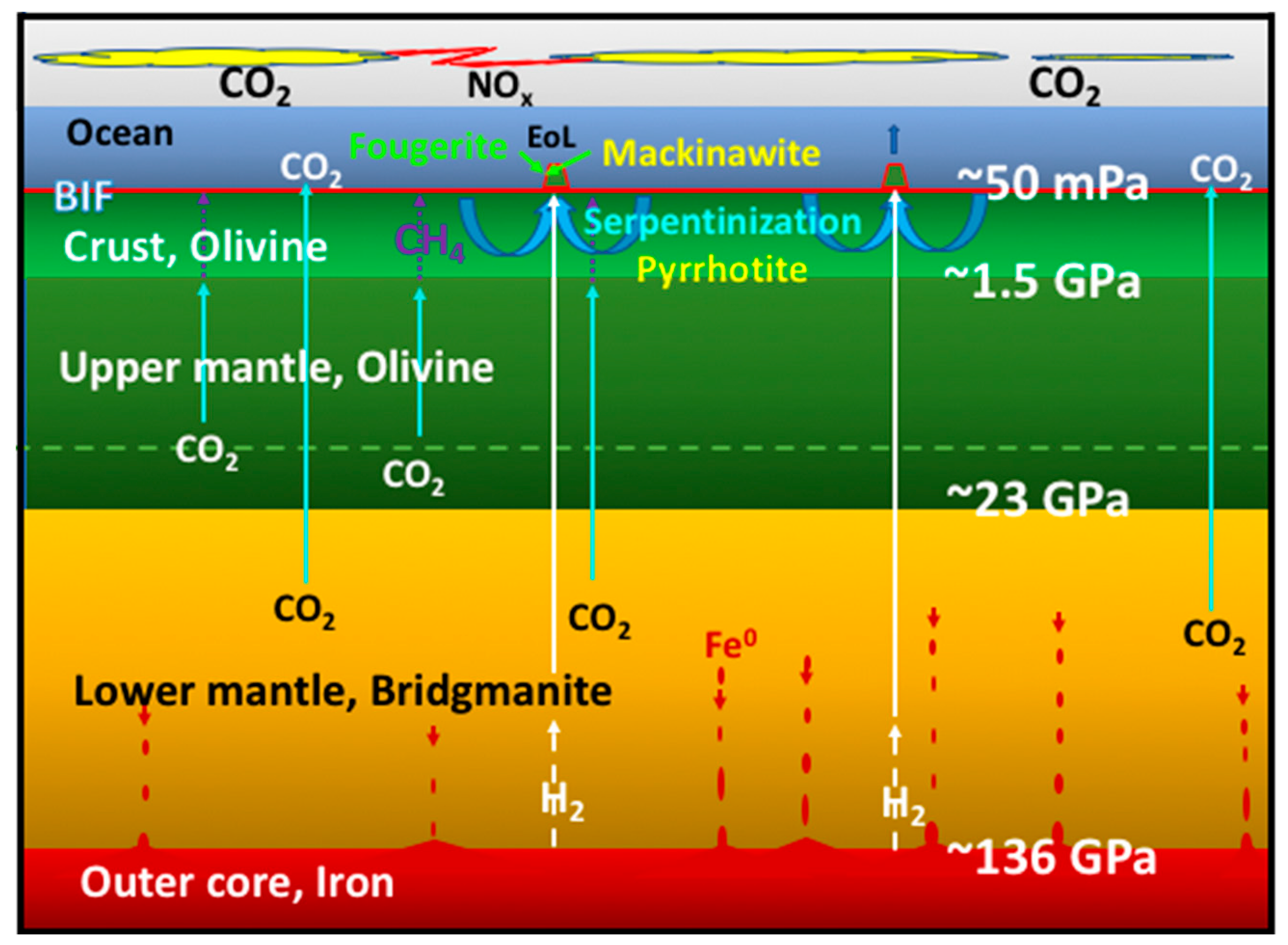
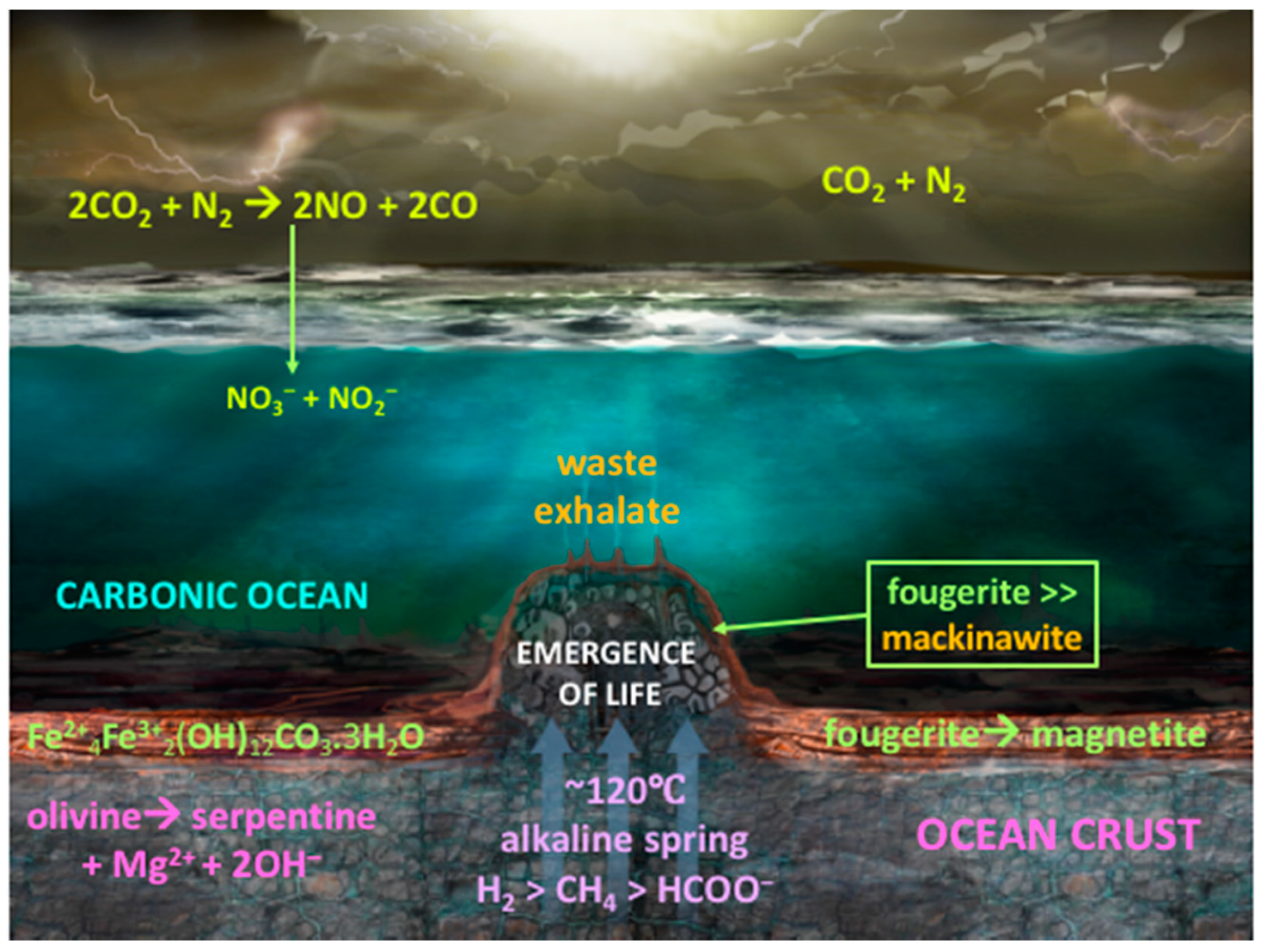
- The second group contains the two mineral precipitates—ferrous iron-rich oxyhydroxides and monosulfides—constituting a membrane that keeps the two contrasting fluids from immediate mixing. In AVT the oxyhydroxides are the disequilibria-converting nano-engines and solid electrolytes, while the monosulfides are the electron conductors and proto-hydrogenases. Combined, they have the potential to mediate the disequilibria and bring life into being (Figure 2) [9].
