Extrusion-Based Bioprinting in a Cost-Effective Bioprinter
Abstract
1. Introduction
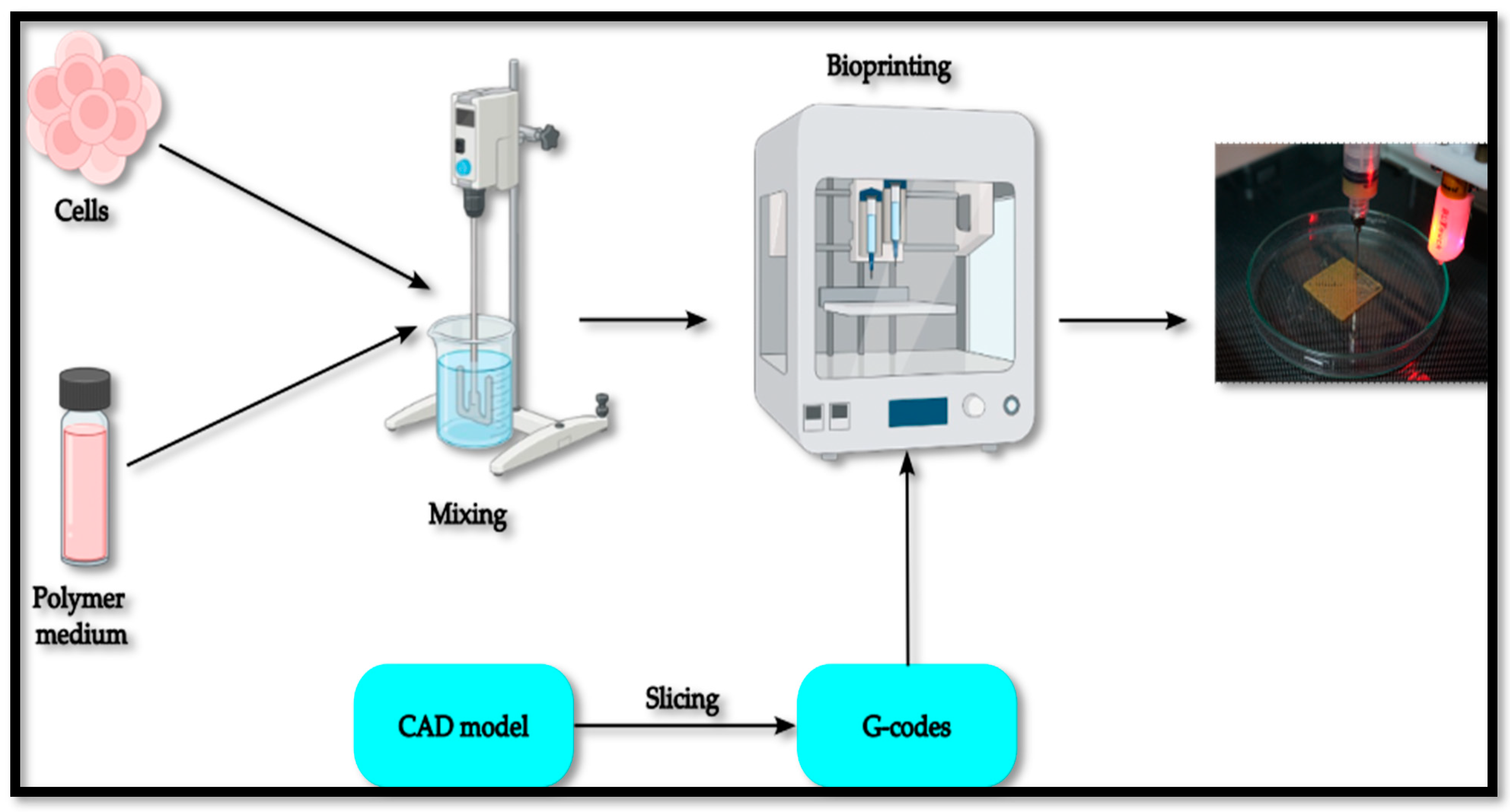
