1. Introduction
Additive manufacturing has many applications in the healthcare sector. For example, medical imaging techniques like Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) scans can transform digital images to computer screens from physical objects. In biomedical engineering, additive manufacturing has enabled applications such as tissue and organ fabrication, the production of implants and prostheses, drug delivery systems, and anatomical structures [
1]. In clinical practice, 3D-printed anatomical models that replicate realistic organ shapes have been used for presurgical planning, allowing surgeons to rehearse and prepare for complex procedures. This approach helps reduce both risk and time during surgery while also enhancing education and training. Moreover, anatomical 3D printing plays a vital role in evaluating the performance of newly developed biomedical products. The challenge lies in developing novel 3D-printable materials that closely replicate the biomechanical properties of real tissues.
PolyJet™ 3D printing is an additive manufacturing process in which layers of acrylic-based photopolymers are deposited onto a build tray at specific coordinates. The liquid resin is dispensed from print heads using controlled piezoelectric pulses, and ultraviolet (UV) lamps mounted on the print block partially cure the resin after each pass. Material is jetted during both left-to-right and right-to-left movements of the print block as shown in
Figure 1 [
2].
A key feature of PolyJet™ technology is its ability to print multiple materials with varying properties into a single build with micron-level precision. For instance, elastomeric and rigid materials can be combined in specific ratios to produce Digital Materials (DMs) with mechanical properties that are intermediate between those of the parent materials. PolyJet™ allows control of both mechanical properties and color through applying specific material mixtures on a point-by-point basis, opening up possibilities for customized and intricate models [
4]. Its printing parameters can be fine-tuned to replicate the haptic feel, responsiveness, and biomechanical performance of real tissues, making it a powerful tool for creating realistic anatomical models for medical training, surgical planning, and device testing [
5].
This technology also allows for the precise creation of complex bio-inspired shapes. The entire 3D printing process can be controlled using the GrabCAD™ Print software version 1.92.17.44384, which employs a voxel-based system to modify the physiological characteristics of preset materials with high precision. The Stratasys J850™ digital anatomy 3D Printer includes in its portfolio of materials three proprietary anatomical digital materials tailored for specific anatomical applications: GelMatrix™, TissueMatrix™, and BoneMatrix™ [
6]. Each material offers distinct mechanical characteristics suited to different uses. These digital materials extend the range of hardness (measured on the Shore A scale), elasticity, and density over the standard portfolio of PolyJet™ materials. The multi-material jetting technology enables the production of realistic, functional, and innovative structures that integrate hard and soft phases with tailored properties—all without compromising the geometric complexity of the designs [
7]. This capability is essential for accurately replicating the mechanical behavior of biological systems.
Tissue-mimicking materials provide an ethical and practical alternative to testing on human tissues, addressing ethical concerns associated with clinical and research development. Typically, phantoms or specialized 3D printing materials are employed for such purposes. The use of 3D modeling in preclinical research and presurgical settings has significantly expanded, enabling enhanced planning for complex procedures, a deeper understanding of underlying pathophysiological conditions, and computational validation of preclinical research designs [
8].
The review by Yildiz et al. presented advances in bioprinting technologies and its current state [
9]. The research presented thermal droplet, laser, and mechanical extrusion bioprinting setups and their ability to fabricate and mimic cellular microenvironments for tissue engineering and regenerative medicine. But mechanical strength and stability are key requirements which are not currently optimized by these methods and need further development. Similarly, the article by Wang et al. also sheds light on similar bioprinting techniques and their application in pharmaceutical and tissue engineering [
10].
Research on multi-material 3D and 4D bioprinting by Chen et al. highlights the different multi-material 3D bioprinting techniques that are used for fabricating heterogenous constructs for tissue engineering applications [
11]. The article suggested that this technique demonstrates significant potential in constructing tissue constructs, although some challenges still remain such as limited multifunction controllability, variance in mechanical properties, and many more.
The benefits of preprocedural planning with 3D models have been extensively documented. For example, digital 3D models enable clinicians to better assess patient-specific factors, such as anatomical asymmetries, the proximity of procedures or devices to critical structures (e.g., nerves or arteries), and potential issues with device biocompatibility. These models improve the precision and safety of clinical interventions [
12]. Despite their utility, most PolyJet™ materials alone lack the mechanical properties required to fully simulate tissue-mimicking materials. This limitation reduces the effectiveness of printed models for applications that depend on highly accurate material jetting to replicate the biomechanical characteristics of human tissues. Further advancements in material development are essential to overcome these constraints and unlock the full potential of 3D printing for tissue-mimicking applications.
The research by Bezek et al. investigated the mechanical properties of tissue-mimicking composites by employing a combination of thermal and mechanical analyses, including dynamic mechanical analysis, Shore hardness testing, puncture testing, and tensile testing [
13]. The base materials used in the study were Stratasys™ TangoPlus™, a clear acrylate-based resin, and a non-curing cleaning fluid (CF) material. The study found that higher concentrations of the non-curing fluid generally resulted in reduced strength and stiffness of the composite material. Additionally, exponential and second-order polynomial models were identified as suitable fits for describing many of the mechanical properties as functions of non-curing fluid concentration. However, the research was limited by the small range of material combinations analyzed, which restricted a comprehensive understanding of how various parameters influence the mechanical properties of tissue-mimicking composites. This highlights the need for further research to explore a broader range of material combinations and parameters to fully characterize the behavior and potential of tissue-mimicking composites in various applications.
The research by Ruiz et al. focuses on the mechanical characterization of multi-material specimens designed and fabricated to replicate bio-inspired hierarchical structures, specifically targeting tendons and ligaments. The mechanical behavior of these structures was evaluated under uniaxial tensile tests, with Digital Image Correlation (DIC) employed to accurately measure large strains in a non-contact manner. Two material combinations were analyzed: VeroWhite™ with Agilus30Clear™ and VeroWhite™ with TissueMatrix™. Additionally, variations in the Shore hardness of Agilus30Clear™ were tested for comparative analysis [
7]. A related study by Lee et al. from Medtronic Inc. explored the use of PolyJet™ 3D printing for tissue-mimicking materials through experimental configurations [
14]. The study quantitatively evaluated solid organ (SO) models under axial loading, comparing their stiffness to that of porcine liver. Similarly, Structural Heart–Myocardium (Myo) configurations were tested under various lubricant conditions to assess lubricity in comparison to porcine epicardium and aorta. Additionally, Subcutaneous Tissue configurations were qualitatively evaluated by preclinical experts during cutting, tunneling, and puncture procedures. The findings revealed that, despite some limitations, digital anatomy materials exhibit tactile properties closer to real tissue than other commercially available 3D-printed materials. Furthermore, DA materials showed lower sample-to-sample variability, providing a level of repeatability that biological tissues cannot achieve although the study was limited to few specific soft tissue types only.
Severseike et al. investigated the ability of 3D-printed synthetic myocardium to replicate the mechanical properties of organic myocardium [
12]. The study found that digital anatomy myocardium demonstrated comparability to porcine myocardium in terms of compliance. For compliance testing, the digital anatomy material was within the same range as porcine myocardium or stiffer, particularly in conditions involving higher stress concentrations or smaller displacements. However, when evaluating suture and cutting properties, the digital anatomy material did not perform as well as porcine myocardium. The material was more prone to tearing during suturing and exhibited higher friction forces during needle insertion and cutting. Despite these limitations, the compliance of the digital anatomy myocardium was much closer to that of real tissue than other 3D-printed materials tested.
Kornfellner et al. analyzed the mechanical properties of 50 3D-printed samples fabricated from commercially available materials in both their pure forms and as matrixinclusion mixed composites. The study involved cubic inclusions of RGD8430-DM™ and RGD8530-DM™ materials, which were used to create matrix-inclusion composites through material jetting [
15]. The results revealed that the performance of multi-materials is influenced by the choice of starting materials, the geometries, and the contact properties between the individual phases within the composite.
In another study, Daneshdoost et al. examined the mechanical tensile performance of two-material composite designs, comparing them based on different volumetric ratios of the two constituent materials—one rigid and strong, and the other soft and ductile [
16]. The researchers concluded that custom digital material composites, when designed with voxel-scale precision, provided better control over mechanical performance compared to traditional layered composites. Specifically, they showed that custom-designed composites offered increased Young’s Modulus and ultimate strength over the standard digital materials preset by the manufacturer.
Several studies have explored how varying the mechanical properties of PolyJet™ printed structures can be achieved irrespective of the tissue-mimicking application. Liu et al. investigated the impact of 3D lattice structures on the mechanical properties of printed parts, specifically analyzing BCC, BCC-Z, FCC, and octet lattices [
2]. They also examined how changes in strut thickness and printability—whether integrated or assembled through snap-fit techniques—affected the material performance. Barclift et al. focused on how the orientation of printed parts relative to the printing bed (X, Y, and Z axes) influenced their mechanical properties [
17]. The study highlighted how variations in orientation could alter the strength and structural integrity of the printed parts, underscoring the importance of print orientation in achieving the desired material performance. Pugalendhi et al. studied the effect of process parameters on the mechanical properties of VeroBlue™ material in PolyJet™ technology [
4]. Two properties were varied: High Quality/High Speed and Matte/Glossy finishes. Their findings concluded that the High Speed-Glossy setting resulted in the highest flexural modulus and strength, which suggests this combination provides an optimal balance for producing strong, functional parts. These studies demonstrate the importance of process optimization in PolyJet™ 3D printing, with material selection, lattice design, print orientation, and finish type all playing significant roles in determining the mechanical properties of the final printed structures. Further research is needed to refine these techniques and enhance the reliability and versatility of PolyJet™ printed materials.
Cross-comparison of real tissue value and PolyJet™ printed tissue values would establish a relationship for suggesting the possible parameter settings/material combination to achieve a specific tissue manufactured with accuracy. Vergen measured the biomechanical properties of human and animal cardiothoracic tissues. Planar Biaxial Testing was used to characterize the changes in axial load and strain of human, porcine, and ovine fossa ovalis following a transseptal puncture [
18]. Additionally, characterization of Human Ascending Aorta and Human and Porcine Pericardium were also done. Grytz et al. mentions the material properties of the posterior human sclera regions [
19]. The elastic response of the posterior human sclera can be characterized by the anisotropic architecture and crimp response of scleral collagen fibrils. Emig et al. outlines a review of existing studies that have measured mechanical properties of myocardial tissue [
20]. They outlined the measured Young’s Modulus reported from various studies between Porcine and Human Myocardial tissue and their variation when the heart is diseased. In a similar manner, Singh et al. provided an extensive review of the literature reporting the mechanical properties of whole-body soft tissues [
21]. They listed tissue properties existing in the literature available online with the variation that is possible with age, gender, etc.
Table 1 below shows a consolidated list of the tissue properties extracted from the available literature.