2. Four Minerals to Set the Stage for Life’s Emergence
2.1. Olivine (Mg>Fe)2SiO4
2.2. Bridgmanite (Mg,Fe)SiO3
2.3. Pyrrhotite Fe(1-x)S (x = 0 to 0.2)
2.4. Serpentine (Mg,Fe,)2-3Si2O5(OH)4
3. Two Minerals to Make Life Happen
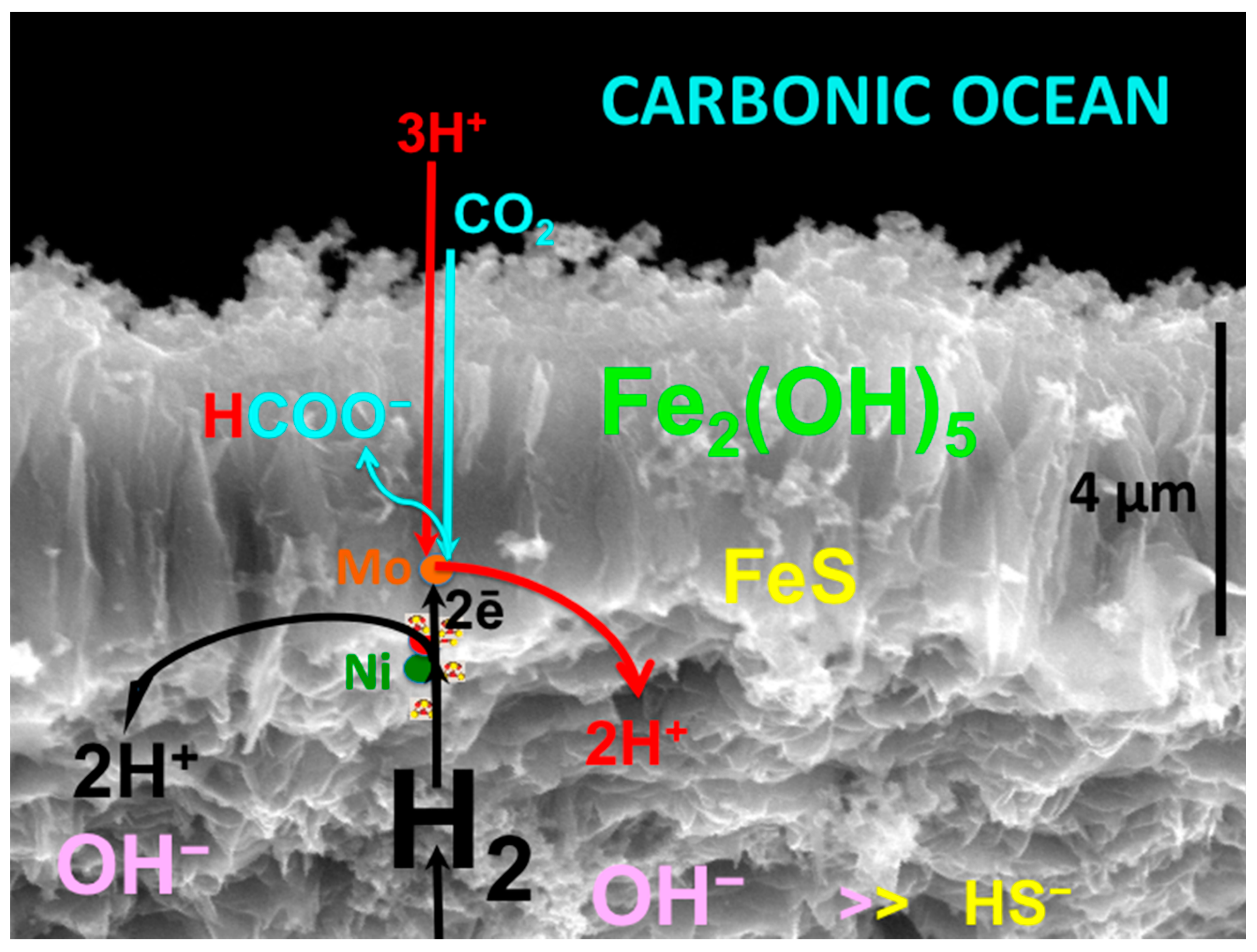
3.1. Fougerite [Fe2+6xFe3+6(x−1)O12H2(7−3x)]2+·[(CO2−)·3H2O]2−
[Fe42+,Fe23+,(OH)12]2+·[SO4·nH2O]2− + 0.25 NH3 + 0.25 OH−
3.2. Mackinawite Fe(Ni)S
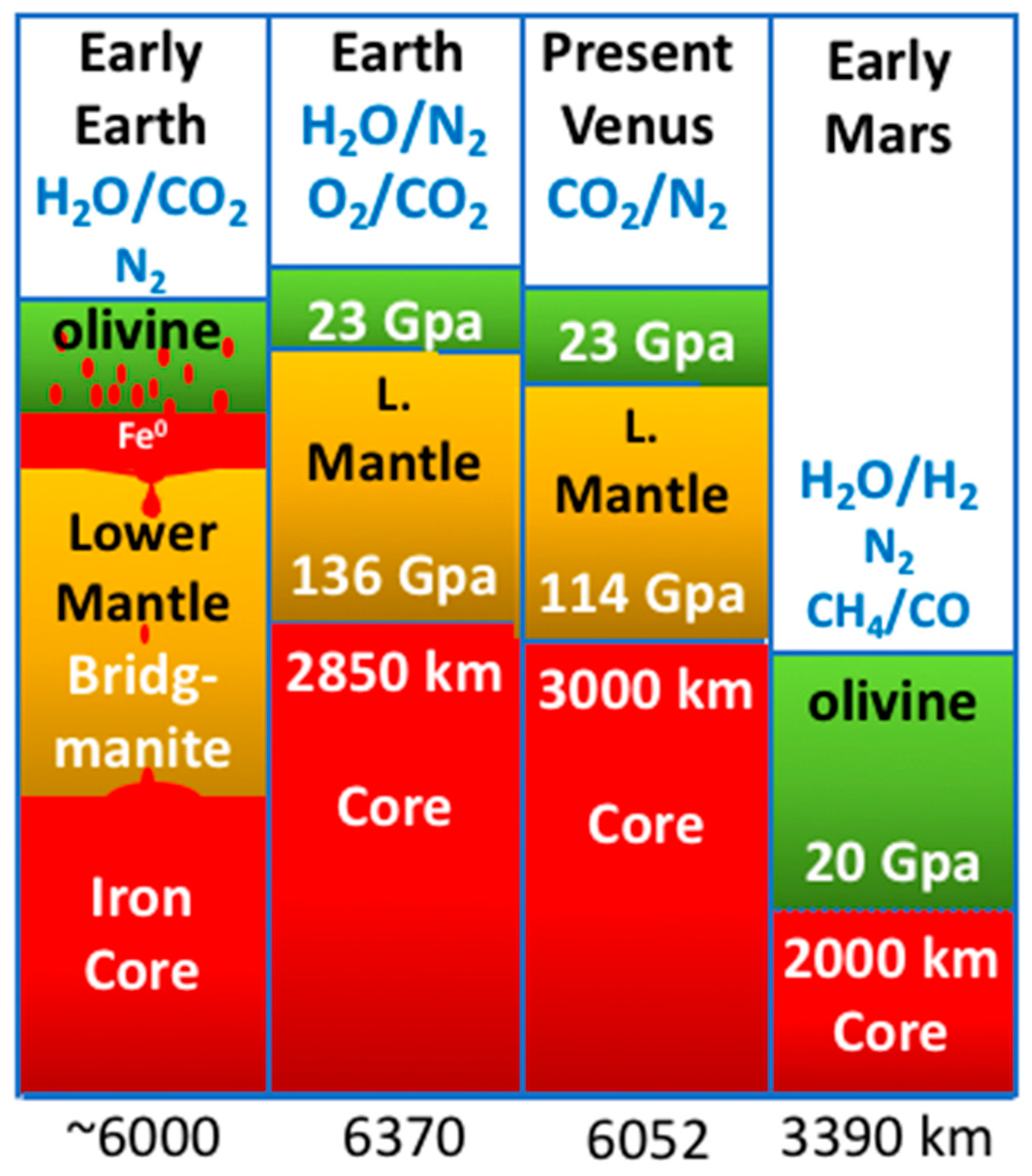
4. The Relevance of Accretion Histories to Astrobiology
5. Discussion
6. Astrobiological Implications
7. Caveats and Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leduc, S. A study of molecular physics. Arch. Roentgen Ray 1911, 16, 202–205. [Google Scholar] [CrossRef]
- Longo, G.; Montevil, M.; Sonnenschein, C.; Soto, A.M. In search of principles for a theory of organisms. J. Biosci. 2015, 40, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Daniel, R.M.; Hall, A.J.; Sherringham, J. A hydrothermally precipitated catalytic iron sulphide membrane as a first step toward life. J. Mol. Evol. 1994, 39, 231–241. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. 1997, 154, 377–402. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.; Wood, B.J. Core formation and the oxidation state of the Earth. Earth Planet. Sci. Lett. 2005, 236, 78–95. [Google Scholar] [CrossRef]
- Russell, M.J. First life. Am. Sci. 2006, 94, 32–39. [Google Scholar] [CrossRef]
- Elkins-Tanton, L.T. Linked magma ocean solidification and atmospheric growth for Earth and Mars. Earth Planet. Sci. Lett. 2008, 271, 181–191. [Google Scholar] [CrossRef]
- Hirschmann, M.M. Magma ocean influence on early atmosphere mass and composition. Earth Planet. Sci. Lett. 2012, 341–344, 48–57. [Google Scholar] [CrossRef]
- Russell, M.J.; Barge, L.M.; Bhartia, R.; Bocanegra, D.; Bracher, P.J.; Branscomb, E.; Kidd, R.; McGlynn, S.; Meier, D.H.; Nitschke, W.; et al. The drive to life on wet and icy worlds. Astrobiology 2014, 14, 308–343. [Google Scholar] [CrossRef]
- Barge, L.M.; Abedian, Y.; Russell, M.J.; Doloboff, I.J.; Cartwright, J.H.; Kidd, R.D.; Kanik, I. From chemical gardens to fuel cells: Generation of electrical potential and current across self-assembling iron mineral membranes. Angew. Chem. Int. Ed. Engl. 2015, 54, 8184–8187. [Google Scholar] [CrossRef]
- Waite, J.H., Jr.; Lewis, W.S.; Magee, B.A.; Lunine, J.I.; McKinnon, W.B.; Glein, C.R.; Mousis, O.; Young, D.T.; Brockwell, T.; Westlake, J.; et al. Liquid water on Enceladus from observations of ammonia and 40Ar in the plume. Nature 2009, 460, 487–490. [Google Scholar] [CrossRef]
- Yung, Y.L.A.M.; Pinto, J.P. Photochemistry of the atmosphere of Titan: Comparison between model and observations. Astrophys. J. Suppl. Ser. 1984, 55, 465–506. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, A.R.; Hurford, T.A.; Barge, L.M.; Bland, M.T.; Bowman, J.S.; Brinckerhoff, W.; Buratti, B.J.; Cable, M.L.; Castillo-Rogez, J.; Collins, G.C.; et al. The NASA roadmap to ocean worlds. Astrobiology 2019, 19, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Hall, A.J. The onset and early evolution of life. In Evolution of Early Earth’s Atmosphere, Hydrosphere, and Biosphere—Constraints from Ore Deposits; Kesler, S.E., Ohmoto, H., Eds.; Geological Society of America Memoir: Boulder, CO, USA, 2006; Volume 198, pp. 1–32. [Google Scholar]
- Armstrong, K.; Frost, D.J.; McCammon, C.A.; Rubie, D.C.; Ballaran, B.T. Deep magma ocean formation set the oxidation state of Earth’s mantle. Science 2019, 365, 903–906. [Google Scholar] [CrossRef]
- Deng, J.; Du, Z.; Karki, B.B.; Ghosh, D.B.; Lee, K.K.M. A magma ocean origin to divergent redox evolutions of rocky planetary bodies and early atmospheres. Nat. Commun. 2020, 11, 2007. [Google Scholar] [CrossRef]
- Tyburczy, J.A.F.B.; Ahrens, T.J. Shock-induced volatile loss from a carbonaceous chondrite: Implications for planetary accretion. Earth Planet. Sci. Lett. 1986, 80, 201–207. [Google Scholar] [CrossRef]
- Cottrell, A. The natural philosophy of engines. Contemp. Phys. 1979, 20, 1–10. [Google Scholar] [CrossRef]
- Javoy, M.; Kaminski, E.; Guyot, F.; Andrault, D.; Sanloup, C.; Moreira, M.; Labrosse, S.; Jambon, A.; Agrinier, P.; Davaille, A.; et al. The chemical composition of the Earth: Enstatite chondrite models. Earth Planet. Sci. Lett. 2010, 293, 259–268. [Google Scholar] [CrossRef]
- Trønnes, R.G.; Baron, M.A.; Eigenmann, K.R.; Guren, M.G.; Heyn, B.H.; Løken, A.; Mohn, C.E. Core formation, mantle differentiation and core-mantle interaction within Earth and the terrestrial planets. Tectonophysics 2019, 760, 165–198. [Google Scholar] [CrossRef]
- Braukmuller, N.; Wombacher, F.; Funk, C.; Munker, C. Earth’s volatile element depletion pattern inherited from a carbonaceous chondrite-like source. Nat. Geosci. 2019, 12, 564–568. [Google Scholar] [CrossRef]
- Wordsworth, R.D. Atmospheric nitrogen evolution on Earth and Venus. Earth Planet. Sci. Lett. 2016, 447, 103–111. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Güdel, M.; Stökl, A.; Lammer, H.; Tu, L.; Kislyakova, K.G.; Lüftinger, T.; Odert, P.; Erkaev, N.V.; Dorfi, E.A. The evolution of stellar rotation and the hydrogen atmospheres of habitable-zone terrestrial planets. Astrophys. J. 2015, 815. [Google Scholar] [CrossRef]
- Ryder, G. Mass flux in the ancient Earth-Moon system and benign implications for the origin of life on Earth. J. Geophys. Res. Planets 2002, 107, 6-1–6-13. [Google Scholar] [CrossRef]
- Foley, B.J.; Smye, A.J. Carbon cycling and habitability of Earth-sized stagnant lid planets. Astrobiology 2018, 18, 873–896. [Google Scholar] [CrossRef]
- López-Puertas, M.; Funke, B.; Gil-López, S.; von Clarmann, T.; Stiller, G.P.; Höpfner, M.; Kellmann, S.; Tsidu, M.G.; Fischer, H.; Jackman, C.H. HNO3, N2O5, and ClONO2 enhancements after the October-November 2003 solar proton events. J. Geophys. Res. Space Phys. 2005, 110. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Heidt, L.E.; Krasnec, J.P.; Pollock, W.H.; Seiler, W. Biomass burning as a source of atmospheric gases CO, H2, N2O, NO, CH3Cl and COS. Nature 1979, 282, 253–256. [Google Scholar] [CrossRef]
- Wong, M.L.; Charnay, B.D.; Gao, P.; Yung, Y.L.; Russell, M.J. Nitrogen oxides in early earth’s atmosphere as electron acceptors for life’s emergence. Astrobiology 2017, 17, 975–983. [Google Scholar] [CrossRef]
- Lingam, M.; Loeb, A. Colloquium: Physical constraints for the evolution of life on exoplanets. Rev. Mod. Phys. 2019, 91. [Google Scholar] [CrossRef]
- Gilat, A. Primordial hydrogen-helium degassing, an overlooked major energy source for internal terrestrial processes. HAIT J. Sci. Eng. 2005, B2, 125–167. [Google Scholar]
- Zgonnik, V. The occurrence and geoscience of natural hydrogen: A comprehensive review. Earth Sci. Rev. 2020, 203. [Google Scholar] [CrossRef]
- Worman, S.L.; Pratson, L.F.; Karson, J.A.; Schlesinger, W.H. Abiotic hydrogen (H2) sources and sinks near the Mid-Ocean Ridge (MOR) with implications for the subseafloor biosphere. Proc. Natl. Acad. Sci. USA 2020, 117, 13283–13293. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.-k.; Mao, W.L. Key problems of the four-dimensional Earth system. Matter Radiat. Extrem. 2020, 5. [Google Scholar] [CrossRef]
