The Effectiveness and Retention Rate of Iguratimod in Japanese Rheumatoid Arthritis Patients with/without Methotrexate in Daily Medical Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Compliance with Ethical Standards
2.3. Demographic Characteristics and the Assessment of Clinical and Laboratory Data
2.4. Assessments of Clinical and Laboratory Data
2.5. Definitions of Disease Activity
2.6. Retention Rate
2.7. Safety and Reasons for Discontinuation
2.8. Statistical Analyses of Patients
3. Results
3.1. Patient Characteristics
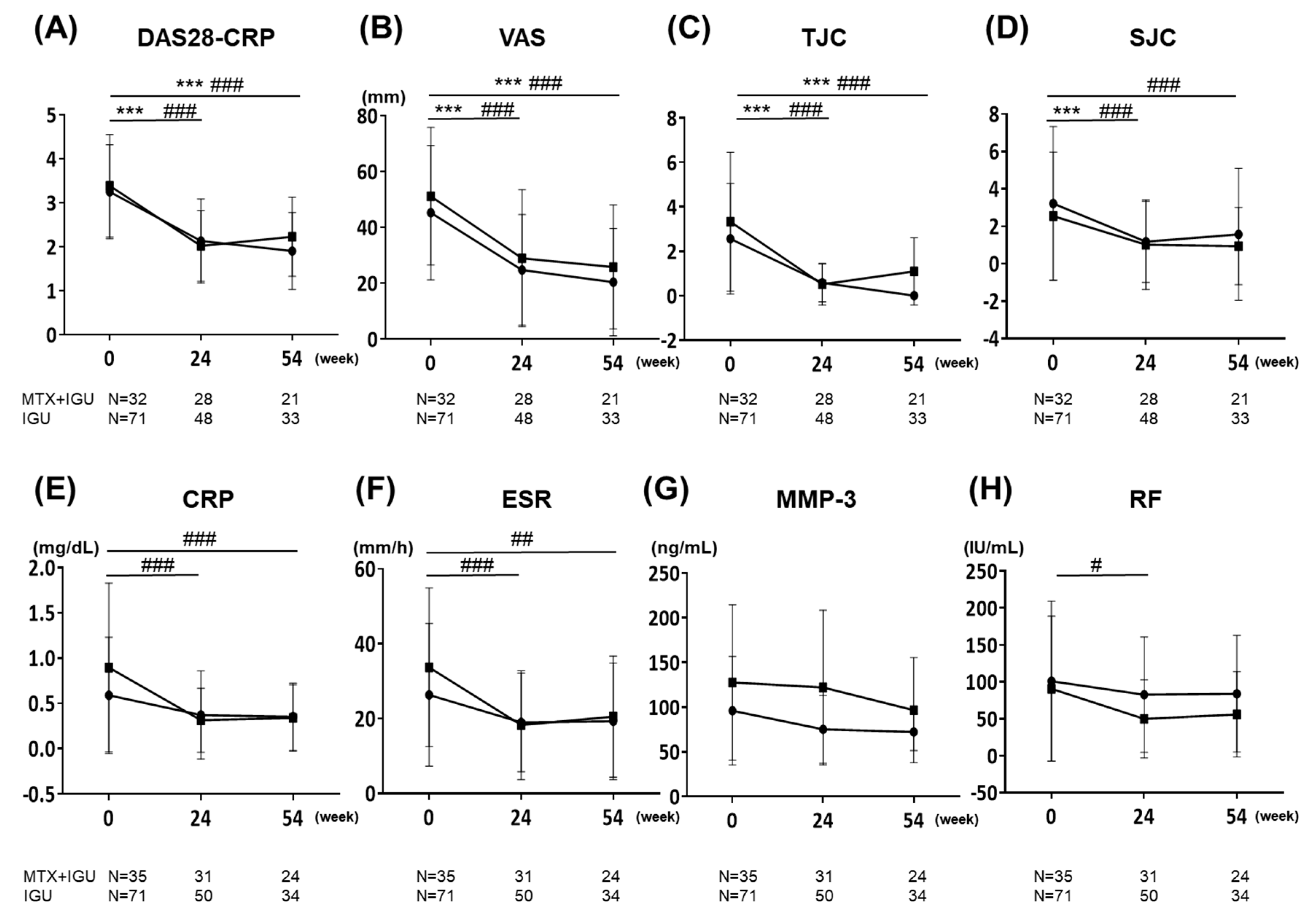
3.2. Clinical Effectiveness
3.3. MTX Treatment
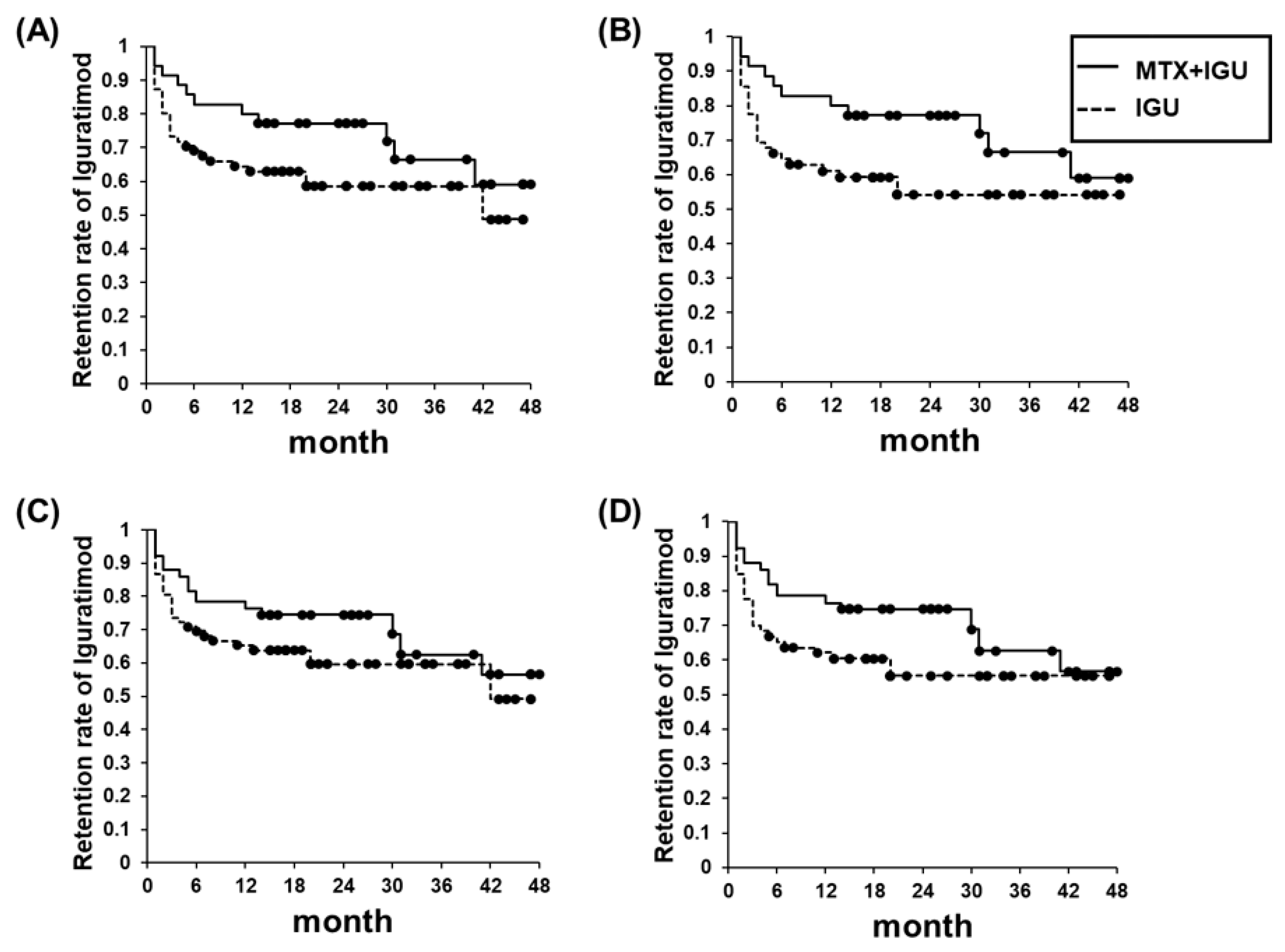
3.4. Retention Rate
3.5. Reasons for Discontinuation; Safety Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coury, F.F.; Weinblatt, M.E. Clinical trials to establish methotrexate as a therapy for rheumatoid arthritis. Clin. Exp. Rheumatol. 2010, 28, 9–12. [Google Scholar]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.W.J.; Burmester, G.R.; Chatzidionysiou, K.; Dougados, M.; Nam, J.L.; Ramiro, S.; Voshaar, M.; Van Vollenhoven, R.F.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhoven, R.F.; Geborek, P.; Forslind, K.; Albertsson, K.; Ernestam, S.; Petersson, I.F.; Chatzidionysiou, K.; Bratt, J.; Swefot Study Group. Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 2012, 379, 1712–1720. [Google Scholar] [CrossRef]
- Kawakami, A.; Tsuboi, M.; Urayama, S.; Matsuoka, N.; Yamasaki, S.; Hida, A.; Aoyagi, T.; Furuichi, I.; Nakashima, T.; Migita, K.; et al. Inhibitory effect of a new anti-rheumatic drug T-614 on costimulatory molecule expression, cytokine production, and antigen presentation by synovial cells. J. Lab. Clin. Med. 1999, 133, 566–574. [Google Scholar] [CrossRef]
- Du, F.; Lü, L.J.; Fu, Q.; Dai, M.; Teng, J.-L.; Fan, W.; Chen, S.-L.; Ye, P.; Shen, N.; Huang, X.-F.; et al. T-614, a novel immunomodulator, attenuates joint inflammation and articular damage in collagen-induced arthritis. Arthritis Res. Ther. 2008, 10, R136. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Aikawa, Y.; Tsubouchi, Y.; Hashiramoto, A.; Yamada, R.; Kawahito, Y.; Inoue, K.I.; Kusaka, Y.; Kondo, M.; Sano, H. Inhibitory effect of T-614 on tumor necrosis factor-α induced cytokine production and nuclear factor-κB activation in cultured human synovial cells. J. Rheumatol. 2001, 28, 2591–2596. [Google Scholar] [PubMed]