While the studies provide insights into characterizing the mechanical properties of 3D-printed materials produced by 3D printing, there is still limited research into how variations in material choices and software parameters impact the mechanical properties of printed objects. Further research is needed to fully understand the influence of these variables on the performance of 3D-printed multi-materials to optimize material and software combinations for specific applications. This research employs a DOE approach to systematically capture the effects of varying PolyJet™ printing parameters on the mechanical properties observed in tensile testing. The resulting data is then validated by comparing the mechanical properties of the printed samples with those of biological tissues available in the literature, ensuring the printed models closely mimic real tissue behavior.
3. Results and Discussion
After tensile testing of the samples, no samples failed in an unintentional manner. There were no failures caused by any mix-ups or any misprints. In this DOE, Analysis of Variance (ANOVA) was derived to analyze the impactful factors and their relative interactions. During this analysis, the statistical software JMP™ Pro 17.2, a subsidiary of SAS Institute, was utilized for analyzing the data. Alongside that was the free trial of DesignExpert™ by Stat-Ease 360, which was also utilized to construct the model and build relationships between factors and responses. In addition to this, Microsoft Excel was used to compare with the existing literature of human tissue values.
Table 6 below shows data of a few random runs that were performed during the DOE.
In the existing literature, research on soft tissues has predominantly focused on their linear and isotropic mechanical properties [
21]. So far, tissues such as the skin, muscles, brain, arteries, breast, liver, spleen, esophagus, tonsils, tongue, pancreas, pelvic tissues, and small intestine have been characterized using isotropic or transversely isotropic hyperelastic models, though typically only at a limited range of strain rates. Expanding these studies to cover broader strain conditions and refining the models could greatly enhance the accuracy of tissue-mimicking materials in medical and research applications, but for the purposes of simplifying this article, linear elastic relationship is assumed and analyzed for tissues.
The factors are annotated according to
Table 7 below for maintaining consistency across the research article. After analyzing the mechanical properties of the samples, the data turned out to be skewed due to many samples being designed for softer tissue types which had low Max Stress, high Max Strain, and low Young’s Modulus, as can be seen in
Table 8,
Table 9 and