- Takahashi, E. Speculations on the Archean mantle: Missing link between komatiite and depleted garnet peridotite. J. Geophys. Res. Solid Earth 1990, 95B, 15941–15954. [Google Scholar] [CrossRef]
- Bédard, J.H. A catalytic delamination-driven model for coupled genesis of Archaean crust and sub-continental lithospheric mantle. Geochim. Cosmochim. Acta 2006, 70, 1188–1214. [Google Scholar] [CrossRef]
- Shock, E.L.; Canovas, P.; Yang, Z.; Boyer, G.; Johnson, K.; Robinson, K.; Fecteau, K.; Windman, T.; Cox, A. Thermodynamics of organic transformations in hydrothermal fluids. Rev. Mineral. Geochem. 2013, 76, 311–350. [Google Scholar] [CrossRef]
- O’Neill, C.; Debaille, V. The evolution of Hadean–Eoarchaean geodynamics. Earth Planet. Sci. Lett. 2014, 406, 49–58. [Google Scholar] [CrossRef]
- Lang, S.Q.; Fruh-Green, G.L.; Bernasconi, S.M.; Brazelton, W.J.; Schrenk, M.O.; McGonigle, J.M. Deeply-sourced formate fuels sulfate reducers but not methanogens at Lost City hydrothermal field. Sci. Rep. 2018, 8, 755. [Google Scholar] [CrossRef]
- White, L.M.; Shibuya, T.; Vance, S.D.; Christensen, L.E.; Bhartia, R.; Kidd, R.; Hoffmann, A.; Stucky, G.D.; Kanik, I.; Russell, M.J. Simulating serpentinization as it could apply to the emergence of life using the JPL hydrothermal reactor. Astrobiology 2020, 20, 307–326. [Google Scholar] [CrossRef]
- Mitchell, P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: Power transmission by proticity. Biochem. Soc. Trans. 1976, 4, 399–430. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Frankenstein or a submarine alkaline vent: Who is responsible for abiogenesis? Part 2: As life is now, so it must have been in the beginning. Bioessays 2018, 40, e1700182. [Google Scholar] [CrossRef]
- Nitschke, W.; Russell, M.J. Hydrothermal focusing of chemical and chemiosmotic energy, supported by delivery of catalytic Fe, Ni, Mo/W, Co, S and Se, forced life to emerge. J. Mol. Evol. 2009, 69, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J. Green rust: The simple organizing ‘seed’ of all life? Life 2018, 8, 35. [Google Scholar] [CrossRef]
- Hudson, R.; de Graaf, R.; Strandoo Rodin, M.; Ohno, A.; Lane, N.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R.; Barge, L.M.; Braun, D.; et al. CO2 reduction driven by a pH gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 22873–22879. [Google Scholar] [CrossRef]
- Kump, L.R.; Seyfried, W.E. Hydrothermal Fe fluxes during the Precambrian: Effect of low oceanic sulfate concentrations and low hydrostatic pressure on the composition of black smokers. Earth Planet. Sci. Lett. 2005, 235, 654–662. [Google Scholar] [CrossRef]
- Mielke, R.E.; Russell, M.J.; Wilson, P.R.; McGlynn, S.E.; Coleman, M.; Kidd, R.; Kanik, I. Design, fabrication, and test of a hydrothermal reactor for origin-of-life experiments. Astrobiology 2010, 10, 799–810. [Google Scholar] [CrossRef]
- Morrison, P.R.; Mojzsis, S.J. Tracing the early emergence of microbial sulfur metabolisms. Geomicrobiol. J. 2020, 1–21. [Google Scholar] [CrossRef]
- Wood, B.J.; Walter, M.J.; Wade, J. Accretion of the Earth and segregation of its core. Nature 2006, 441, 825–833. [Google Scholar] [CrossRef]
- Meyssami, B.; Balaban, M.O.; Teixeira, A.A. Prediction of pH in model systems pressurized with carbon-dioxide. Biotechnol. Prog. 1992, 8, 149–154. [Google Scholar] [CrossRef]
- Kusakabe, M.; Tanyileke, G.Z.; McCord, S.A.; Schladow, S.G. Recent pH and CO2 profiles at Lakes Nyos and Monoun, Cameroon: Implications for the degassing strategy and its numerical simulation. J. Volcanol. Geotherm. Res. 2000, 97, 241–260. [Google Scholar] [CrossRef]
- Cartigny, P.; Pineau, F.; Aubaud, C.; Javoy, M. Towards a consistent mantle carbon flux estimate: Insights from volatile systematics (H2O/Ce, δD, CO2/Nb) in the North Atlantic mantle (14° N and 34° N). Earth Planet. Sci. Lett. 2008, 265, 672–685. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, M.B.; Rodolfo, R.S.; Lapus, M.R.; Cabria, H.B.; Fullon, J.; Gojunco, G.R.; Breecker, D.O.; Cantarero, D.M.; Evaristo, J.; Siringan, F.P.; et al. Submarine groundwater and vent discharge in a volcanic area associated with coastal acidification. Geophys. Res. Lett. 2020, 47. [Google Scholar] [CrossRef]
- Shock, E.L. Geochemical constraints on the origin of organic compounds in hydrothermal systems. Origins Life Evol. Biosph. 1990, 20, 331–367. [Google Scholar] [CrossRef]
- Shock, E.L. Chemical environments of submarine hydrothermal systems. Orig. Life Evol. Biosph. 1992, 22, 67–107. [Google Scholar] [CrossRef] [PubMed]
- Mielke, R.E.; Robinson, K.J.; White, L.M.; McGlynn, S.E.; McEachern, K.; Bhartia, R.; Kanik, I.; Russell, M.J. Iron-sulfide-bearing chimneys as potential catalytic energy traps at life’s emergence. Astrobiology 2011, 11, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Seyfried, W.E., Jr.; Lowell, R.P.; Liu, Y.; Liang, J.; Guo, Z.; Ding, K.; Zhang, H.; Liu, J.; Qiu, L.; et al. Deep high-temperature hydrothermal circulation in a detachment faulting system on the ultra-slow spreading ridge. Nat. Commun. 2020, 11, 1300. [Google Scholar] [CrossRef]
- German, C.R.; Petersen, S.; Hannington, M.D. Hydrothermal exploration of mid-ocean ridges: Where might the largest sulfide deposits be forming? Chem. Geol. 2016, 420, 114–126. [Google Scholar] [CrossRef]
- Arrhenius, G.O.; Gedulin, B.; Mojzsis, S. Phosphate in models for chemical evolution. In Chemical Evolution and Origin of Life; Ponnamperuma, C., Chela-Flores, J., Eds.; Harpers Brothers: New York, NY, USA, 1993; pp. 23–40. [Google Scholar]
- Halevy, I.; Alesker, M.; Schuster, E.M.; Popovitz-Biro, R.; Feldman, Y. A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nat. Geosci. 2017, 10, 135–139. [Google Scholar] [CrossRef]
- White, L.M.; Bhartia, R.; Stucky, G.D.; Kanik, I.; Russell, M.J. Mackinawite and greigite in ancient alkaline hydrothermal chimneys: Identifying potential key catalysts for emergent life. Earth Planet. Sci. Lett. 2015, 430, 105–114. [Google Scholar] [CrossRef]
- Russell, M.J.; Nitschke, W.; Branscomb, E. The inevitable journey to being. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20120254. [Google Scholar] [CrossRef]
- Astumian, R.D. Stochastically pumped adaptation and directional motion of molecular machines. Proc. Natl. Acad. Sci. USA 2018, 115, 9405–9413. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef]
- Branscomb, E.; Biancalani, T.; Goldenfeld, N.; Russell, M. Escapement mechanisms and the conversion of disequilibria; the engines of creation. Phys. Rep. 2017, 677, 1–60. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. Escapement mechanisms: Efficient free energy transduction by reciprocally-coupled gating. Proteins 2020, 88, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Sagawa, T.; Parrondo, J.M. Imitating chemical motors with optimal information motors. Phys. Rev. Lett. 2013, 111, 010602. [Google Scholar] [CrossRef] [PubMed]
- Šešelja, D.; Straßer, C. Epistemic justification in the context of pursuit: A coherentist approach. Synthese 2014, 191, 3111–3141. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Ryabchikov, I.D.; Wirth, R. A primary natrocarbonatitic association in the Deep Earth. Mineral. Petrol. 2015, 110, 387–398. [Google Scholar] [CrossRef]
