Ephrin Receptors (Eph): EphA1, EphA5, and EphA7 Expression in Uveal Melanoma—Associations with Clinical Parameters and Patient Survival
Abstract
1. Introduction
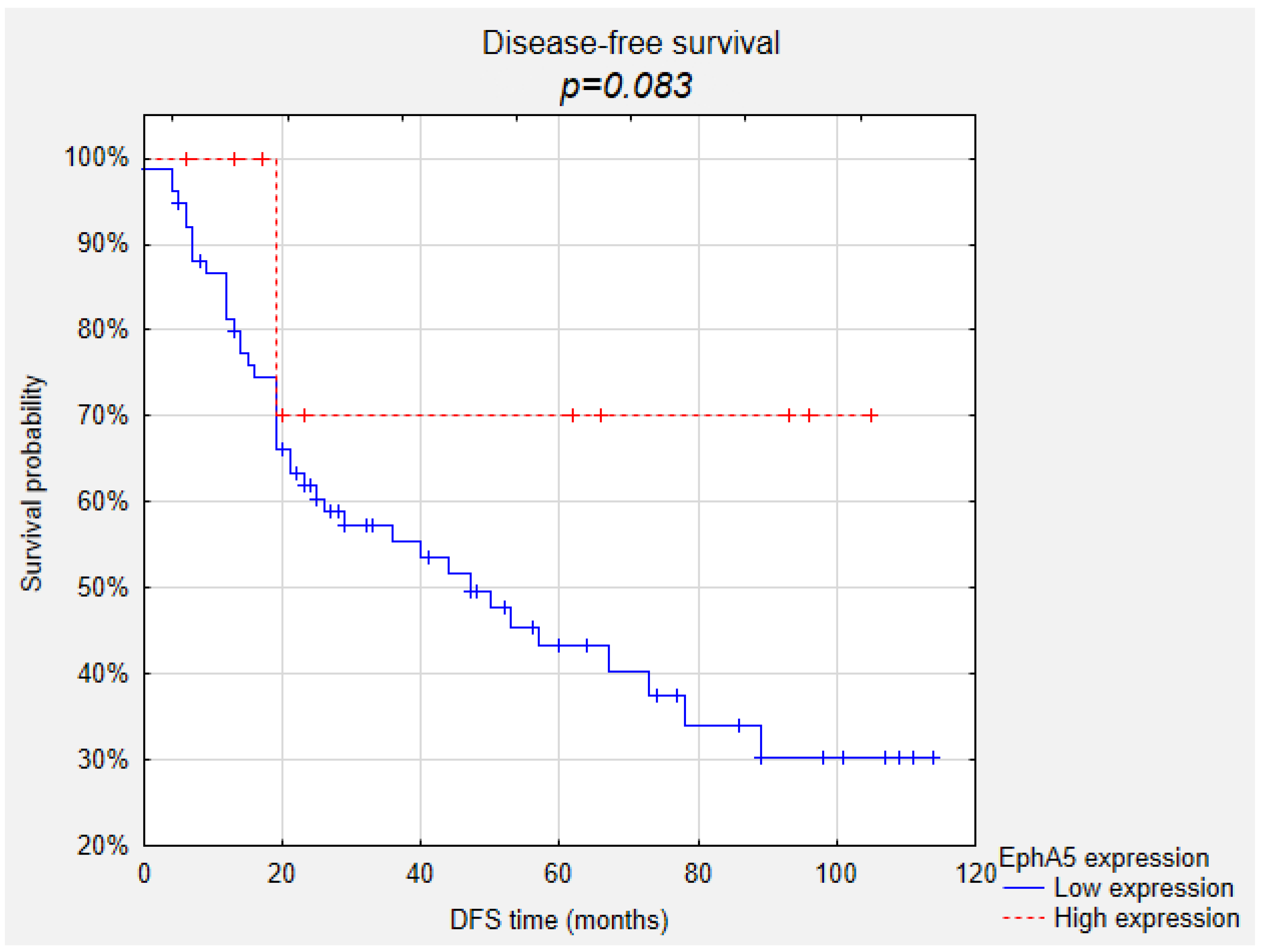
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Statistical analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.-M.; Paci, E. Incidence of Uveal Melanoma in Europe. Ophthalmology 2007, 114, 2309–2315.e2. [Google Scholar] [CrossRef] [PubMed]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kivela, T.; Simpson, E.R.; Grossniklaus, H.E.; Jager, M.J.; Singh, A.D.; Caminal, J.M.; Pavlick, A.C.; Kujala, E.; Coupland, S.E.; Finger, P. Uveal melanoma. In AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2017; pp. 805–817. [Google Scholar]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef]
- Himanen, J.-P.; Saha, N.; Nikolov, D.B. Cell–cell signaling via Eph receptors and ephrins. Curr. Opin. Cell Biol. 2007, 19, 534–542. [Google Scholar] [CrossRef]
- Surawska, H.; Ma, P.C.; Salgia, R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004, 15, 419–433. [Google Scholar] [CrossRef]
- Nievergall, E.; Lackmann, M.; Janes, P.W. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell. Mol. Life Sci. 2012, 69, 1813–1842. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef]
- Tognolini, M.; Hassan-Mohamed, I.; Giorgio, C.; Zanotti, I.; Lodola, A. Therapeutic perspectives of Eph–ephrin system modulation. Drug Discov. Today. 2014, 19, 661–669. [Google Scholar] [CrossRef]
- Brantley, D.M.; Cheng, N.; Thompson, E.J.; Lin, Q.; Brekken, R.A.; E Thorpe, P.; Muraoka, R.S.; Cerretti, D.P.; Pozzi, A.; Jackson, D.; et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene 2002, 21, 7011–7026. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, S.; Klijanienko, J.; Giaginis, C.; Alexandrou, P.; Patsouris, E.; Sastre-Garau, X. Ephrin receptor (Eph) -A1, -A2, -A4 and -A7 expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients survival. Pathol Oncol Res. 2014, 20, 277–284. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsourouflis, G.; Zizi-Serbetzoglou, A.; Kouraklis, G.; Chatzopoulou, E.; Dimakopoulou, K.; Theocharis, S. Clinical significance of Ephrin (Eph)-A1, -A2, -A4, -A5 and -A7 receptors in pancreatic ductal adenocarcinoma. Pathol. Oncol. Res. 2010, 16, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Tsoukalas, N.; Bournakis, E.; Alexandrou, P.; Kavantzas, N.; Patsouris, E.; Theocharis, S. Ephrin (Eph) receptor A1, A4, A5 and A7 expression in human non-small cell lung carcinoma: Associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Clin. Pathol. 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-Q.; Zhang, J.-Y.; Bai, C.-Y.; Xu, X.-E.; Wu, J.-Y.; Chen, B.; Wu, Z.-Y.; Wang, S.-H.; Shen, J.; Shen, J.-H.; et al. Low EphA7 expression correlated with lymph node metastasis and poor prognosis of patients with esophageal squamous cell carcinoma. Acta Histochem. Cytochem. 2015, 48, 75–81. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Dong, Y.; Shen, Z.; Ma, H.; Wang, X.; Shi, S.; Wu, J.; Lu, G.; Peng, L.; et al. High expression of EphA1 in esophageal squamous cell carcinoma is associated with lymph node metastasis and advanced disease. APMIS 2013, 121, 30–37. [Google Scholar] [CrossRef]
- Li, D.; Xiang, B.; Ying, X.; Ying, X.; Dong, H. Correlation analysis of EphA7 expression with clinico-pathological parameters and prognosis in tongue squamous cell carcinoma. Shanghai Kou Qiang Yi Xue 2014, 23, 575–579. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25543601 (accessed on 14 August 2019).
- Hess, A.R.; Margaryan, N.V.; Seftor, E.A.; Hendrix, M.J.C. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: Role of the Eph receptors. Dev. Dyn. 2007, 236, 3283–3296. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kato, K.; Hasegawa, T.; Ikeda, S. An agonistic antibody to EPHA2 εxhibits antitumor effects on human melanoma cells. Anticancer Res. 2018, 38, 3273–3282. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Cao, G.; Zhang, X.; Xu, H.; Xu, H.; Wang, J. Expression of the EphA1 protein is associated with Fuhrman nuclear grade in clear cell renal cell carcinomas. Int J. Clin. Exp. Pathol. 2015, 8, 6821–6827. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26261568 (accessed on 14 August 2019).