Table 10 below. The data is represented separately for ease in comparison among the existing preset standard in the upcoming sections.
3.1. Maximum Stress
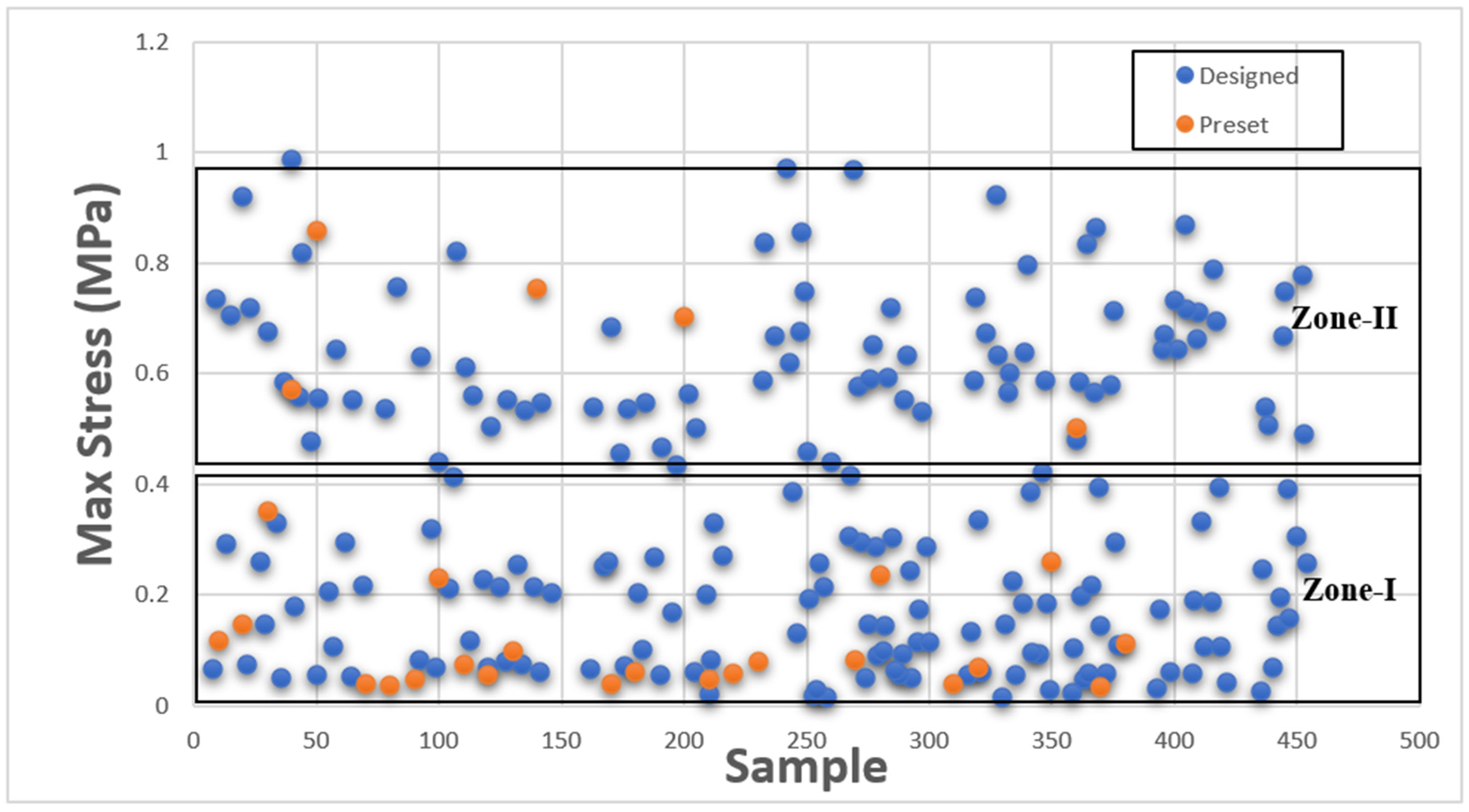
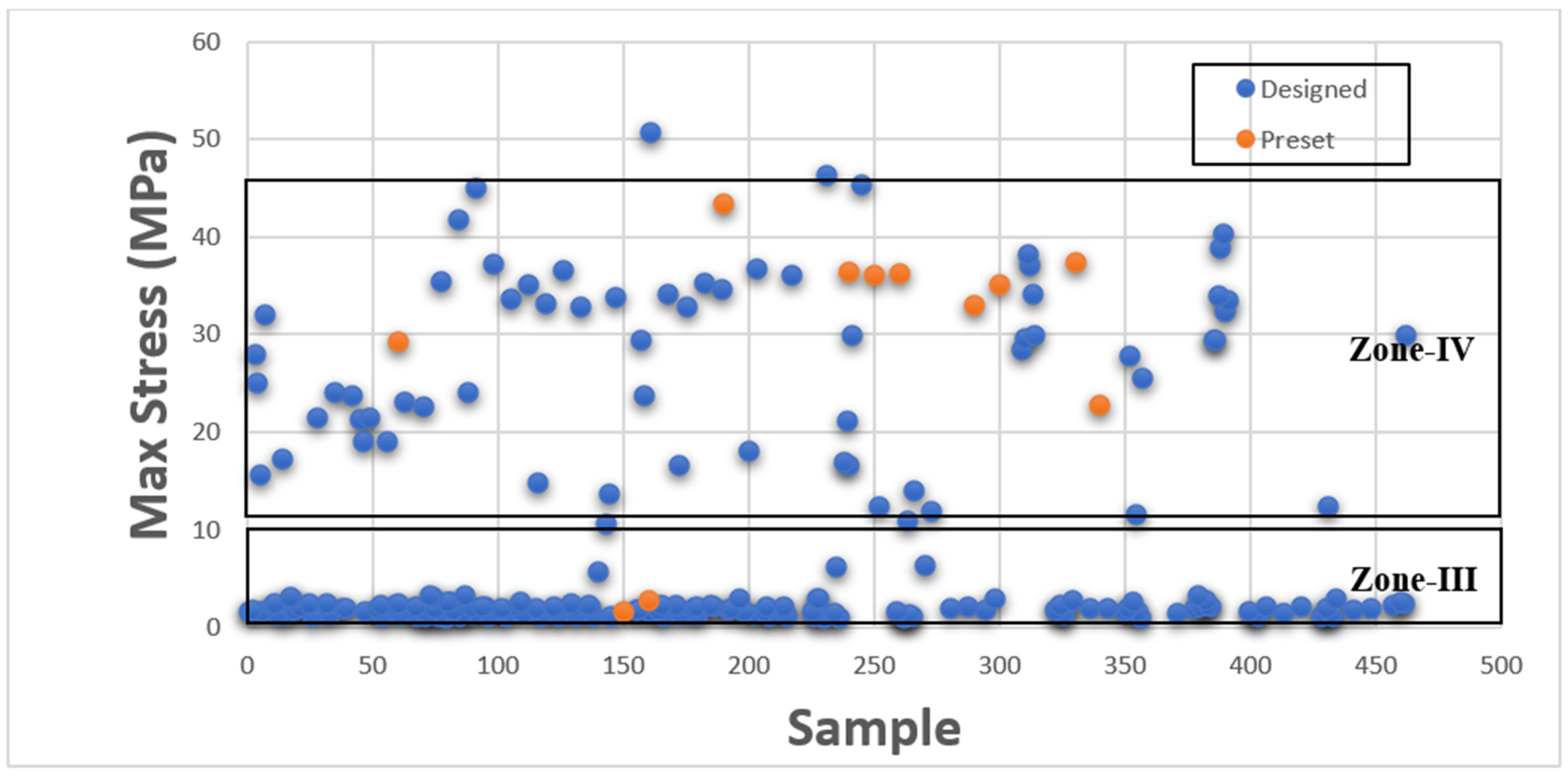
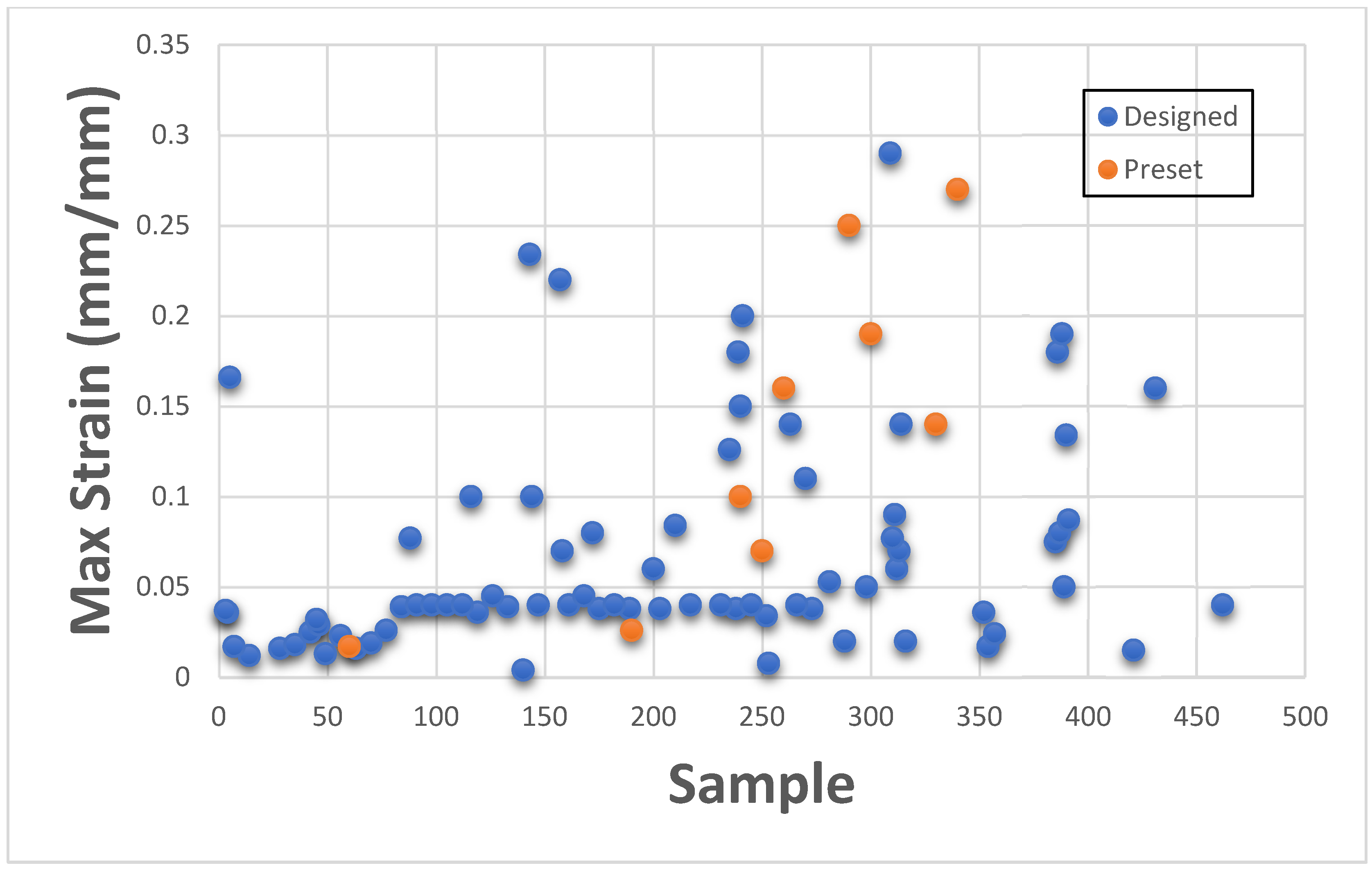
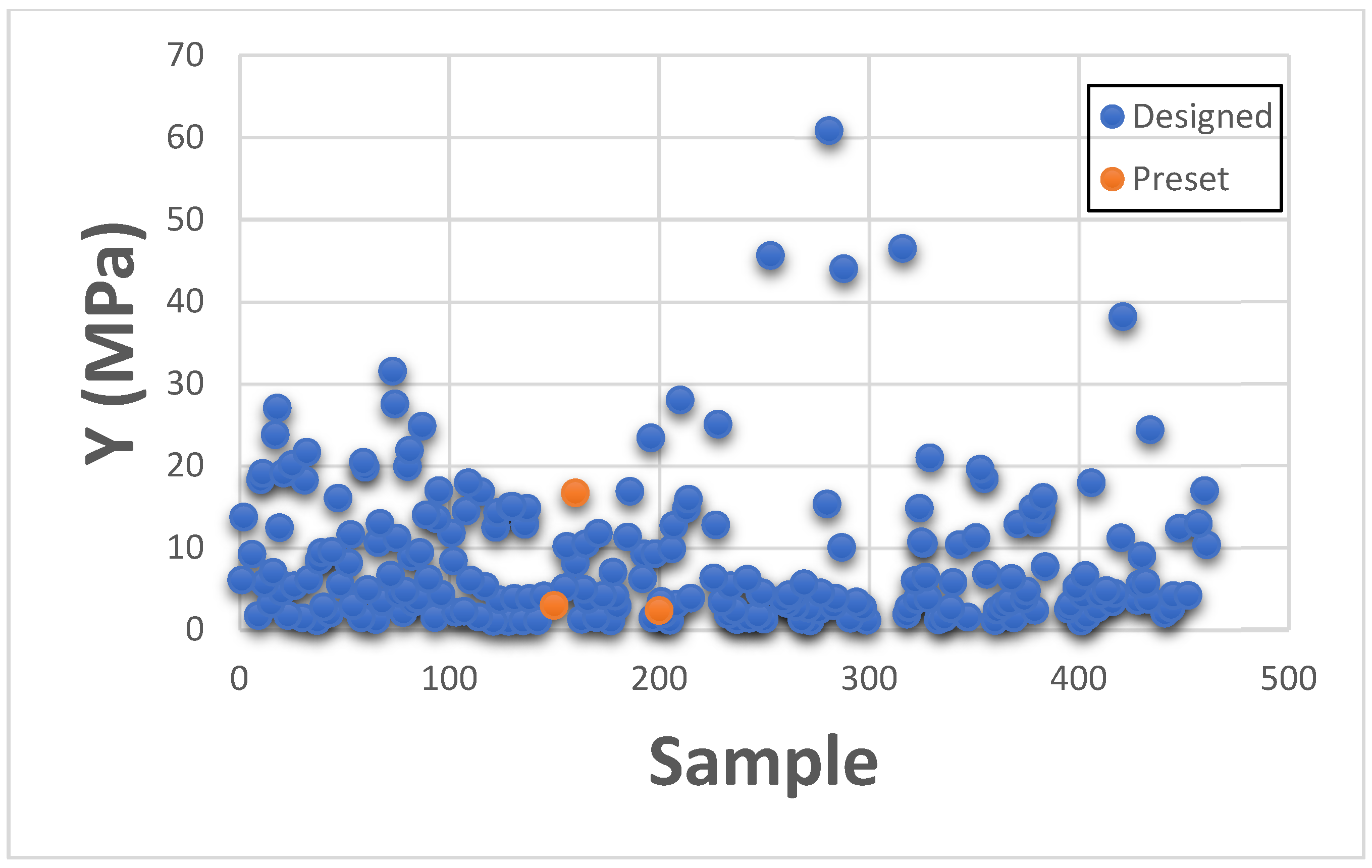
This section presents Maximum Stress results individually in comparison with existing GrabCAD™ tissue presets. The tested samples resulted in a wide range of Max Stress values between 0.016 MPa and 50.69 MPa. The samples can be divided into several groups based on the Max Stress values shown in subsequent
Figure 4 and
Figure 5.
These figures show the resulting Max Stress values overlayed with the existing preset standard tested parts from Stratasys™. The figures are divided into four zones-I, II, III, and IV. Zone I indicate the samples which had low ultimate tensile strength value. Based on tensile values of biological tissue derived from the literature, these are usually ultra-soft tissues like liver tissue, lung tissue, low rigidity structural heart frame, low stiffness Myocardium, Suturable vessel wall, extensible valve chordae, soft ligaments, nerves, etc. Zone II includes ascending aorta, pericardium, stomach tissue, moderately rigid structural heart frame, stiff Myocardium, soft valve annulus tissue, and general anatomically dense connective tissues. Zone III includes stiff valve annulus, pulmonary valve tissue, aortic valve, esophagus, fossa ovalis, stiff ascending aorta, stiff pericardium, etc. Finally, Zone IV consists of rigid tissue materials such as varying porosity bones, skull, vertebrae, tendons, ligaments, etc.
This indicates that there are combinations of settings that can broaden the range of Max Stresses beyond what is available in the standard GrabCAD™ settings.
Interaction Plots and ANOVA: Max Stress
A linear model was fitted into the analysis. To investigate the primary influence of input parameters on individual response, an ANOVA with a 95% confidence interval was used. The ANOVA values for Max Stress are shown in
Table 11. If the
p-value is less than 0.05, the parameter is significant in terms of responses as highlighted in the ANOVA tables. Due to the complexity of the DOE, which includes mixture–mixture factorial design, the ANOVA table has been analyzed in a similar manner. After analyzing the significant terms, it is observed that using VeroPureWhite™ in the outer layer (B) and background (F) has the highest impact on the obtained Max Stress values. While the dependence of Max Stress between outer layer and background layer is governed by the OT(K). It determines which mixture will have the most impact on the response among the two as the volume of mixture 1 and mixture 2 constitute depends upon it.