- Majumdar, A.; Wu, M.; Pan, Y.; Iitaka, T.; Tse, J.S. Structural dynamics of basaltic melt at mantle conditions with implications for magma oceans and superplumes. Nat. Commun. 2020, 11, 4815. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.; Bass, J.D.; Ricard, Y.; Mattern, E.; Bukowinsky, M.S. On the bulk composition of the lower mantle: Predictions and limitations from generalized inversion of radial seismic profiles. Geophys J. Int. 2007, 170, 764–780. [Google Scholar] [CrossRef]
- Proskurowski, G.; Lilley, M.D.; Kelley, D.S.; Olson, E.J. Low temperature volatile production at the Lost City Hydrothermal Field, evidence from a hydrogen stable isotope geothermometer. Chem. Geol. 2006, 229, 331–343. [Google Scholar] [CrossRef]
- Tosca, N.J.; Jiang, C.Z.; Rasmussen, B.; Muhling, J. Products of the iron cycle on the early Earth. Free Radic. Biol. Med. 2019, 140, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, C.A. Phosphoran pyroxene and olivine in silicate inclusions in natural iron-carbon alloy, Disko-Island, Greenland. Geochim. Cosmochim. Acta 1984, 48, 1115–1126. [Google Scholar] [CrossRef]
- Smyth, J.R.; Frost, D.J.; Nestola, F.; Holl, C.M.; Bromiley, G. Olivine hydration in the deep upper mantle: Effects of temperature and silica activity. Geophys. Res. Lett. 2006, 33, 15. [Google Scholar] [CrossRef]
- Welsch, B.; Hammer, J.; Hellebrand, E. Phosphorus zoning reveals dendritic architecture of olivine. Geology 2014, 42, 867–870. [Google Scholar] [CrossRef]
- Veter, M.; Foley, S.F.; Mertz-Kraus, R.; Groschopf, N. Trace elements in olivine of ultramafic lamprophyres controlled by phlogopite-rich mineral assemblages in the mantle source. Lithos 2017, 292–293, 81–95. [Google Scholar] [CrossRef]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of pyrophosphate and its relevance to prebiotic evolution. Nature 1991, 352, 516–519. [Google Scholar] [CrossRef]
- Milman-Barris, M.S.; Beckett, J.R.; Baker, M.B.; Hofmann, A.E.; Morgan, Z.; Crowley, M.R.; Vielzeuf, D.; Stolper, E. Zoning of phosphorus in igneous olivine. Contrib. Mineral. Petrol. 2008, 155, 739–765. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef]
- Haldorsen, S.; Akan, H.; Çelik, B.; Heun, M. The climate of the Younger Dryas as a boundary for Einkorn domestication. Veg. Hist. Archaeobotany 2011. [Google Scholar] [CrossRef]
- Stow, D. Vanished Ocean: How Tethys Reshaped the World; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef]
- Maruyama, S.; Santosh, M.; Zhao, D. Superplume, supercontinent, and post-perovskite: Mantle dynamics and anti-plate tectonics on the Core–Mantle Boundary. Gondwana Res. 2007, 11, 7–37. [Google Scholar] [CrossRef]
- Kondo, N.; Yoshino, T.; Matsukage, K.N.; Kogiso, T. Major element composition of an Early Enriched Reservoir: Constraints from 142Nd/144Nd isotope systematics in the early Earth and high-pressure melting experiments of a primitive peridotite. Prog. Earth Planet. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Bell, D.; Rossman, G.; Maldener, J.; Endisch, D.; Rauch, F. Hydroxide in olivine: A quantitative determination of the absolute amount and calibration of the IR spectrum. J. Geophys. Res. 2003, 108, 2105. [Google Scholar] [CrossRef]
- Jacobsen, S.D.; Smyth, J.R. Effect of water on the sound velocities of ringwoodite in the transition zone. Geophys Monogr. Am. Geophys. Union 2006, 168, 131. [Google Scholar]
- Binns, R.A.; Davis, R.J.; Reed, S.J.B. Ringwoodite, natural (Mg,Fe)2SiO4 spinel in the Tenham meteorite. Nature 1969, 221, 943–944. [Google Scholar] [CrossRef]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Genda, H. Origin of Earth’s oceans: An assessment of the total amount, history and supply of water. Geochem. J. 2016, 50, 27–42. [Google Scholar] [CrossRef]
- Mao, Z.; Lin, J.F.; Yang, J.; Inoue, T.; Prakapenka, V.B. Effects of the Fe3+ spin transition on the equation of state of bridgmanite. Geophys. Res. Lett. 2015, 42, 4335–4342. [Google Scholar] [CrossRef]
- Wood, B.J. Phase transformations and partitioning relations in peridotite under lower mantle conditions. Earth Planet. Sci. Lett. 2000, 174, 341–354. [Google Scholar] [CrossRef]
- Liu, X.; Sui, Z.; Fei, H.; Yan, W.; Ma, Y.; Ye, Y. IR Features of hydrous Mg2SiO4-ringwoodite, unannealed and annealed at 200–600 °C and 1 atm, with implications to hydrogen defects and water-coupled cation disorder. Minerals 2020, 10, 499. [Google Scholar] [CrossRef]
- Trail, D.; Watson, E.B.; Tailby, N.D. The oxidation state of Hadean magmas and implications for early Earth’s atmosphere. Nature 2011, 480, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Tschauner, O.; Ma, C.; Beckett, J.R.; Prescher, C.; Prakapenka, V.B.; Rossman, G.R. Mineralogy. Discovery of bridgmanite, the most abundant mineral in Earth, in a shocked meteorite. Science 2014, 346, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Hansen, V.L. Global tectonic evolution of Venus, from exogenic to endogenic over time, and implications for early Earth processes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 26. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M. Redox conditions on small bodies, the Moon and Mars. Rev. Mineral. Geochem. 2008, 68, 493–510. [Google Scholar] [CrossRef]
- Mahieux, A.; Vandaele, A.C.; Robert, S.; Wilquet, V.; Drummond, R.; Montmessin, F.; Bertaux, J.L. Densities and temperatures in the Venus mesosphere and lower thermosphere retrieved from SOIR on board Venus Express: Carbon dioxide measurements at the Venus terminator. J. Geophys. Res. 2012, 117, E07001. [Google Scholar] [CrossRef]
- Rivoldini, A.; Van Hoolst, T.; Verhoeven, O.; Mocquet, A.; Dehant, V. Geodesy constraints on the interior structure and composition of Mars. Icarus 2011, 213, 451–472. [Google Scholar] [CrossRef]
- Fei, Y.; Stagno, V. The redox boundaries of earth’s interior. Elements 2020, 16, 167–172. [Google Scholar] [CrossRef]
- Wetzel, D.T.; Rutherford, M.J.; Jacobsen, S.D.; Hauri, E.H.; Saal, A.E. Degassing of reduced carbon from planetary basalts. Proc. Natl. Acad. Sci. USA 2013, 110, 8010–8013. [Google Scholar] [CrossRef]
- Stixrude, L.; Lithgow-Bertelloni, C. Geophysics of chemical heterogeneity in the mantle. Annu. Rev. Earth Planet. Sci. 2012, 40, 569–595. [Google Scholar] [CrossRef]
- Bindi, L.S.; Shim, S.H.; Sharp, T.G.; Xie, X. Evidence for the charge disproportionation of iron in extraterrestrial bridgmanite. Sci. Adv. 2020, 6, eaay7893. [Google Scholar] [CrossRef]
- Ma, C.; Tschauner, O.; Beckett, J.R.; Liu, Y.; Rossman, G.R.; Sinogeikin, S.V.; Smith, J.S.; Taylor, L.A. Ahrensite, γ-Fe2SiO4, a new shock-metamorphic mineral from the Tissint meteorite: Implications for the Tissint shock event on Mars. Geochim. Cosmochim. Acta 2016, 184, 240–256. [Google Scholar] [CrossRef]
- Tiwari, K.; Ghosh, S.; Miyahara, M.; Ray, D. High pressure polymorphs in katol l6 chondrite: Deciphering thermal history and shock conditions. In Geophysical Research Abstracts; 2019; p. 18592. Available online: https://meetingorganizer.copernicus.org/EGU2019/EGU2019-18592-2.pdf (accessed on 15 November 2020).