- Inokuchi, M.; Nakagawa, M.; Baogok, N.; Takagi, Y.; Tanioka, T.; Gokita, K.; Okuno, K.; Kojima, K. Prognostic significance of high EphA1-4 expression levels in locally advanced gastric cancer. Anticancer Res. 2018, 38, 1685–1693. [Google Scholar] [CrossRef]
- Nakagawa, M.; Inokuchi, M.; Takagi, Y.; Kato, K.; Sugita, H.; Otsuki, S.; Kojima, K.; Uetake, H.; Sugihara, K. Erythropoietin-producing hepatocellular A1 is an independent prognostic factor for gastric cancer. Ann Surg. Oncol. 2015, 22, 2329–2335. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Sheng, Z.; Li, G.; Ma, H.; Wang, X.; Zhang, R.; Lu, G.; Hu, Q.; Sugimura, H.; et al. Downregulation of EphA1 in colorectal carcinomas correlates with invasion and metastasis. Mod. Pathol. 2009, 22, 151–160. [Google Scholar] [CrossRef][Green Version]
- Wu, J.-C.; Sun, B.-S.; Ren, N.; Ye, Q.-H.; Qin, L.-X. Genomic aberrations in hepatocellular carcinoma related to osteopontin expression detected by array-CGH. J. Cancer Res. Clin. Oncol. 2010, 136, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Wei, X.; Wang, J. EphA5 protein, a potential marker for distinguishing histological grade and prognosis in ovarian serous carcinoma. J. Ovarian Res. 2016, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H.; Wu, Z.; Chen, X.; Wang, J. Decreased expression of EphA5 is associated with Fuhrman nuclear grade and pathological tumour stage in ccRCC. Int. J. Exp. Pathol. 2017, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, Y.; Ma, C.; Qiu, Z.; Zhang, X.; Kang, Z.; Wu, Z.; Wang, H.; Xu, X.; Zhang, H.; et al. Downregulation of EphA5 by promoter methylation in human prostate cancer. BMC Cancer 2015, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kataoka, H.; Suzuki, M.; Sato, N.; Nakamura, R.; Tao, H.; Maruyama, K.; Isogaki, J.; Kanaoka, S.; Ihara, M.; et al. Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene 2005, 24, 5637–5647. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Ma, H.; Bao, Y.; Wang, X.; Zhou, H.; Sheng, Z.; Sugimura, H.; Jin, J.; Zhou, X. Differential expression of EphA7 receptor tyrosine kinase in gastric carcinoma. Hum. Pathol. 2007, 38, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Fokas, E.; Juricko, J.; You, A.; Rose, F.; Pagenstecher, A.; Engenhart-Cabillic, R.; An, H.-X. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer 2008, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Barquilla, A.; Pasquale, E.B. Eph receptors and ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Oricchio, E.; Nanjangud, G.; Wolfe, A.L.; Schatz, J.H.; Mavrakis, K.J.; Jiang, M.; Liu, X.; Bruno, J.; Heguy, A.; Olshen, A.B.; et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell 2011, 147, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Placzek, W.J.; Stebbins, J.L.; Mitra, S.; Noberini, R.; Koolpe, M.; Zhang, Z.; Dahl, R.; Pasquale, E.B.; Pellecchia, M. Novel targeted system to deliver chemotherapeutic drugs to EphA2-expressing cancer cells. J. Med. Chem. 2012, 55, 2427–2436. [Google Scholar] [CrossRef]