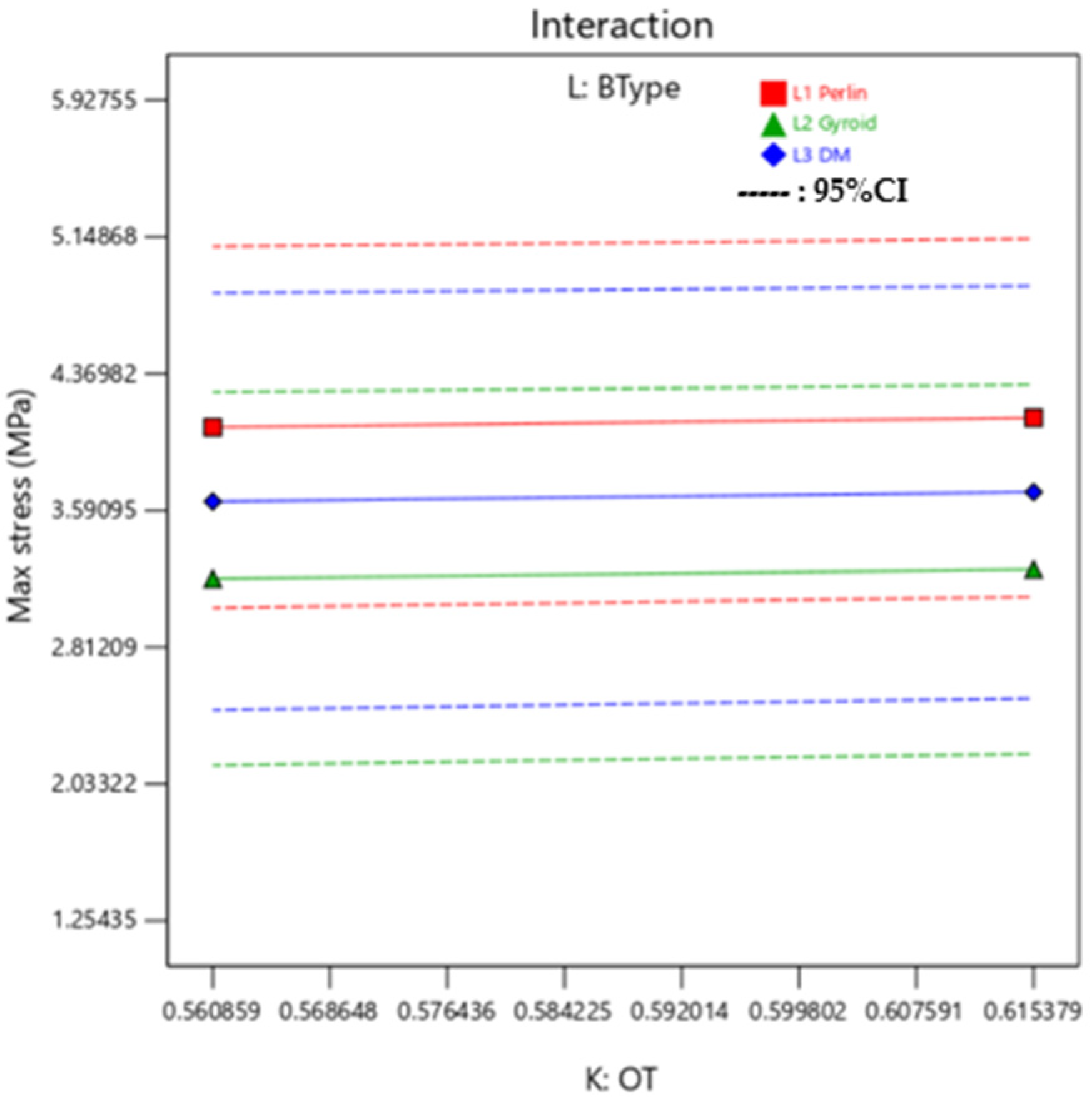
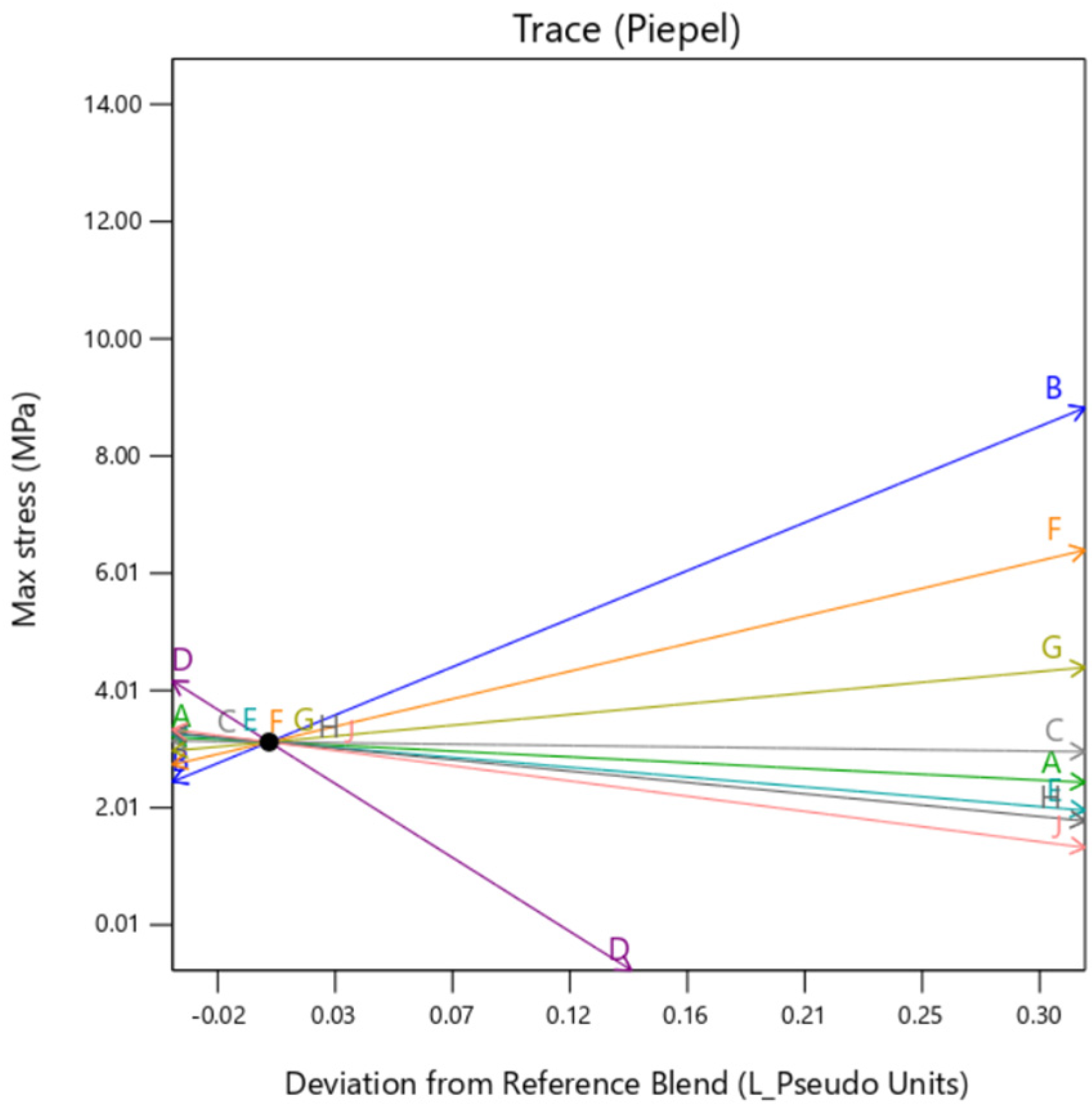
Additionally, it can be observed in
Figure 6, that is the interaction plot for the median setting of outer layer mixture and background layer mixture, there is minimal variation in Max Stress with the background type variation. In this interaction plot, the lines are almost parallel, which indicates there is not a good relationship between the BType (L) factor and its interaction with OT (K) on the value of Max Stress. Irrespective of changing the BType, Max Stress remains constant while OT is varied.
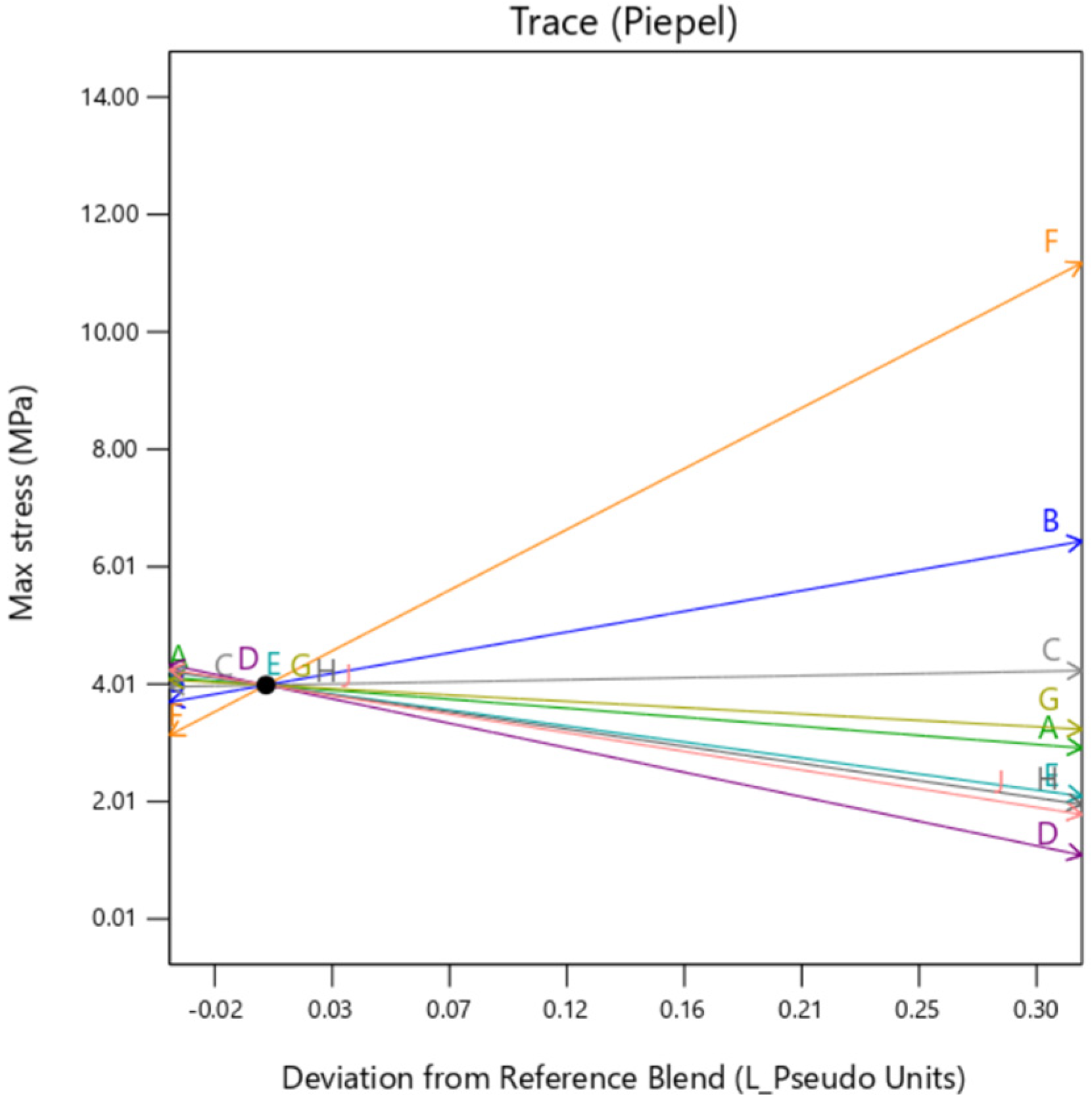
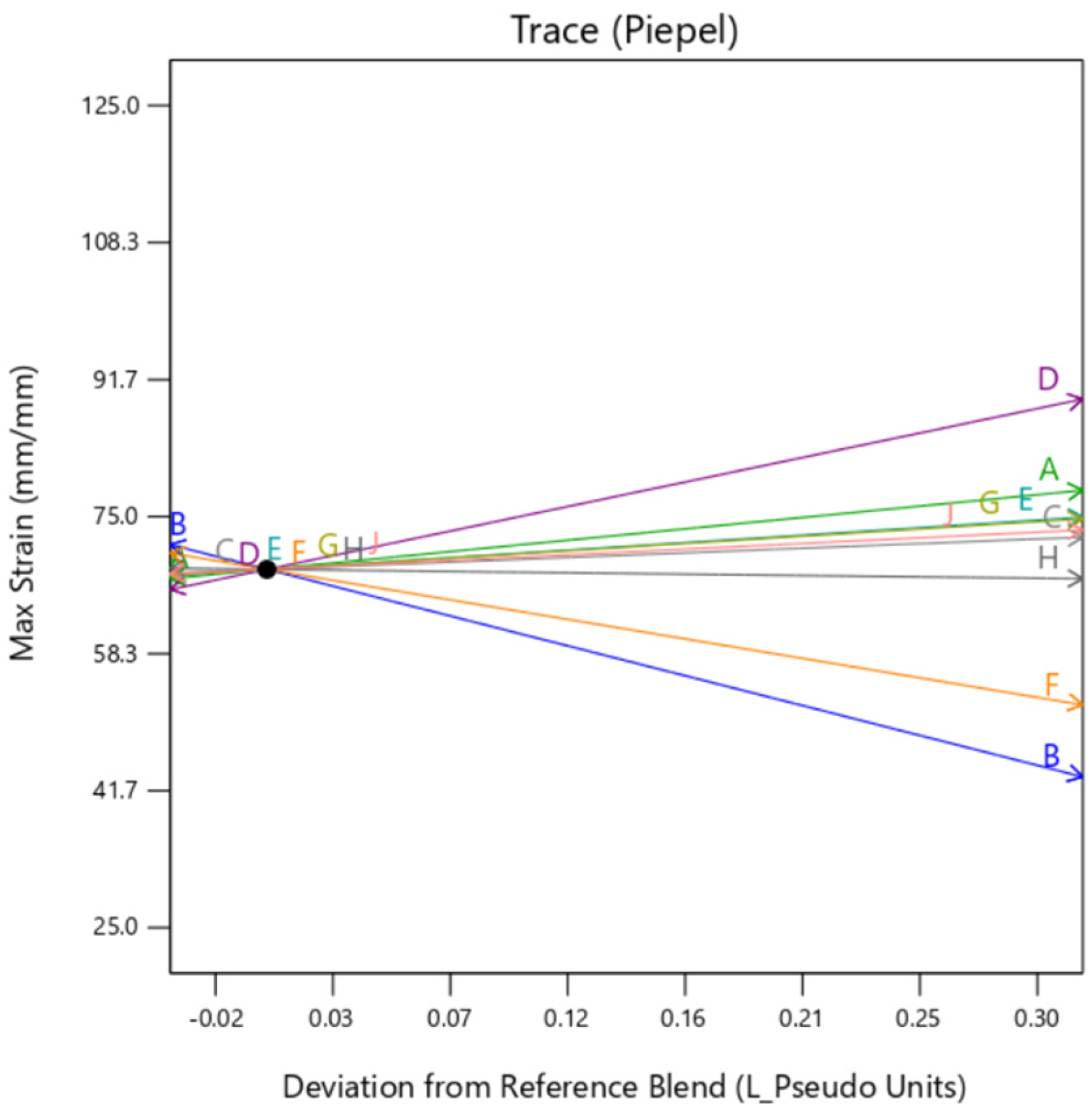
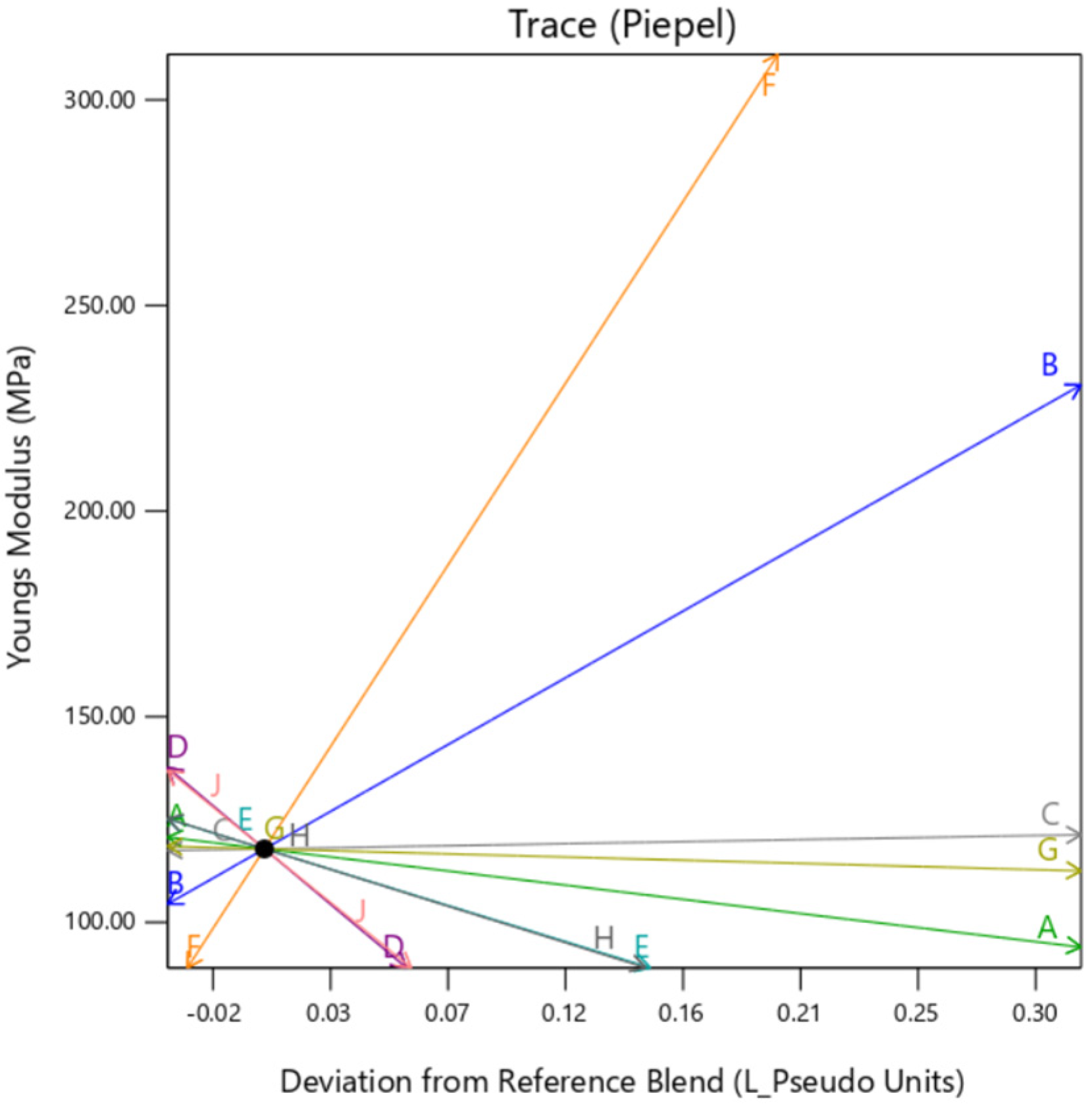
Individual trace plots are also presented below in
Figure 7,
Figure 8 and
Figure 9. They show the individual impact of the mixture factors and its sensitivity on the response when they are varied from a reference point, which is termed as “Deviation from Reference blend”. In this article, the reference blend is