- Luo, Q.; Sun, Y.; Liu, W.; Qian, C.; Jin, B.; Tao, F.; Gu, Y.; Wu, X.; Shen, Y.; Xu, Q. A Novel Disease-Modifying Antirheumatic Drug, Iguratimod, Ameliorates Murine Arthritis by Blocking IL-17 Signaling, Distinct from Methotrexate and Leflunomide. J. Immunol. 2013, 191, 4969–4978. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamamoto, T.; Aikawa, Y.; Kizawa, K.; Muramoto, K.; Matsuno, H.; Muraguchi, A. Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatology 2003, 42, 1365–1371. [Google Scholar] [CrossRef]
- Aikawa, Y.; Yamamoto, M.; Yamamoto, T.; Morimoto, K.; Tanaka, K. An anti-rheumatic agent T-614 inhibits NF-κB activation in LPS- and TNF-α-stimulated THP-1 cells without interfering with IκBα degradation. Inflamm. Res. 2002, 51, 188–194. [Google Scholar] [CrossRef]
- Hara, M.; Abe, T.; Sugawara, S.; Mizushima, Y.; Hoshi, K.; Irimajiri, S.; Hashimoto, H.; Yoshino, S.; Matsui, N.; Nobunaga, M.; et al. Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: A controlled, multicenter, double-blind, parallel-group study. Mod. Rheumatol. 2007, 17, 1–9. [Google Scholar] [CrossRef]
- Nozaki, Y.; Inoue, A.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Efficacy of iguratimod vs. salazosulfapyridine as the first-line csDMARD for rheumatoid arthritis. Mod. Rheumatol. 2020, 30, 249–258. [Google Scholar] [CrossRef]
- Hara, M.; Ishiguro, N.; Katayama, K.; Kondo, M.; Sumida, T.; Mimori, T.; Soen, S.; Nagai, K.; Yamaguchi, T.; Yamamoto, K.; et al. Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: An open-label extension of a randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 2014, 24, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, N.; Yamamoto, K.; Katayama, K.; Kondo, M.; Sumida, T.; Mimori, T.; Soen, S.; Nagai, K.; Yamaguchi, T.; Hara, M.; et al. Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: A randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 2013, 23, 430–439. [Google Scholar] [CrossRef]