- Goldschmidt, V.M. Geochemical aspects of the origin of complex organic molecules on Earth, as precursors to organic life. New Biol. 1952, 12, 97–105. [Google Scholar]
- Ismailova, L.; Bykova, E.; Bykov, M.; Cerantola, V.; McCammon, C.; Ballaran, T.B.; Bobrov, A.; Sinmyo, R.; Dubrovinskaia, N.; Glazyrin, K.; et al. Stability of Fe, Al-bearing bridgmanite in the lower mantle and synthesis of pure Fe-bridgmanite. Sci. Adv. 2016, 2, e1600427. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.R.; O’Day, P.A. Production of CO2 and H2 by diking-eruptive events at mid-ocean ridges: Implications for abiotic organic synthesis and global geochemical cycling. Int. Geol. Rev. 2000, 42, 673–683. [Google Scholar] [CrossRef]
- Wade, J.; Wood, B.J. The oxidation state and mass of the Moon-forming impactor. Earth Planet. Sci. Lett. 2016, 442, 186–193. [Google Scholar] [CrossRef]
- Frost, D.J.; Mann, U.; Asahara, Y.; Rubie, D.C. The redox state of the mantle during and just after core formation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 4315–4337. [Google Scholar] [CrossRef]
- Frost, D.J.; Liebske, C.; Langenhorst, F.; McCammon, C.A.; Trønnes, R.G.; Rubie, D.C. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature 2004, 428, 409–412. [Google Scholar] [CrossRef]
- Townsend, J.P.; Tsuchiya, J.; Bina, C.R.; Jacobsen, S.D. Water partitioning between bridgmanite and postperovskite in the lowermost mantle. Earth Planet. Sci. Lett. 2016, 454, 20–27. [Google Scholar] [CrossRef]
- Williams, H.M.; Wood, B.J.; Wade, J.; Frost, D.J.; Tuff, J. Isotopic evidence for internal oxidation of the Earth’s mantle during accretion. Earth Planet. Sci. Lett. 2012, 321, 54–63. [Google Scholar] [CrossRef]
- Herd, C.D.; Borg, L.E.; Jones, J.H.; Papike, J.J. Oxygen fugacity and geochemical variations in the Martian basalts: Implications for Martian basalt petrogenesis and the oxidation state of the upper mantle of Mars. Geochim. Cosmochim. Acta 2002, 66, 2025–2036. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Schrader, C.M.; McCoy, T.J. The primary fO2 of basalts examined by the Spirit rover in Gusev Crater, Mars: Evidence for multiple redox states in the martian interior. Earth Planet. Sci. Lett. 2013, 384, 198–208. [Google Scholar] [CrossRef]
- Shibazaki, Y.; Ohtani, E.; Terasaki, H.; Suzuki, A.; Funakoshi, K. Hydrogen partitioning between iron and ringwoodite: Implications for water transport into the Martian core. Earth Planet. Sci. Lett. 2009, 287, 463–470. [Google Scholar] [CrossRef]
- Taylor, G.J. The bulk composition of Mars. Geochemistry 2013, 73, 401–420. [Google Scholar] [CrossRef]
- Yoshizaki, T.; McDonough, W.F. The composition of Mars. Geochim. Cosmochim. Acta 2020, 273, 137–162. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. On the inevitable emergence of life on Mars. In The Search for Life on Mars; Hiscox, J.A., Ed.; The British Interplanetary Society: London, UK, 1999; pp. 26–36. [Google Scholar]
- Vaughan, D.J.; Craig, J.R. Mineral. Chemistry of Natural Sulfides; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Staude, S.; Barnes, S.J.; Markl, G. Interspinifex Ni sulfide ore from Victor South-McLeay, Kambalda, Western Australia. Miner. Depos. 2020. [Google Scholar] [CrossRef]
- Appel, P.W.U. Mineral Occurrences in the 3.6 Ga Old Isua Supracrustal Belt, West Greenland. In Developments in Precambrian Geology; Naqvi, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 8, pp. 593–603. [Google Scholar]
- Hall, A.J. Pyrite pyrrhotine redox reactions in nature. Mineral. Mag. 1986, 50, 223–229. [Google Scholar] [CrossRef]
- Appel, P.W.U. On the early Archaean Isua iron-formation, West Greenland. Precamb. Res. 1980, 11, 73–87. [Google Scholar] [CrossRef]
- Macleod, G.; McKeown, C.; Hall, A.J.; Russell, M.J. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig. Life Evol. Biosph. 1994, 24, 19–41. [Google Scholar] [CrossRef]
- Lowell, R.P.; Rona, P.A. Seafloor hydrothermal systems driven by the serpentinization of peridotite. Geophys. Res. Lett. 2002, 29, 1531. [Google Scholar] [CrossRef]
- Escartin, J.; Hirth, G.; Evans, B. Strength of slightly serpentinized peridotites: Implications for the tectonics of oceanic lithosphere. Geology 2001, 29, 1023–1026. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Turner, D. In vitro growth of iron sulphide chimneys: Possible culture chambers for origin-of-life experiments. Terra Nova 1989, 1, 238–241. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, W.; Russell, M.J. Beating the acetyl coenzyme A-pathway to the origin of life. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20120258. [Google Scholar] [CrossRef] [PubMed]
- Tutolo, B.M.; Seyfried, W.E., Jr.; Tosca, N.J. A seawater throttle on H2 production in Precambrian serpentinizing systems. Proc. Natl. Acad. Sci. USA 2020, 117, 14756–14763. [Google Scholar] [CrossRef] [PubMed]
- Williams, Q.; Hemley, R.J. Hydrogen in the deep Earth. Ann. Rev. Earth Planet. Sci. 2001, 29, 365–418. [Google Scholar] [CrossRef]
- Isaev, E.I.; Skorodumova, N.V.; Ahuja, R.; Vekilov, Y.K.; Johansson, B. Dynamical stability of Fe-H in the Earth’s mantle and core regions. Proc. Natl. Acad. Sci. USA 2007, 104, 9168–9171. [Google Scholar] [CrossRef]
- Bali, E.; Audetat, A.; Keppler, H. Water and hydrogen are immiscible in Earth’s mantle. Nature 2013, 495, 220–222. [Google Scholar] [CrossRef]
- Nédélec, A.; Monnereau, M.; Toplis, M.J. The Hadean-Archaean transition at 4 Ga: From magma trapping in the mantle to volcanic resurfacing of the Earth. Terra Nova 2017, 29, 218–223. [Google Scholar] [CrossRef]
- Russell, M.J.; Couples, G.D.; Lewis, H.; Pasava, J.; Kribek, B.; Zak, K. SEDEX genesis and super-deep boreholes: Can hydrostatic pressures exist down to the brittle-ductile boundary? In Mineral. Deposits: From Their Origin to Their Environmental Impact; Balkema, A.A., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 315–318. [Google Scholar]
- McDermott, J.M.; Seewald, J.S.; German, C.R.; Sylva, S.P. Pathways for abiotic organic synthesis at submarine hydrothermal fields. Proc. Natl. Acad. Sci. USA 2015, 112, 7668–7672. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Shen, C.-C.; Kelley, D.S.; Cheng, H.; Edwards, R.L. U–Th systematics and 230Th ages of carbonate chimneys at the Lost City Hydrothermal Field. Geochim. Cosmochim. Acta 2011, 75, 1869–1888. [Google Scholar] [CrossRef]
- Ménez, B. Abiotic hydrogen and methane: Fuels for life. Elements 2020, 16, 39–46. [Google Scholar] [CrossRef]
- Grozeva, N.G.; Klein, F.; Seewald, J.S.; Sylva, S.P. Chemical and isotopic analyses of hydrocarbon-bearing fluid inclusions in olivine-rich rocks. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20180431. [Google Scholar] [CrossRef] [PubMed]
- Etiope, G.; Lollar, S.B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Russell, M.J.; Nitschke, W. Methane: Fuel or exhaust at the emergence of life? Astrobiology 2017, 17, 1053–1066. [Google Scholar] [CrossRef]
- Kelley, D.S.; Karson, J.A.; Blackman, D.K.; Früh-Green, G.L.; Butterfield, D.A.; Lilley, M.D.; Olson, E.J.; Schrenk, M.O.; Roe, K.K.; Lebon, G.T.E.A. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature 2001, 412, 145–149. [Google Scholar] [CrossRef]
- Kelley, D.S.; Karson, J.A.; Früh-Green, G.L.; Yoerger, D.R.; Shank, T.M.; Butterfield, D.A.; Hayes, J.M.; Schrenk, M.O.; Olson, E.J.; Proskurowski, G.; et al. A serpentinite-hosted ecosystem: The lost city hydrothermal field. Science 2005, 307, 1428–1434. [Google Scholar] [CrossRef]
- Duval, S.K.; Baymann, F.; Schoepp-Cothenet, B.; Branscomb, E.; Russell, M.J.; Nitschke, W. Minerals and the emergence of life. In Metals in Life Sciences; Kroneck, P., Torres, S.M.E., Eds.; Walter de Gruyter: Berlin, Germany, 2020. [Google Scholar]
- Génin, J.-M.R.; Aïssa, R.; Géhin, A.; Abdelmoula, M.; Benali, O.; Ernstsen, V.; Ona-Nguema, G.; Upadhyay, C.; Ruby, C. Fougerite and FeII–III hydroxycarbonate green rust; ordering, deprotonation and/or cation substitution; structure of hydrotalcite-like compounds and mythic ferrosic hydroxide. Solid State Sci. 2005, 7, 545–572. [Google Scholar] [CrossRef]
- Mackay, A.L. Some aspects of the topochemistry of the iron oxides and hydroxides. In Reactivity of Solids: Proceedings of the Fourth International Symposium on the Reactivity of Solids, Amsterdam, The Netherlans, 30 May–4 June 1960; Elsevier: Amsterdam, The Netherlands, 1960; pp. 571–583. [Google Scholar]