set at the mixture where all the constituents are taken at equal quantity %. Here, the outer layer thickness is fixed at a nominal setting of 0.5 mm. It is important to note here that except when the background type is set at
gyroid, the trace is similar for all the factors. That means setting the background to
gyroid enhances the outer layer dependency of the sample, irrespective of the mixture constituting the background. It is to be noted here that the model extrapolates the value of Max Stress below zero as there is no boundary condition preventing that. Since this is a hypothetical condition, it can be ignored.
3.2. Max Strain
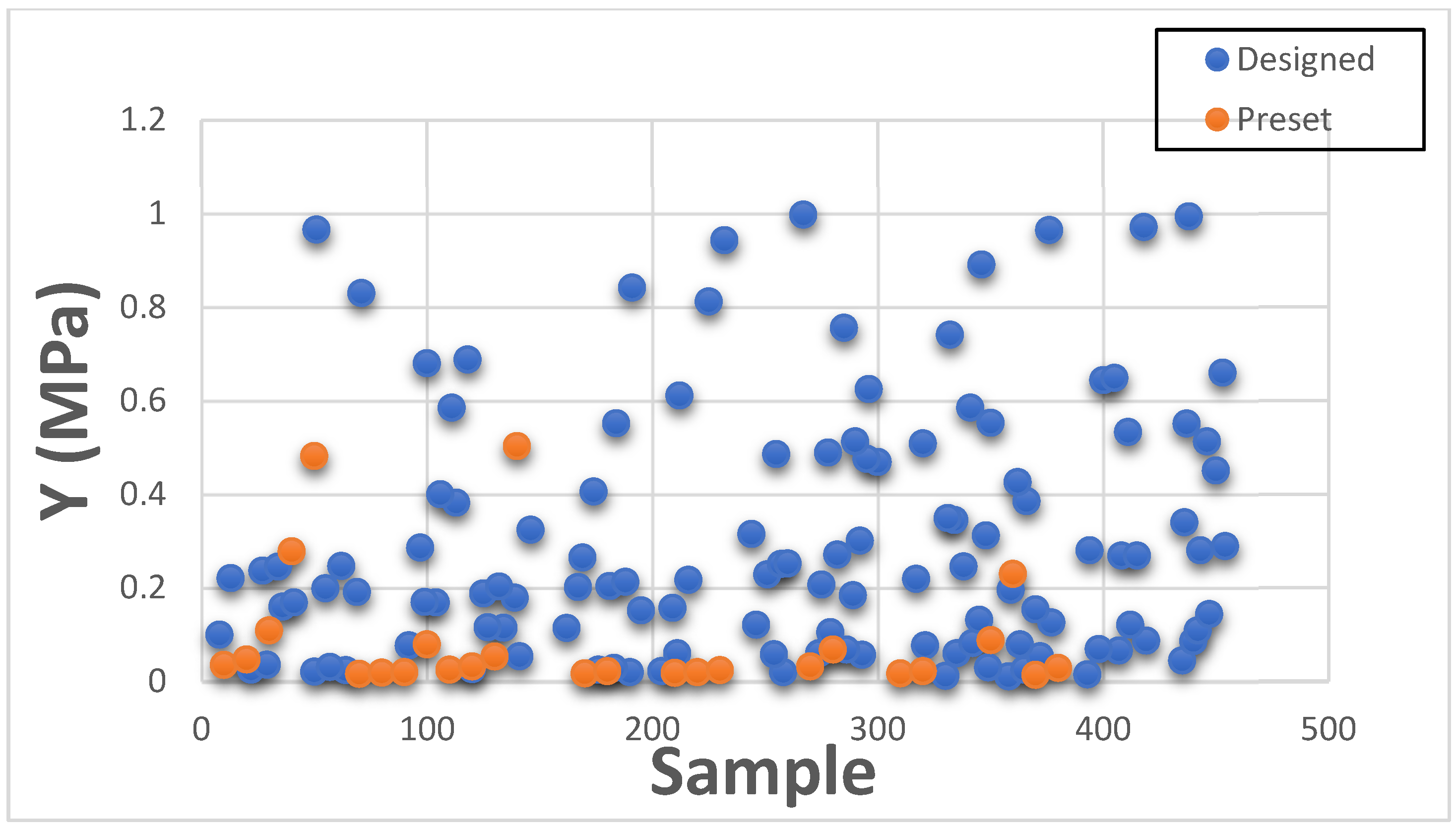
This section presents Maximum Strain results individually for categorizing with existing GrabCAD™ presets. The tested samples resulted in a wide range of Max Strain values ranging between 0.004 to 424.16. The vast number of runs can be divided into several distinct groups based on the existing tested tensile parts made from preset materials.