- Hutchinson, D. Classification criteria: The 1987 American Rheumatism Association revised criteria for the classification of rheumatoid arthritis. CPD Rheumatol. 1999, 1, 13–14. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Inoue, E.; Yamanaka, H.; Hara, M.; Tomatsu, T.; Kamatani, N. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann. Rheum. Dis. 2007, 66, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Robins, M.; James, M.A.H. Causal Inference—What if. Found. Agnostic Stat. 2020, 235–281. [Google Scholar] [CrossRef]
- Xia, Z.; Lyu, J.; Hou, N.; Song, L.; Li, X.; Liu, H. Iguratimod in combination with methotrexate in active rheumatoid arthritis: Therapeutic effects. Zeitschrift für Rheumatologie 2016, 75, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-W.; Zhang, X.-L.; Mao, S.-Y.; Shang, J.-J.; Shi, X.-D. Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: A randomized controlled trial. Clin. Rheumatol. 2015, 34, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Yonemoto, Y.; Suto, T.; Okura, C.; Takagishi, K. Efficacy at 52 weeks of daily clinical use of iguratimod in patients with rheumatoid arthritis. Mod. Rheumatol. 2015, 25, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Ishikawa, J. Iguratimod, a synthetic disease modifying anti-rheumatic drug inhibiting the activation of NF-κB and production of RANKL: Its efficacy, radiographic changes, safety and predictors over two years’ treatment for Japanese rheumatoid arthritis patients. Mod. Rheumatol. 2019, 29, 418–429. [Google Scholar] [CrossRef]
- Suto, T.; Yonemoto, Y.; Okamura, K.; Sakane, H.; Takeuchi, K.; Tamura, Y.; Kaneko, T.; Ayabe, K.; Chikuda, H. The three-year efficacy of iguratimod in clinical daily practice in patients with rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 775–781. [Google Scholar] [CrossRef]
- Mimori, T.; Harigai, M.; Atsumi, T.; Fujii, T.; Kuwana, M.; Matsuno, H.; Momohara, S.; Takei, S.; Tamura, N.; Takasaki, Y.; et al. Safety and effectiveness of iguratimod in patients with rheumatoid arthritis: Final report of a 52-week, multicenter postmarketing surveillance study. Mod. Rheumatol. 2019, 29, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Mimori, T.; Harigai, M.; Atsumi, T.; Fujii, T.; Kuwana, M.; Matsuno, H.; Momohara, S.; Takei, S.; Tamura, N.; Takasaki, Y.; et al. Safety and effectiveness of 24-week treatment with iguratimod, a new oral disease-modifying antirheumatic drug, for patients with rheumatoid arthritis: Interim analysis of a post-marketing surveillance study of 2679 patients in Japan. Mod. Rheumatol. 