- Bernal, J.D.; Dasgupta, D.R.; Mackay, A.L. The oxides and hydroxides of iron and their structural inter-relationships. Clay Miner. Bull. 1959, 4, 15–30. [Google Scholar] [CrossRef]
- Trolard, F.; Bourrié, G. Fougerite a natural layered double hydroxide in gley soil: Habitus, structure, and some properties. In Clay Minerals in Nature—Their Characterization, Modification and Application; InTechOpen: London, UK, 2012; pp. 171–188. [Google Scholar] [CrossRef]
- Duval, S.; Baymann, F.; Schoepp-Cothenet, B.; Trolard, F.; Bourrié, G.; Grauby, O.; Branscomb, E.; Russell, M.J.; Nitschke, W. Fougerite: The not so simple progenitor of the first cells. Interface Focus 2019, 9, 20190063. [Google Scholar] [CrossRef]
- Duval, S.; Branscomb, E.; Trolard, F.; Bourrié, G.; Grauby, O.; Heresanu, V.; Schoepp-Cothenet, B.; Zuchan, K.; Russell, M.J.; Nitschke, W. On the why’s and how’s of clay minerals’ importance in life’s emergence. Appl. Clay Sci. 2020, 195. [Google Scholar] [CrossRef]
- Arrhenius, G.O. Crystals and life. Helv. Chim. Acta 2003, 86, 1569–1586. [Google Scholar] [CrossRef]
- Mloszewska, A.M.; Pecoits, E.; Cates, N.L.; Mojzsis, S.J.; O’Neil, J.; Robbins, L.J.; Konhauser, K.O. The composition of Earth’s oldest iron formations: The Nuvvuagittuq Supracrustal Belt (Québec, Canada). Earth Planet. Sci. Lett. 2012, 317–318, 331–342. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Harrison, T.M.; Pidgeon, R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth’s surface 4300 Myr ago. Nature 2001, 409, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Asimakidou, T.; Makridis, A.; Veintemillas-Verdaguer, S.; Morales, M.P.; Kellartzis, I.; Mitrakas, M.; Vourlias, G.; Angelakeris, M.; Simeonidis, K. Continuous production of magnetic iron oxide nanocrystals by oxidative precipitation. Chem. Eng. J. 2020, 393. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Koch, C.B.; Nancke-Krogh, H.; Borggaard, O.K.; Sørensen, J. Abiotic nitrate reduction to ammonium: Key role of green rust. Environ. Sci. Technol. 1996, 30, 2053–2056. [Google Scholar] [CrossRef]
- Roldan, A.; Hollingsworth, N.; Roffey, A.; Islam, H.U.; Goodall, J.B.; Catlow, C.R.; Darr, J.A.; Bras, W.; Sankar, G.; Holt, K.B.; et al. Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. Chem. Commun. 2015, 51, 7501–7504. [Google Scholar] [CrossRef]
- Barge, L.M.; Flores, E.; Baum, M.M.; VanderVelde, D.G.; Russell, M.J. Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc. Natl. Acad. Sci. USA 2019, 116, 4828–4833. [Google Scholar] [CrossRef]
- Arrabito, G.; Pezzilli, R.; Prestopino, G.; Medaglia, P.G. Layered double hydroxides in bioinspired nanotechnology. Crystals 2020, 10, 602. [Google Scholar] [CrossRef]
- Nitschke, W.; McGlynn, S.E.; Milner-White, E.J.; Russell, M.J. On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochim. Biophys. Acta 2013, 1827, 871–881. [Google Scholar] [CrossRef]
- Muñoz-Santiburcio, D.; Marx, D. Chemistry in nanoconfined water. Chem. Sci. 2017, 8, 3444–3452. [Google Scholar] [CrossRef]
- Muñoz-Santiburcio, D.; Marx, D. Nanoconfinement in slit pores enhances water self-dissociation. Phys. Rev. Lett. 2017, 119, 056002. [Google Scholar] [CrossRef] [PubMed]
- Boesenberg, J.S.; Hewins, R.H. An experimental investigation into the metastable formation of phosphoran olivine and pyroxene. Geochim. Cosmochim. Acta 2010, 74, 1923–1941. [Google Scholar] [CrossRef]
- Finer, J.T.; Simmons, R.M.; Spudich, J.A. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature 1994, 368, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Martinez, V.; Forró, C.; Polesel-Maris, J.; Vörös, J.; Zambelli, T. Integration of silver nanowires into SU-8 hollow cantilevers for piezoresistive-based sensing. Sens. Actuators A Phys. 2020, 301. [Google Scholar] [CrossRef]
- Brockman, J.M.; Su, H.; Blanchard, A.T.; Duan, Y.; Meyer, T.; Quach, M.E.; Glazier, R.; Bazrafshan, A.; Bender, R.L.; Kellner, A.V.; et al. Live-cell super-resolved PAINT imaging of piconewton cellular traction forces. Nat. Methods 2020, 17, 1018–1024. [Google Scholar] [CrossRef]
- Mullet, M.; Khare, V.; Ruby, C. XPS study of Fe(II)-Fe(III) (oxy)hydroxycarbonate green rust compounds. Surf. Interface Anal. 2008, 40, 323–328. [Google Scholar] [CrossRef]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Role of mineral surfaces in prebiotic chemical evolution. In silico quantum mechanical studies. Life 2019, 9, 10. [Google Scholar] [CrossRef]
- Thyveetil, M.A.; Coveney, P.V.; Greenwell, H.C.; Suter, J.L. Computer simulation study of the structural stability and materials properties of DNA-intercalated layered double hydroxides. J. Am. Chem. Soc. 2008, 130, 4742–4756. [Google Scholar] [CrossRef]
- Wander, M.C.F.; Rosso, K.M.; Schoonen, M.A.A. Structure and charge hopping dynamics in green rust. J. Phys. Chem. C 2007, 111, 11414–11423. [Google Scholar] [CrossRef]
- Fracchia, M.; Visibile, A.; Ahlberg, E.; Vertova, A.; Minguzzi, A.; Ghigna, P.; Rondinini, S. α- and γ-FeOOH: Stability, reversibility, and nature of the active phase under hydrogen evolution. ACS Appl. Energy Mater. 2018, 1, 1716–1725. [Google Scholar] [CrossRef]
- Brown, A.I.; Sivak, D.A. Theory of nonequilibrium free energy transduction by molecular machines. Chem. Rev. 2020, 120, 434–459. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. The origin of life and the formation and organizing functions of natural membranes. In The Origin of Life on the Earth; Oparin, A.I., BraunshteÎN, A.E., PasynskiÎ, A.G., Pavlovskaya, T.E., Eds.; Pergamon Press: New York, NY, USA, 1959; pp. 437–443. [Google Scholar] [CrossRef]
- Hoffmann, P.M. Life’s Ratchet: How Molecular Machines Extract Order from Chaos; Basic Books: New York, NY, USA, 2012. [Google Scholar]
- Heidary, N.; Kornienko, N.; Kalathil, S.; Fang, X.; Ly, K.H.; Greer, H.F.; Reisner, E. Disparity of cytochrome utilization in anodic and cathodic extracellular electron transfer pathways of geobacter sulfurreducens biofilms. J. Am. Chem. Soc. 2020, 142, 5194–5203. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.E.; Shevchenko, E.V.; Park, J.; Lee, E.; Xie, J.; Chen, B.; Zhong, Y.; Filatov, A.S.; Anderson, J.S. Synthesis, modular composition, and electrochemical properties of lamellar iron sulfides. J. Mater. Chem. A 2020, 8, 15834–15844. [Google Scholar] [CrossRef]
- Barge, L.M.; Jones, J.-P.; Pagano, J.J.; Martinez, E.; Bescup, J. Three-dimensional analysis of a simulated prebiotic hydrothermal chimney. ACS Earth Space Chem. 2020, 4, 1663–1669. [Google Scholar] [CrossRef]
- Bourdoiseau, J.A.; Jeannin, M.; Sabot, R.; Rémazeilles, C.; Refait, P. Characterisation of mackinawite by Raman spectroscopy: Effects of crystallisation, drying and oxidation. Corros. Sci. 2008, 50, 3247–3255. [Google Scholar] [CrossRef]
- Rickard, D.; Griffith, A.; Oldroyd, A.; Butler, I.B.; Lopez-Capel, E.; Manning, D.A.C.; Apperley, D.C. The composition of nanoparticulate mackinawite, tetragonal iron(II) monosulfide. Chem. Geol. 2006, 235, 286–298. [Google Scholar] [CrossRef]
- Sano, Y.; Kyono, A.; Yoneda, Y.; Isaka, N.; Takagi, S.; Yamamoto, G.I. Structure changes of nanocrystalline mackinawite under hydrothermal conditions. J. Mineral. Petrol. Sci. 2020, 115, 261–275. [Google Scholar] [CrossRef]
- Arakaki, T.; Morse, J.W. Coprecipitation and adsorption of Mn (II) with mackinawite (FeS) under conditions similar to those found in anoxic sediments. Geochim. Cosmochim. Acta 1993, 57, 9–14. [Google Scholar] [CrossRef]
- Morse, J.W.; Arakaki, T. Adsorption and coprecipitation of divalent metals with mackinawite (FeS). Geochim. Cosmochim. Acta 1993, 57, 3635–3640. [Google Scholar] [CrossRef]
- Volbeda, A.; Fontecilla-Camps, J.C. Catalytic nickel–iron–sulfur clusters: From minerals to enzymes. In Bioorganometallic Chemistry; Berlin, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 57–82. [Google Scholar]