Figure 10 and
Figure 11 shown below depict the values plotted separately based on low strain values of 0.004 to 1.0 and high strain values of 1.1 to 424.16.
Figure 11 consists of samples designed to mimic bone tissue, extremely stiff structural heart frame, skull, vertebrae, long bones, calcified tissues, etc., while
Figure 10 consists of all the remaining soft tissues of the body.
Thus, the DOE also successfully encompasses and expands the existing preset standard material range with Max Strain values too.
Interaction Plots and ANOVA: Max Strain
After fitting the Linear fit model, it was observed that the quantity of Agilus30Clear™ in the outer layer (A) and background layer (E) has the highest influence on the Max strain values, as suggested by ANOVA in
Table 12. No other factor has a significant impact on the response in this case. This clearly suggested that while designing a sample to mimic a specific tissue, the amount of Agilus30Clear™ included in the mixture should be monitored and governed in accordance with Max Stress and Young’s Modulus values. The non-impact of interaction between OT and BType has also been observed in the interaction plot in
Figure 12, while it can also be seen that using
DM as BType has a slight impact on the resultant strain values.
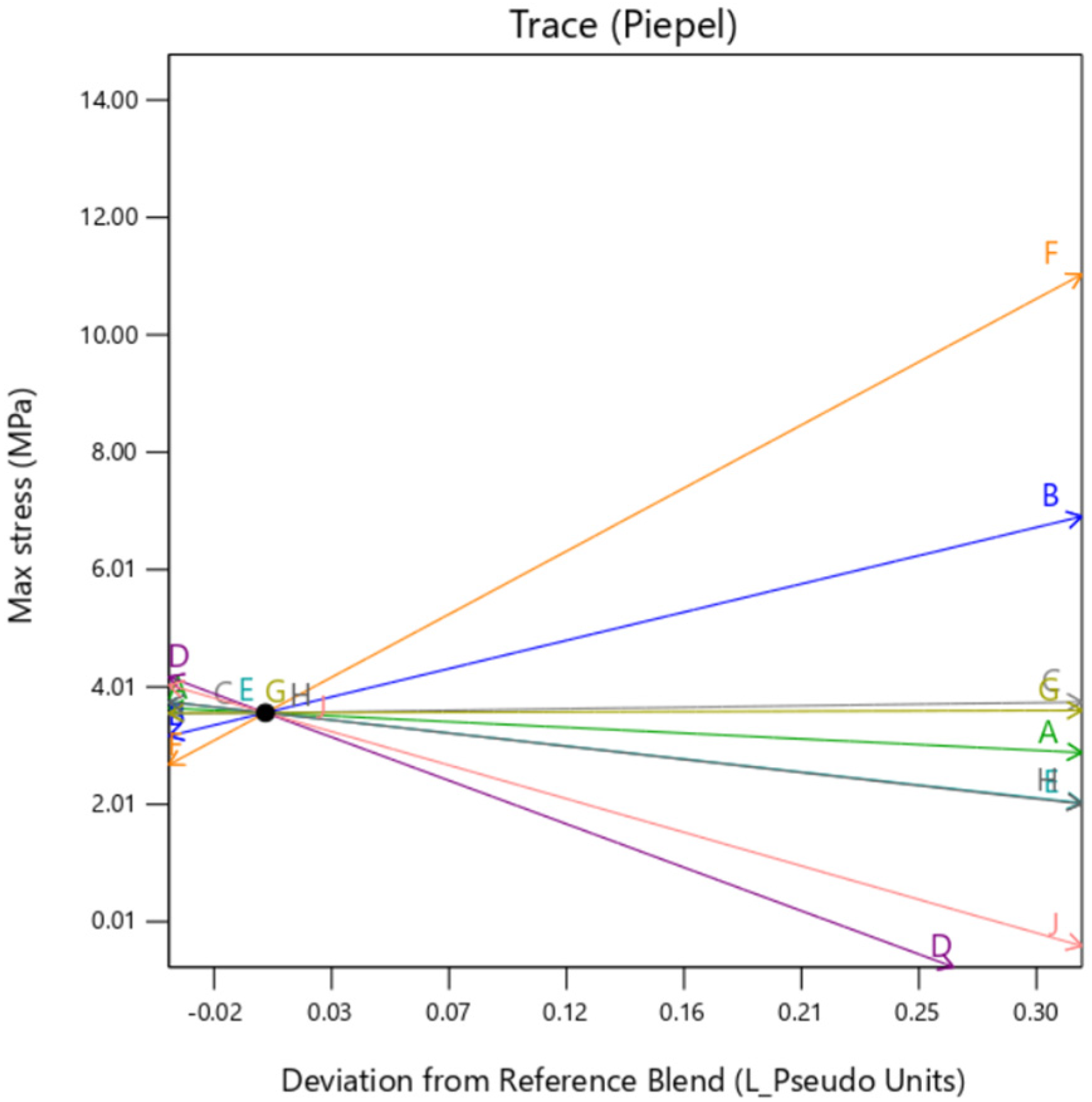
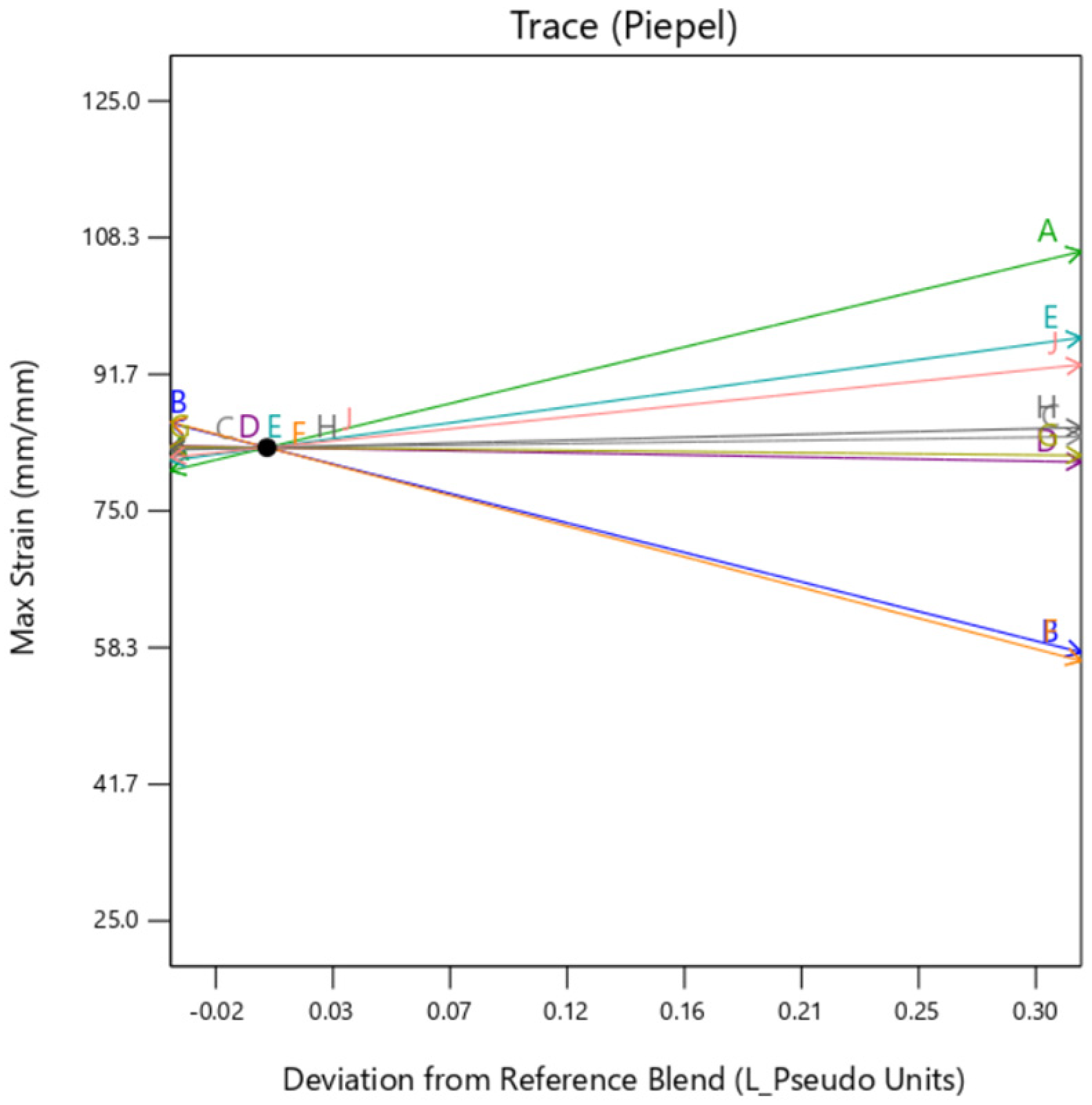
The individual trace plots shown below in
Figure 13,
Figure 14 and
Figure 15 depicts the similarity between the graphs of
DM and
perlin.
Gyroid background shows some dependency on the TissueMatrix™(D) too for the outer layer as can be observed by the slope of trace D in
Figure 14 in contrast to
Figure 13 and
Figure 15. This is due to the increased dependency on the Agilus30Clear™(A) in the overall sample as
gyroid infill utilizes both mixture 1 and mixture 2 as its constituents, which inadvertently increases net quantity of mixture 1 in the overall sample.