- Cai, W.; Ebrahimnejad, A.; Chen, K.; Cao, Q.; Li, Z.; Tice, D.A.; Chen, X. Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2024–2036. [Google Scholar] [CrossRef] [PubMed]
- Detre, S.; Saclani Jotti, G.; Dowsett, M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef]
- Vukoja, V.; Brandenbusch, T.; Tura, A.; Nassar, K.; Rohrbach, D.J.M.; Luke, M.; Grisanti, S. Expression of EphA2 in metastatic and non-metastatic primary uveal melanoma. Klin. Monbl. Augenheilkd. 2016, 232, 290–297. [Google Scholar] [CrossRef]
- Chen, L.-X.; Sun, B.-C.; Li, X.; He, Y.-J.; Song, G.-X. Overexpression of the receptor tyrosine kinase EphA2 in choroidal melanoma: Correlation with vesculogenic mimicry and prognosis. Chin. J. Ophthalmol. 2012, 48, 985–990. (In Chinese) [Google Scholar] [CrossRef]





| EphA1 | EphA5 | EphA7 | ||
|---|---|---|---|---|
| Reaction Intensity | Low | 69 (7.4%) | 79 (86.8%) | 82 (91.1%) |
| High | 19 (21.6%) | 12 (13.2%) | 8 (8.9%) | |
| Percentage of Positive Cells | Low | 68 (77.3%) | 83 (91.2%) | 78 (86.7%) |
| High | 20 (22.7%) | 8 (8.8%) | 12 (13.3%) | |
| Total Expression | Low | 62 (70.5%) | 78 (85.7%) | 76 (84.4%) |
| High | 26 (29.5%) | 13 (14.3%) | 14 (15.6%) | |
| Clinicopathological Parameters | EphA1 Low Expression (0–2) | EphA1 High Expression (3–6) | p-Value |
|---|---|---|---|
| Age | 0.143 | ||
| Mean 63, 63 | |||
| Gender | 0.210 | ||
| Male | 28 | 8 | |
| Female | 34 | 18 | |
| Tumor Size | 0.048 | ||
| ≤9.0 mm | 4 | 2 | |
| 9.1–12.0 mm | 8 | 8 | |
| 12.1–15.0 mm | 24 | 10 | |
| >15.0 mm | 26 | 6 | |
| Ciliary Body Involvement | 0.762 | ||
| No | 36 | 16 | |
| Yes | 26 | 10 | |
| Intrascleral Extension | 0.083 | ||
| No | 11 | 1 | |
| Yes | 51 | 25 | |
| Extra-Scleral Extension | 0.030 | ||
| No | 52 | 26 | |
| Yes | 10 | 0 | |
| Histopathological Grade | 0.690 | ||
| G1 | 17 | 8 | |
| G2 | 31 | 13 | |
| G3 | 14 | 5 | |
| Mitotic Index/40 hpf | 0.042 | ||
| 0–5 | 40 | 21 | |
| 6–10 | 13 | 3 | |
| >10 | 7 | 0 | |
| Chromosome 3 Loss | 0.064 | ||
| No | 8 | 6 | |
| Yes | 39 | 9 | |
| Metastases | 0.322 | ||
| No | 31 | 16 | |
| Yes | 31 | 10 | |
| Posterior Pole Involvement | 0.612 | ||
| No | 47 | 21 | |
| Yes | 15 | 5 | |
| Retinal Detachment | 0.487 | ||
| No | 36 | 13 | |
| Yes | 26 | 13 | |
| Vitreous Hemorrhage | 0.014 | ||
| No | 56 | 18 | |
| Yes | 6 | 8 |
| Clinicopathological Parameters | EphA5 Low Expression (0–2) | EphA5 High Expression (3–6) | p-Value |
|---|---|---|---|
| Age | 0.683 | ||
| Mean 64, 34 | |||
| Gender | 0.163 | ||
| Male | 34 | 3 | |
| Female | 44 | 10 | |
| Tumor Size | 0.269 | ||
| ≤9.0 mm | 5 | 2 | |
| 9.1–12.0 mm | 14 | 2 | |
| 12.1–15.0 mm | 28 | 6 | |
| >15.0 mm | 31 | 3 | |
| Ciliary Body Involvement | 0.341 | ||
| No | 43 | 9 | |
| Yes | 35 | 4 | |
| Intrascleral Extension | 0.463 | ||
| No | 12 | 1 | |
| Yes | 66 | 12 | |
| Extra-Scleral Extension | 0.171 | ||
| No | 68 | 13 | |
| Yes | 10 | 0 | |
| Histopathological Grade | 0.169 | ||