- Wilkin, R.T.; Beak, D.G. Uptake of nickel by synthetic mackinawite. Chem. Geol. 2017, 462, 15–29. [Google Scholar] [CrossRef]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubi, E.; Lane, N. The origin of life in alkaline hydrothermal vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, R.; Dimov, N.; Szita, N.; Jordan, S.F.; Lane, N. Possible mechanisms of CO2 reduction by H2 via prebiotic vectorial electrochemistry. Interface Focus 2019, 9, 20190073. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Hu, W.; Zhou, L.; Shi, W.; Song, S.; Lei, Y.; Wang, S.; Zhang, H. 3D Fe3S4 flower-like microspheres: High-yield synthesis via a biomolecule-assisted solution approach, their electrical, magnetic and electrochemical hydrogen storage properties. Dalton. Trans. 2009, 9246–9252. [Google Scholar] [CrossRef] [PubMed]
- Kadirvel, P.; Subramanian, A.; Sridharan, N.; Subramanian, S.; Vimaladhasan, S.; Anishetty, S. Molecular dynamics simulation study of Plasmodium falciparum and Escherichia coli SufA: Exploration of conformational changes possibly involved in iron-sulfur cluster transfer. J. Biomolec. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Santos-Carballal, D.; Roldan, A.; De Leeuw, N.H. CO2 reduction to acetic acid on the greigite Fe3S4{111} surface. Faraday Discuss. 2020. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochim. Biophys. Acta-Bioenerg. 2013, 1827, 62–78. [Google Scholar] [CrossRef]
- Pinske, C.; Sargent, F. Exploring the directionality of Escherichia coli formate hydrogenlyase: A membrane-bound enzyme capable of fixing carbon dioxide to organic acid. Microbiologyopen 2016, 5, 721–737. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G.; Hall, A.J.; Russell, M.J. Chapter 9 Mineral theories of the origin of life and an iron sulfide example. Orig. life Evol. Biosph. 1992, 22, 161–180. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Why the Submarine Alkaline Vent is the Most Reasonable Explanation for the Emergence of Life. Bioessays 2019, 41. [Google Scholar] [CrossRef]
- Piani, L.; Marrocchi, Y.; Rigaudier, T.; Vacher, L.G.; Thomassin, D.; Marty, B. Earth’s water may have been inherited from material similar to enstatite chondrite meteorites. Science 2020, 369, 1110–1113. [Google Scholar] [CrossRef]
- Jacquet, E.; Pignatale, F.C.; Chaussidon, M.; Charnoz, S. Fingerprints of the protosolar cloud collapse in the Solar System. II. Nucleosynthetic anomalies in meteorites. Astrophys. J. 2019, 884, 32. [Google Scholar] [CrossRef]
- Raymond, S.N.; Quinn, T.; Lunine, J.I. High-resolution simulations of the final assembly of Earth-like planets 2: Water delivery and planetary habitability. Astrobiology 2007, 7, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Kruijer, T.S.; Kleine, T.; Borg, L.E. The great isotopic dichotomy of the early Solar System. Nat. Astron. 2019, 4, 32–40. [Google Scholar] [CrossRef]
- Nesvorný, D. Dynamical evolution of the early Solar System. Ann. Rev. Astron. Astrophys. 2018, 56, 137–174. [Google Scholar] [CrossRef]
- Kane, S.R.; Vervoort, P.; Horner, J.; Pozuelos, F.J. Could the migration of Jupiter have accelerated the atmospheric evolution of venus? Planet. Sci. J. 2020, 1. [Google Scholar] [CrossRef]
- Bahcall, J.N.; Pinsonneault, M.H.; Basu, S. Solar models: Current epoch and time dependences, neutrinos, and helioseismological properties. Astrophys. J. 2001, 555, 990–1012. [Google Scholar] [CrossRef]
- Palubski, I.Z.; Shields, A.L.; Deitrick, R. Habitability and Water Loss Limits on Eccentric Planets Orbiting Main-sequence Stars. arXiv 2020, arXiv:2001.02228. [Google Scholar] [CrossRef]
- Luger, R.; Barnes, R. Extreme water loss and abiotic O2 buildup on planets throughout the habitable zones of M dwarfs. Astrobiology 2015, 15, 119–143. [Google Scholar] [CrossRef]
- Edwards, C.S.; Ehlmann, B.L. Carbon sequestration on Mars. Geology 2015, 43, 863–866. [Google Scholar] [CrossRef]
- Hu, R.; Kass, D.M.; Ehlmann, B.L.; Yung, Y.L. Tracing the fate of carbon and the atmospheric evolution of Mars. Nat. Commun. 2015, 6, 10003. [Google Scholar] [CrossRef]
- Wong, M.L.; Friedson, A.J.; Willacy, K.; Shia, R.L.; Yung, Y.L.; Russell, M.J. A methane-rich early Mars: Implications for habitability and the emergence of life. In Proceedings of the Habitable Worlds 2017: A System Science Workshop, Laramie, Wyoming, 13–17 November 2017. LPI Contrib. No. 1965. [Google Scholar]
- Putzig, N.E.; Smith, I.B.; Perry, M.R.; Foss, F.J., 2nd; Campbell, B.A.; Phillips, R.J.; Seu, R. Three-dimensional radar imaging of structures and craters in the Martian polar caps. Icarus 2018, 308, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Bultel, B.; Viennet, J.C.; Poulet, F.; Carter, J.; Werner, S.C. Detection of carbonates in martian weathering profiles. J. Geophys. Res. Planets 2019, 124, 989–1007. [Google Scholar] [CrossRef]
- Heard, A.W.; Kite, E.S. A probabilistic case for a large missing carbon sink on Mars after 3.5 billion years ago. Earth Planet. Sci. Lett. 2020, 531. [Google Scholar] [CrossRef]
- Urata, R.A.; Toon, O.B. Simulations of the martian hydrologic cycle with a general circulation model: Implications for the ancient martian climate. Icarus 2013, 226, 229–250. [Google Scholar] [CrossRef]
- Khan, A.; Liebske, C.; Rozel, A.; Rivoldini, A.; Nimmo, F.; Connolly, J.A.D.; Plesa, A.C.; Giardini, D. A geophysical perspective on the bulk composition of mars. J. Geophys. Res. Planets 2018, 123, 575–611. [Google Scholar] [CrossRef]
- O’Rourke, J.G.; Shim, S.H. Hydrogenation of the martian core by hydrated mantle minerals with implications for the early dynamo. J. Geophys. Res. Planets 2019, 124, 3422–3441. [Google Scholar] [CrossRef]
- Plesa, A.C.; Padovan, S.; Tosi, N.; Breuer, D.; Grott, M.; Wieczorek, M.A.; Spohn, T.; Smrekar, S.E.; Banerdt, W.B. The thermal state and interior structure of Mars. Geophys. Res. Lett. 2018, 45, 12198–112209. [Google Scholar] [CrossRef]
- Hooks, M.R.; Webster, P.; Weber, J.M.; Perl, S.; Barge, L.M. Effects of amino acids on iron-silicate chemical garden precipitation. Langmuir 2020, 36, 5793–5801. [Google Scholar] [CrossRef]
- Mayen, L.; Jensen, N.D.; Desbord, M.; Laurencin, D.; Gervais, C.; Bonhomme, C.; Smith, M.E.; Porcher, F.; Elkaim, E.; Charvillat, C.; et al. Advances in the synthesis and structure of α-canaphite: A multitool and multiscale study. CrystEngComm 2020, 22, 3130–3143. [Google Scholar] [CrossRef]
- Wang, Q.; Barge, L.M.; Steinbock, O. Microfluidic production of pyrophosphate catalyzed by mineral membranes with steep pH gradients. Chemistry 2019, 25, 4732–4739. [Google Scholar] [CrossRef]
- Zeng, S.-L.; Wang, H.-X.; Dong, C. Synthesis and electrical conductivity of nanocrystalline tetragonal FeS. Chin. Phys. B 2014, 23. [Google Scholar] [CrossRef]
- Li, Y.; Kitadai, N.; Nakamura, R. Chemical diversity of metal sulfide minerals and its implications for the origin of life. Life 2018, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J. Life is a verb, not a noun. Geology 2017, 45, 1143–1144. [Google Scholar] [CrossRef]
- Barge, L.M.; Flores, E.; VanderVelde, D.G.; Weber, J.M.; Baum, M.M.; Castonguay, A. Effects of Geochemical and Environmental Parameters on Abiotic Organic Chemistry Driven by Iron Hydroxide Minerals. J. Geophys. Res. Planets 2020, 125. [Google Scholar] [CrossRef]
- Russell, M.J.; Daia, D.E.; Hall, A.J. The emergence of life from FeS bubbles at alkaline hot springs in an acid ocean. In Thermophiles: The Keys to Molecular Evolution and the Origin of Life? Adams, M.W.W., Ljungdahl, L.G., Wiegel, J., Eds.; Taylor and Francis: London, UK; Washington, DC, USA, 1998; pp. 77–126. [Google Scholar]
- Preiner, M.; Igarashi, K.; Muchowska, K.B.; Yu, M.; Varma, S.J.; Kleinermanns, K.; Nobu, M.K.; Kamagata, Y.; Tuysuz, H.; Moran, J.; et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 2020, 4, 534–542. [Google Scholar] [CrossRef]
- Martin, W.F. Older than genes: The acetyl CoA pathway and origins. Front. Microbiol. 2020, 11, 817. [Google Scholar] [CrossRef]
- Endres, R.G. Entropy production selects nonequilibrium states in multistable systems. Sci. Rep. 2017, 7, 14437. [Google Scholar] [CrossRef]
- Cartwright, J.H.E.; Russell, M.J. The origin of life: The submarine alkaline vent theory at 30. Interface Focus 2019, 9. [Google Scholar] [CrossRef]
- Cook, J.; Endres, R.G. Thermodynamics of switching in multistable non-equilibrium systems. J. Chem. Phys. 2020, 152. [Google Scholar] [CrossRef]
- Lane, N. Why are cells powered by proton gradients? Nat. Educ. 2010, 3, 18. [Google Scholar]