3.3. Young’s Modulus
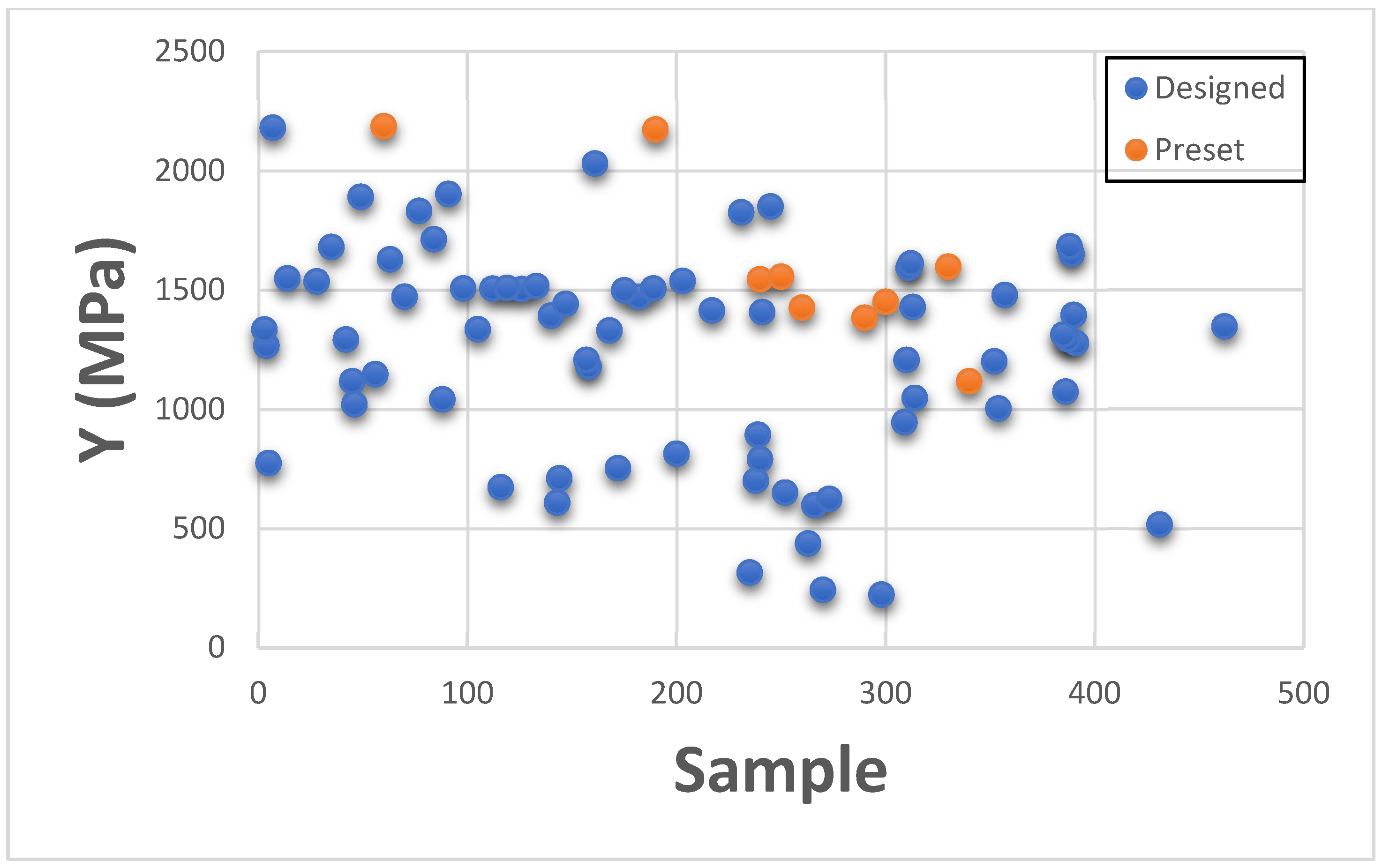
This section presents Young’s Modulus results individually for categorizing with existing GrabCAD™ presets. The tested samples resulted in a wide range of Young’s Modulus values ranging between 0.011 MPa to 2180 MPa. The vast number of runs can be divided into several distinct groups based on the existing tested tensile parts made from preset materials.
The
Figure 16,
Figure 17 and
Figure 18 shown below are the resulting Young’s Modulus values overlapped with the existing preset standard tested parts from Stratasys.
Figure 16 contains the samples which had low ultimate Young’s Modulus value. These are generally softer tissues like Liver tissue, Lung tissue, low to mid rigidity structural heart frame, low to mid stiffness Myocardium, Suturable vessel wall, extensible Valve chordae, soft ligaments, nerves, liver tissue, lung tissue, esophagus, stomach tissue, kidney tissue etc.
Figure 17 includes stiffened valve annulus, pulmonary valve tissue, aortic valve, soft skin tissue, Sclera tissue, temporal muscle tissue, general anatomical dense connective tissues etc. Finally,
Figure 18 consists of rigid tissue materials such as high rigidity structural heart frame, varying porosity bones, skull, vertebrae, tendons, ligaments, rigid skin tissue, long bone tissue, etc.
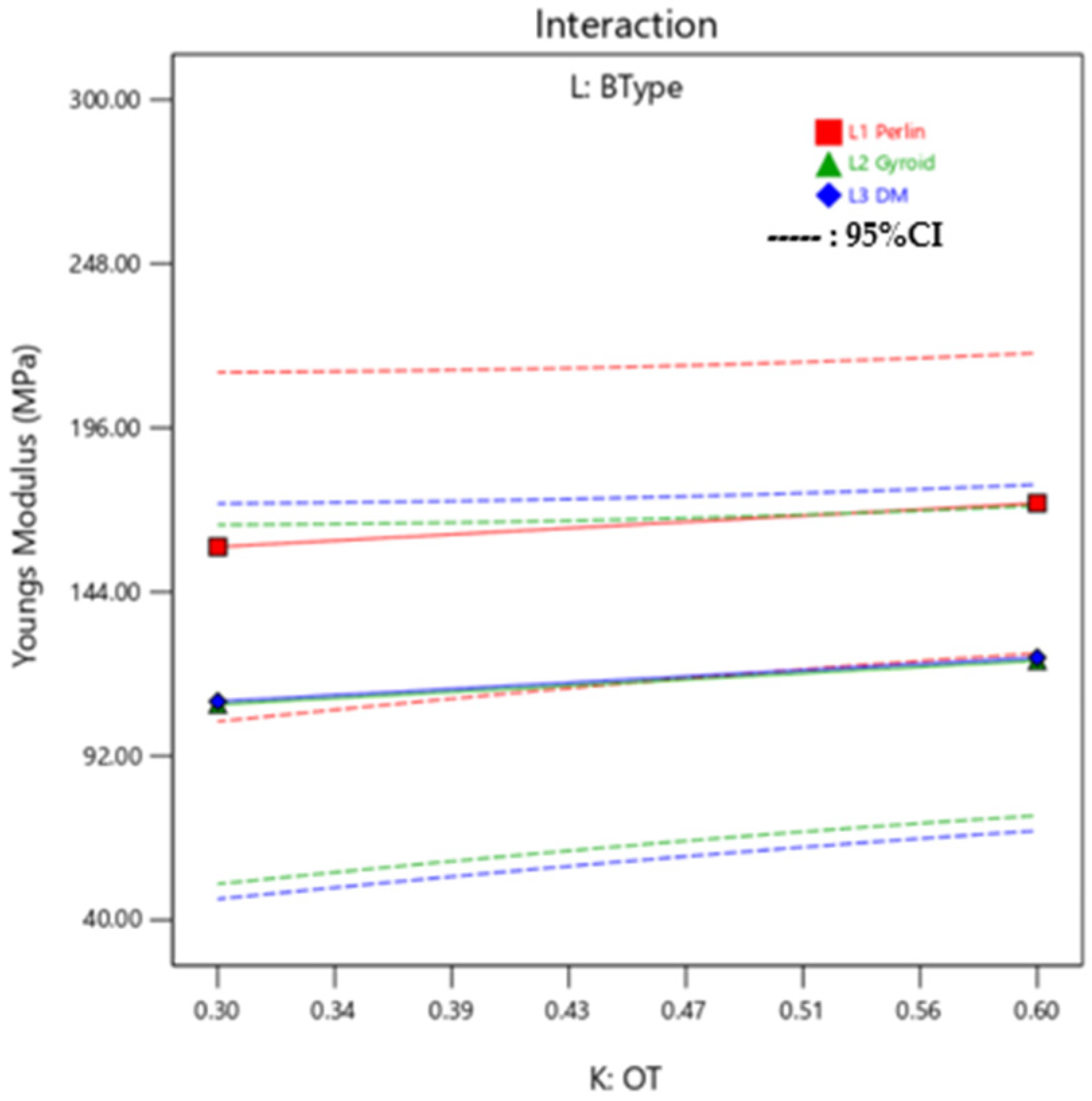
Interaction Plots and ANOVA: Young’s Modulus
The linear model fitting suggested that the ratio of Agilus30Clear™ and VeroPureWhite™ in the Background layer (A and B) and Outer Layer (E and F) have the highest influence on the Young’s Modulus of the sample. As suggested by the ANOVA in
Table 13, all the materials have a substantial impact on the Young’s Modulus, it is due to the collective mixture of the Outer Layer and background layer that makes the influence substantial. Young’s Modulus is also highly affected by the OT (K) as it impacts the dependency between Mixture 1 and Mixture 2. As can be seen in the interaction plot in
Figure 19, there is little variation on response effected by BType.