| G1 | 19 | 7 | |
| G2 | 42 | 3 | |
| G3 | 17 | 3 | |
| Mitotic Index/40hpf | 0.075 | ||
| 0–5 | 50 | 12 | |
| 6–10 | 16 | 1 | |
| >10 | 7 | 0 | |
| Chromosome 3 Loss | <0.001 | ||
| No | 8 | 6 | |
| Yes | 47 | 3 | |
| Metastases | 0.010 | ||
| No | 36 | 11 | |
| Yes | 42 | 2 | |
| Posterior Pole Involvement | 0.121 | ||
| No | 63 | 8 | |
| Yes | 15 | 5 | |
| Retinal Detachment | 0.606 | ||
| No | 42 | 8 | |
| Yes | 36 | 5 | |
| Vitreous Hemorrhage | 0.013 | ||
| No | 69 | 8 | |
| Yes | 9 | 5 |
| Clinicopathological Parameters | EphA7 Low Expression (0–2) | EphA7 High Expression (3–6) | p-Value |
|---|---|---|---|
| Age | 0.479 | ||
| Mean 64, 18 | |||
| Gender | 0.722 | ||
| Male | 31 | 5 | |
| Female | 45 | 9 | |
| Tumor Size | 0.425 | ||
| ≤9.0 mm | 3 | 3 | |
| 9.1–12.0 mm | 16 | 1 | |
| 12.1–15.0 mm | 27 | 7 | |
| >15.0 mm | 30 | 3 | |
| Ciliary Body Involvement | 0.969 | ||
| No | 43 | 8 | |
| Yes | 33 | 6 | |
| Intrascleral Extension | 0.094 | ||
| No | 13 | 0 | |
| Yes | 63 | 14 | |
| Extra-Scleral Extension | 0.175 | ||
| No | 67 | 14 | |
| Yes | 9 | 0 | |
| Histopathological Grade | 0.366 | ||
| G1 | 20 | 5 | |
| G2 | 38 | 7 | |
| G3 | 18 | 2 | |
| Mitotic Index/40 hpf | 1.000 | ||
| 0–5 | 50 | 11 | |
| 6–10 | 15 | 2 | |
| >10 | 6 | 1 | |
| Chromosome 3 Loss | 0.744 | ||
| No | 11 | 2 | |
| Yes | 44 | 6 | |
| Metastases | 0.283 | ||
| No | 37 | 9 | |
| Yes | 39 | 5 | |
| Posterior Pole Involvement | 0.043 | ||
| No | 62 | 8 | |
| Yes | 14 | 6 | |
| Retinal Detachment | 0.649 | ||
| No | 43 | 7 | |
| Yes | 33 | 7 | |
| Vitreous hemorrhage | 0.509 | ||
| No | 65 | 11 | |
| Yes | 11 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajdzis, M.; Theocharis, S.; Gajdzis, P.; Cassoux, N.; Gardrat, S.; Donizy, P.; Klijanienko, J.; Kaczmarek, R. Ephrin Receptors (Eph): EphA1, EphA5, and EphA7 Expression in Uveal Melanoma—Associations with Clinical Parameters and Patient Survival. Life 2020, 10, 225. https://doi.org/10.3390/life10100225
Gajdzis M, Theocharis S, Gajdzis P, Cassoux N, Gardrat S, Donizy P, Klijanienko J, Kaczmarek R. Ephrin Receptors (Eph): EphA1, EphA5, and EphA7 Expression in Uveal Melanoma—Associations with Clinical Parameters and Patient Survival. Life. 2020; 10(10):225. https://doi.org/10.3390/life10100225
Chicago/Turabian StyleGajdzis, Malgorzata, Stamatios Theocharis, Pawel Gajdzis, Nathalie Cassoux, Sophie Gardrat, Piotr Donizy, Jerzy Klijanienko, and Radoslaw Kaczmarek. 2020. "Ephrin Receptors (Eph): EphA1, EphA5, and EphA7 Expression in Uveal Melanoma—Associations with Clinical Parameters and Patient Survival" Life 10, no. 10: 225. https://doi.org/10.3390/life10100225
APA StyleGajdzis, M., Theocharis, S., Gajdzis, P., Cassoux, N., Gardrat, S., Donizy, P., Klijanienko, J., & Kaczmarek, R. (2020). Ephrin Receptors (Eph): EphA1, EphA5, and EphA7 Expression in Uveal Melanoma—Associations with Clinical Parameters and Patient Survival. Life, 10(10), 225. https://doi.org/10.3390/life10100225