- Preiner, M.; Xavier, J.C.; Sousa, F.L.; Zimorski, V.; Neubeck, A.; Lang, S.Q.; Greenwell, H.C.; Kleinermanns, K.; Tuysuz, H.; McCollom, T.M.; et al. Serpentinization: Connecting geochemistry, ancient metabolism and industrial hydrogenation. Life 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.; Stanger, G. Hydrogen generation from mantle source rocks in Oman. Earth Planet. Sci. Lett. 1983, 66, 315–320. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. From geochemistry to biochemistry: Chemiosmotic coupling and transition element clusters in the onset of life and photosynthesis. Geochem. News 2002, 113, 6–12. [Google Scholar]
- Refait, P.; Drissi, H.; Marie, Y.; Genin, J.M.R. The substitution of Fe2+ ions by Ni2+ ions in green rust one compounds. Hyperfine Interact. 1994, 90, 389–394. [Google Scholar] [CrossRef]
- Shock, E.L.; Sassani, D.C.; Willis, M.; Sverjensky, D.A. Inorganic species in geologic fluids: Correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim. Cosmochim. Acta 1997, 61, 907–950. [Google Scholar] [CrossRef]
- Lin, S.; Popp, R.K. Solubility and complexing of Ni in the system NiO-H2O-HCl. Geochim. Cosmochim. Acta 1984, 48, 2713–2722. [Google Scholar] [CrossRef]
- Mitchell, P. Metabolism, transport, and morphogenesis: Which drives which? J. Gen. Microbiol. 1962, 29, 25–37. [Google Scholar] [CrossRef]
- Milner-White, E.J. Protein three-dimensional structures at the origin of life. Interface Focus 2019, 9, 20190057. [Google Scholar] [CrossRef]
- Szilagyi, R.K.; Hanscam, R.; Shepard, E.M.; McGlynn, S.E. Natural selection based on coordination chemistry: Computational assessment of [4Fe-4S]-maquettes with non-coded amino acids. Interface Focus 2019, 9, 20190071. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. Melting in the Earth’s deep upper mantle caused by carbon dioxide. Nature 2006, 440, 659–662. [Google Scholar] [CrossRef]
- Yung, Y.L.; McElroy, M.B. Fixation of nitrogen in the prebiotic atmosphere. Science 1979, 203, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Ducluzeau, A.-L.; van Lis, R.; Duval, S.; Schoepp-Cothenet, B.; Russell, M.J.; Nitschke, W. Was nitric oxide the first deep electron sink? Trends Biochem. Sci. 2009, 34, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, A.; Mousis, O.; Waite, J.H.; Picaud, S. Possible evidence for a methane source in Enceladus’ ocean. Geophys. Res. Lett. 2015, 42, 1334–1339. [Google Scholar] [CrossRef]
- Dorofeeva, V.A. Genesis of volatile components at Saturn’s regular satellites. Origin of Titan’s atmosphere. Geochem. Intern. 2016, 54, 7–26. [Google Scholar] [CrossRef]
- Girard, J.A.; Amulele, G.; Farla, R.; Mohiuddin, A.; Karato, S.I. Shear deformation of bridgmanite and magnesiowüstite aggregates at lower mantle conditions. Science 2016, 351, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Li, M.; McCammon, C.; Lee, K.K.M. Redox-induced lower mantle density contrast and effect on mantle structure and primitive oxygen. Nat. Geosci. 2016, 9, 723–727. [Google Scholar] [CrossRef]
- Righter, K.; Herd, C.D.K.; Boujibar, A. Redox Processes in early earth accretion and in terrestrial bodies. Elements 2020, 16, 161–166. [Google Scholar] [CrossRef]
- Zolotov, M.; Kargel, J. On the chemical composition of europa’s icy shell, ocean, and underlying rocks. Geology 2009. [Google Scholar] [CrossRef]
- Wadhwa, M. Redox state of Mars’ upper mantle and crust from Eu anomalies in shergottite pyroxenes. Science 2001, 291, 1527–1530. [Google Scholar] [CrossRef]
- Herd, C.D. The oxygen fugacity of olivine-phyric Martian basalts and the components within the mantle and crust of Mars. Meteor. Planet. Sci. 2003, 38, 1793–1805. [Google Scholar] [CrossRef]
- Wordsworth, R.; Kalugina, Y.; Lokshtanov, S.; Vigasin, A.; Ehlmann, B.; Head, J.; Sanders, C.; Wang, H. Transient reducing greenhouse warming on early Mars. Geophys. Res. Lett. 2017, 44, 665–671. [Google Scholar] [CrossRef]
- Deng, Z.; Moynier, F.; Villeneuve, J.; Jensen, N.K.; Liu, D.; Cartigny, P.; Mikouchi, T.; Siebert, J.; Agranier, A.; Chaussidon, M.; et al. Early oxidation of the martian crust triggered by impacts. Sci. Adv. 2020, 6, eabc4941. [Google Scholar] [CrossRef] [PubMed]
- Ootsubo, T.; Kawakita, H.; Hamada, S.; Kobayashi, H.; Yamaguchi, M.; Usui, F.; Nakagawa, T.; Ueno, M.; Ishiguro, M.; Sekiguchi, T.; et al. Akari near-Infrared Spectroscopic Survey for CO2 in 18 Comets. Astrophys. J. 2012, 752. [Google Scholar] [CrossRef]
- Hand, K.P.; Sotin, C.; Hayes, A.; Coustenis, A. On the habitability and future exploration of ocean worlds. Space Sci. Rev. 2020, 216. [Google Scholar] [CrossRef]
- Russell, M.J.; Murray, A.E.; Hand, K.P. The possible emergence of life and differentiation of a shallow biosphere on irradiated icy worlds: The example of Europa. Astrobiology 2017, 17, 1265–1273. [Google Scholar] [CrossRef]
- Vance, S.D.; Hand, K.P.; Pappalardo, R.T. Geophysical controls of chemical disequilibria in Europa. Geophys. Res. Lett. 2016, 43, 4871–4879. [Google Scholar] [CrossRef]
- Nealson, K.H. The limits of life on Earth and searching for life on Mars. J. Geophys. Res. Planets 1997, 102, 23675–23686. [Google Scholar] [CrossRef]
- Rogers, L.A. Most 1.6 earth-radius planets are not rocky. Astrophys. J. 2015, 801. [Google Scholar] [CrossRef]
- Cowan, N.B.; Abbot, D.S. Water cycling between ocean and mantle: Super-earths need not be waterworlds. Astrophys. J. 2014, 781. [Google Scholar] [CrossRef]
- Narita, N.; Enomoto, T.; Masaoka, S.; Kusakabe, N. Titania may produce abiotic oxygen atmospheres on habitable exoplanets. Sci. Rep. 2015, 5, 13977. [Google Scholar] [CrossRef]
- Noack, L.; Höning, D.; Rivoldini, A.; Heistracher, C.; Zimov, N.; Journaux, B.; Lammer, H.; Van Hoolst, T.; Bredehöft, J.H. Water-rich planets: How habitable is a water layer deeper than on Earth? Icarus 2016, 277, 215–236. [Google Scholar] [CrossRef]
- Meadows, V.S. Reflections on O2 as a biosignature in exoplanetary atmospheres. Astrobiology 2017, 17, 1022–1052. [Google Scholar] [CrossRef]
- Ponce, A. Radionuclide-induced defect sites in iron-bearing minerals may have accelerated the emergence of life. Interface Focus 2019, 9, 20190085. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.L.; Jansen, M.; Abbot, D.S. Oceanographic considerations for exoplanet life detection. Astrophys. J. 2020, 895. [Google Scholar] [CrossRef]

| Mineral | Contribution and Consequence | References |
|---|---|---|
| Olivine | Upper mantle/crust: precursor to bridgmanite & serpentine | [74,75,76,77,79,80] |
| Bridgmanite | Lower mantle mineral produced by metamorphism of Fe2+/Mg-silicate so forcing disproportionation of the Fe2+ as bridgmanite purloins Fe3+, effectively oxidizing the lower mantle as the orphaned Fe0 gravitates to the core | [92,95,103,105,107] |
| Pyrrhotite | Source of bisulfide (HS−) in the alkaline hydrothermal solutions | [3,39,46,120,121,123,124,125,192] |
| Serpentine | Hydration of olivine generates highly alkaline submarine springs with pH contrast with Hadean ocean of ~6 pH units | [3,4,38,39,55,126,127,128,129,130,131,136,137,142,143,144] |
| Fougerite | Dominant precipitate at vent, sufficiently complex as a membrane to have acted as embryonic life’s first disequilibria converter (as a general reductase, aminase, and possibly a polymerase and pyrophosphatase), H2 generator and proton transfer wire | [41,43,59,60,73,146,147,150,151,152,155,156,159,167,193] |
| Mackinawite | Subsidiary mineral acting as hydrogenase and electron wire | [42,56,61,160,177,178,179,180,181,182,184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, M.J.; Ponce, A. Six ‘Must-Have’ Minerals for Life’s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite. Life 2020, 10, 291. https://doi.org/10.3390/life10110291
Russell MJ, Ponce A. Six ‘Must-Have’ Minerals for Life’s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite. Life. 2020; 10(11):291. https://doi.org/10.3390/life10110291
Chicago/Turabian StyleRussell, Michael J., and Adrian Ponce. 2020. "Six ‘Must-Have’ Minerals for Life’s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite" Life 10, no. 11: 291. https://doi.org/10.3390/life10110291
APA StyleRussell, M. J., & Ponce, A. (2020). Six ‘Must-Have’ Minerals for Life’s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite. Life, 10(11), 291. https://doi.org/10.3390/life10110291