2017, 27, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Abe, T.; Sugawara, S.; Mizushima, Y.; Hoshi, K.; Irimajiri, S.; Hashimoto, H.; Yoshino, S.; Matsui, N.; Nobunaga, M. Long-term safety study of iguratimod in patients with rheumatoid arthritis. Mod. Rheumatol. 2007, 17, 10–16. [Google Scholar] [CrossRef] [PubMed]

| MTX+IGU Group n = 35 | IGU Group n = 71 | p-Value | |
|---|---|---|---|
| Age | 56.9 ± 16.5 | 64.8 ± 13.5 | 0.02 * |
| Disease duration, months | 65.4 ± 70.6 | 58.9 ± 69.8 | 0.30 |
| Dose of MTX, mg/week | 7.8 ± 3.2 | - | - |
| PSL use at baseline, % | 14.3 | 22.5 | 0.32 |
| SASP use at baseline, % | 45.7 | 32.4 | 0.18 |
| BUC use at baseline, % | 5.7 | 7 | 0.80 |
| TAC use at baseline, % | 0 | 8.5 | 0.17 |
| DAS28-CRP | 3.3 ± 1.1 | 3.4 ± 1.2 | 0.62 |
| Pt VAS, 0–100 mm | 45.2 ± 24.0 | 51.1 ± 24.6 | 0.24 |
| TJC | 2.6 ± 2.5 | 3.3 ± 3.1 | 0.21 |
| SJC | 2.9 ± 3.7 | 1.8 ± 1.9 | 0.80 |
| CRP, mg/dL | 0.6 ± 0.6 | 0.8 ± 0.8 | 0.21 |
| ESR, mm/h | 26.4 ± 19.1 | 33.7 ± 21.2 | 0.10 |
| MMP-3, ng/mL | 96.0 ± 60.6 | 127.6 ± 86.9 | 0.15 |
| RF positive, %, titer, IU/mL | 68.6, 100.9 ± 108.3 | 77.5, 90.7 ± 98.1 | 0.32, 0.85 |
| ACPA positive, %, titer, U/mL | 71.4, 176.6 ± 178.6 | 74.6, 91.5 ± 86.8 | 0.13, 0.17 |
| Difference in the Changes from Baseline to 24 Weeks (95% CI) | p-Value | Difference in the Changes from Baseline to 54 Weeks (95% CI) | p-Value | |
|---|---|---|---|---|
| (a) no Age Adjustment | ||||
| DAS28-CRP | 0.2 (−0.4–0.7) | 0.58 | −0.2 (−0.9–0.5) | 0.52 |
| Pt VAS, 0–100 mm | 5.8 (−8.2–19.9) | 0.41 | 1.0 (−13.0–15.0) | 0.88 |
| TJC | 0.4 (−1.1–1.9) | 0.58 | 0.4 (−1.4–2.1) | 0.67 |
| SJC | 0.0 (−1.6–1.6) | 0.97 | 0.9 (−1.3–3.0) | 0.41 |
| CRP, mg/dL | 0.0 (−1.0–0.9) | 0.96 | −0.3 (−1.2–0.7) | 0.59 |
| ESR, mm/h | 2.7 (−4.5–9.9) | 0.45 | −0.3 (−8.7–8.0) | 0.94 |
| MMP-3, ng/mL | −73.7 (−261.0–113.5) | 0.42 | −103.1 (−310.0–103.7) | 0.52 |
| RF, IU/mL | 22.6 (−26.9–72.1) | 0.36 | −0.2 (−0.9–0.5) | 0.88 |
| (b) with Age Adjustment | ||||
| DAS28-CRP | 0.1 (−0.4–0.7) | 0.60 | 0.3 (−1.0–0.32) | 0.31 |
| Pt VAS, 0–100 mm | 6.3 (−6.8–19.5) | 0.34 | −0.1 (−13.9–13.6) | 0.98 |