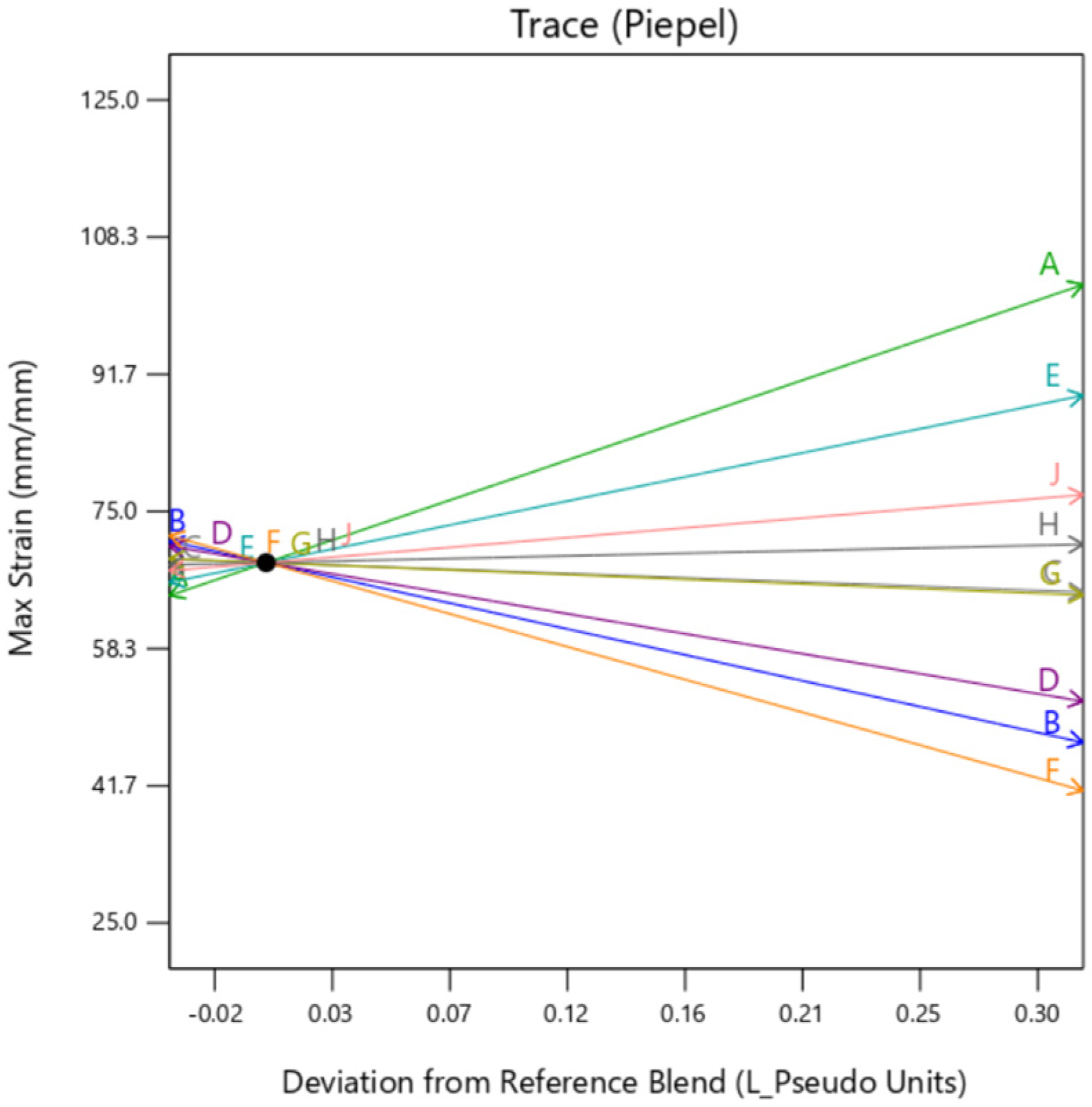
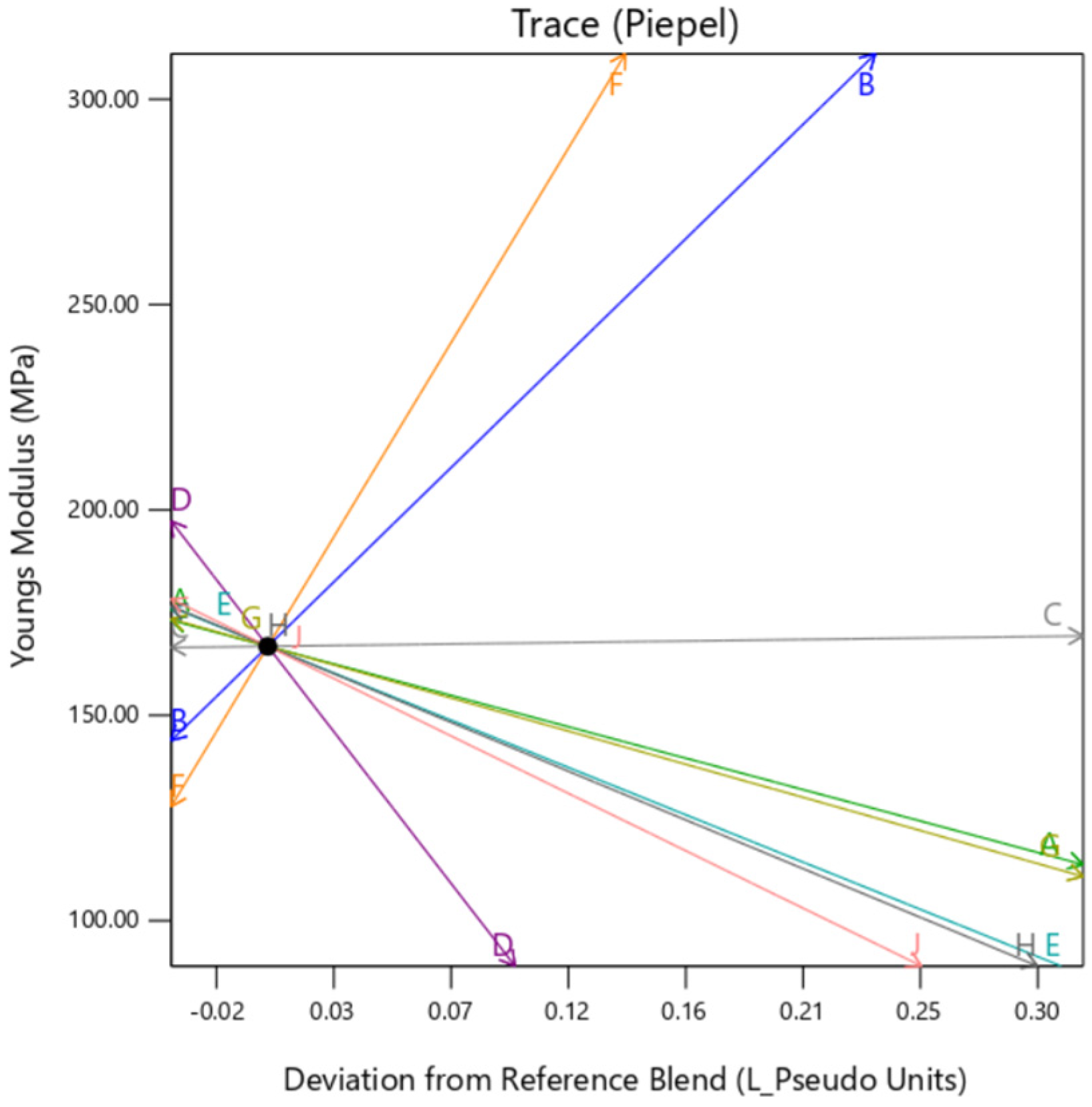
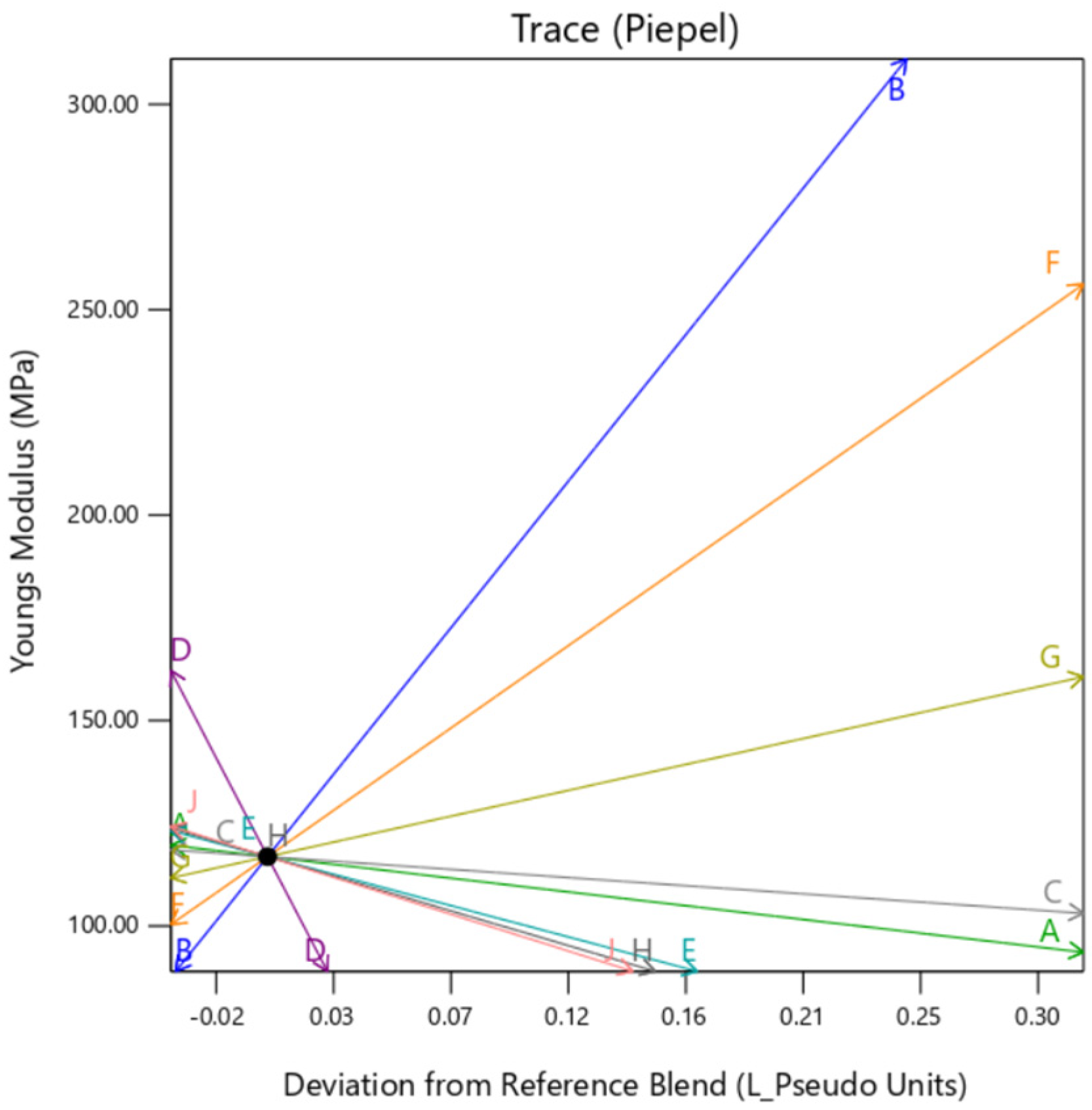
The individual trace plots in
Figure 20,
Figure 21 and
Figure 22 affirm the slight variation caused by
gyroid type background in comparison to
perlin and
DM. This clearly suggests that although the infill for all the samples is 100% across the DOE,
gyroid infill creates some discrepancies.
3.4. Optimization and Prediction
To check the validity of the results, several use cases of the tissues have also been tested. This section overviews the parameter/material settings required to generate liver tissue- and tendon tissue-mimicking materials. These tissue types exist on the opposite spectrum mechanistically as liver tissue is relatively soft whereas tendon tissue is relatively hard. The statistics model uses the tissue mechanical properties of Max Stress, Max Strain and Young’s Modulus as inputs based on the literature. As a result, DesignExpert outputs the recommended parameters to achieve the specific tissue types.
3.4.1. Liver Tissue
According to the literature, the liver tissue’s Max Stress value ranges from 0.011 to 0.2 MPa, the Max Strain values range from 200 to 400, and the Young’s Modulus values range from 0.010 to 0.05 MPa [
21]. These values depend upon the donor’s age, height, weight, health etc. These values are also listed in
Table 14 below.
These values are used in the optimization toolbox of the Design Expert and
Table 15 shows the recommended parameter/material settings, indicated by the red dot on each subplot, that are required to obtain an accurate liver tissue mimicking material. These values can also be tweaked in either direction based on the learnings of the impact of specific parameters on the responses to obtain a particular value between LSL and USL. The model suggests various solutions which depend upon the total range of LSL and USL. Based on the requirement of tissue, any specific parameter can be chosen accordingly.
3.4.2. Tendon Tissue
According to the literature, the tendon tissue’s Max Stress value ranges from 100 to 140 MPa, the Max Strain values range from 0.1 to 0.5, and the Young’s Modulus values range from 1000 to 1500 MPa [
21]. These values depend upon different factors such as a specimen’s age, height, weight, health, etc. These values are also listed in
Table 16 below.
In a similar manner, as explained in the aforementioned section for liver tissue, the model suggests various different settings to achieve the target values between the Max Stress, Max Strain, and Young’s Modulus for tendon tissue as shown in
Table 17. According to the requirement, a specific setting can be chosen and used for making the model. Since the mechanical properties provided in this case are broad, the model generates multiple solutions to satisfy the values within the given range.