| TJC | 0.4 (−1.0–1.8) | 0.59 | 0.2 (−1.5–1.9) | 0.78 |
| SJC | −0.2 (−1.8–1.3) | 0.76 | 0.5 (−1.6–2.5) | 0.64 |
| CRP, mg/dL | −0.2 (−1.2–0.8) | 0.68 | −0.5 (−1.5–0.5) | 0.34 |
| ESR, mm/h | 0.7 (−6.7–8.0) | 0.86 | −2.7 (−11.2–5.9) | 0.53 |
| MMP-3, ng/mL | −50.0 (−243.4–143.4) | 0.60 | −78.1 (−288.4–132.2) | 0.45 |
| RF, IU/mL | 15.6 (−33.3–64.5) | 0.52 | 42.0 (−45.3–129.4) | 0.34 |
| Reasons for IGU Discontinuation | MTX+IGU Group n = 35 | IGU Group n = 71 | p-Value |
|---|---|---|---|
| SAE | 6 | 20 | 0.21 |
| Liver dysfunction | 0 | 12 | 0.01 * |
| Eruption | 2 | 2 | 0.46 |
| Gastritis | 0 | 1 | 0.48 |
| Nausea | 1 | 0 | 0.15 |
| Diarrhea | 0 | 1 | 0.48 |
| Paresthesia | 1 | 1 | 0.61 |
| Pneumonia | 0 | 1 | 0.48 |
| Hypoglobulinemia | 1 | 0 | 0.15 |
| Thrombocytopenia | 0 | 1 | 0.48 |
| Lymphadenopathy | 1 | 1 | 0.61 |
| Others | 4 | 9 | - |
| No improvement | 3 | 6 | 0.98 |
| Improvement | 1 | 1 | 0.61 |
| Own interruption | 0 | 1 | 0.48 |
| Death (due to other underlying diseases) | 0 | 1 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, A.; Nozaki, Y.; Hirooka, Y.; Kinoshita, K.; Chiba, Y.; Funauchi, M.; Matsumura, I. The Effectiveness and Retention Rate of Iguratimod in Japanese Rheumatoid Arthritis Patients with/without Methotrexate in Daily Medical Care. Life 2020, 10, 261. https://doi.org/10.3390/life10110261
Inoue A, Nozaki Y, Hirooka Y, Kinoshita K, Chiba Y, Funauchi M, Matsumura I. The Effectiveness and Retention Rate of Iguratimod in Japanese Rheumatoid Arthritis Patients with/without Methotrexate in Daily Medical Care. Life. 2020; 10(11):261. https://doi.org/10.3390/life10110261
Chicago/Turabian StyleInoue, Asuka, Yuji Nozaki, Yasuaki Hirooka, Koji Kinoshita, Yasutaka Chiba, Masanori Funauchi, and Itaru Matsumura. 2020. "The Effectiveness and Retention Rate of Iguratimod in Japanese Rheumatoid Arthritis Patients with/without Methotrexate in Daily Medical Care" Life 10, no. 11: 261. https://doi.org/10.3390/life10110261
APA StyleInoue, A., Nozaki, Y., Hirooka, Y., Kinoshita, K., Chiba, Y., Funauchi, M., & Matsumura, I. (2020). The Effectiveness and Retention Rate of Iguratimod in Japanese Rheumatoid Arthritis Patients with/without Methotrexate in Daily Medical Care. Life, 10(11), 261. https://doi.org/10.3390/life10110261