2. Materials and Methods
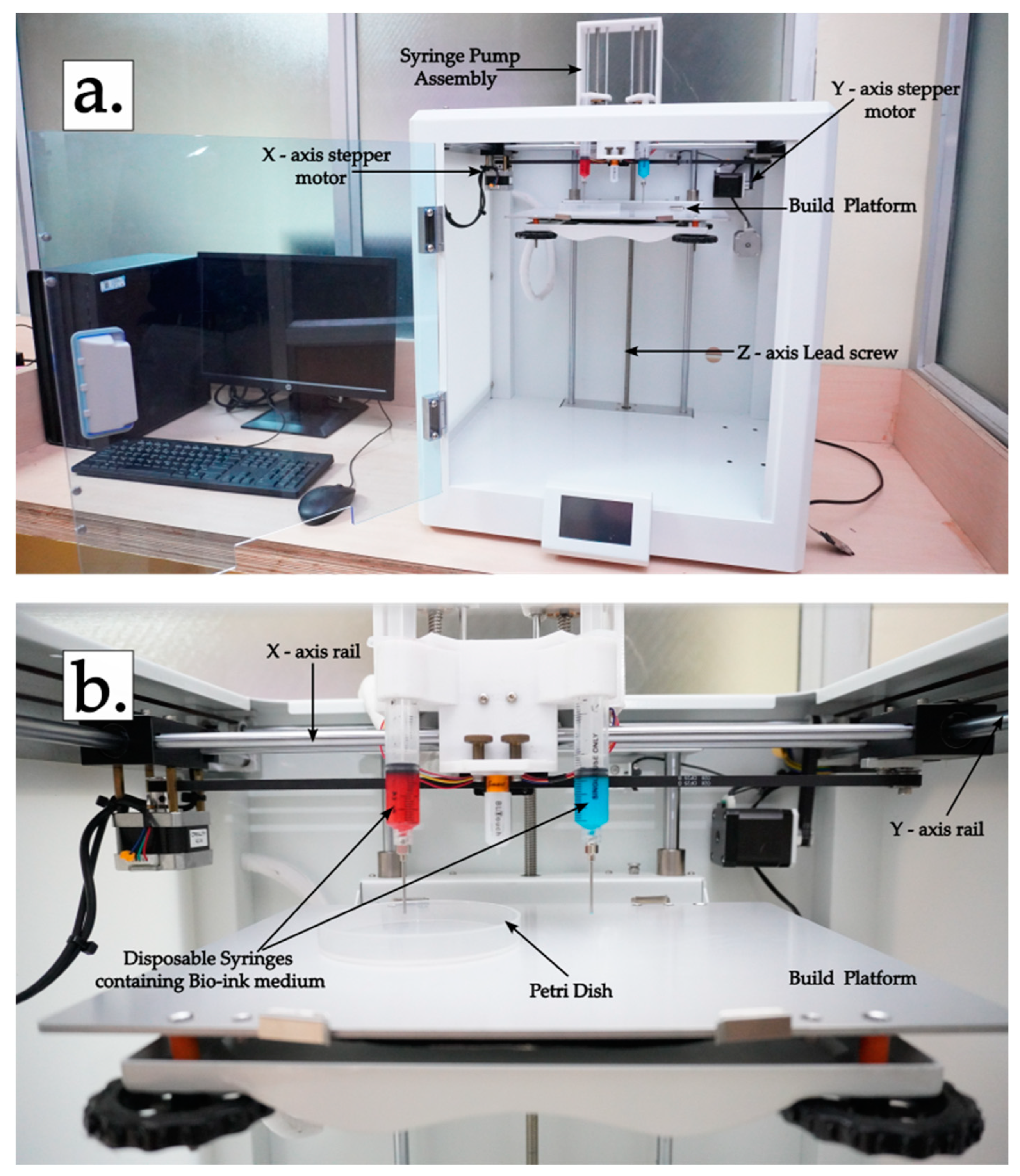
3. Development and Testing of Customized Bioprinter
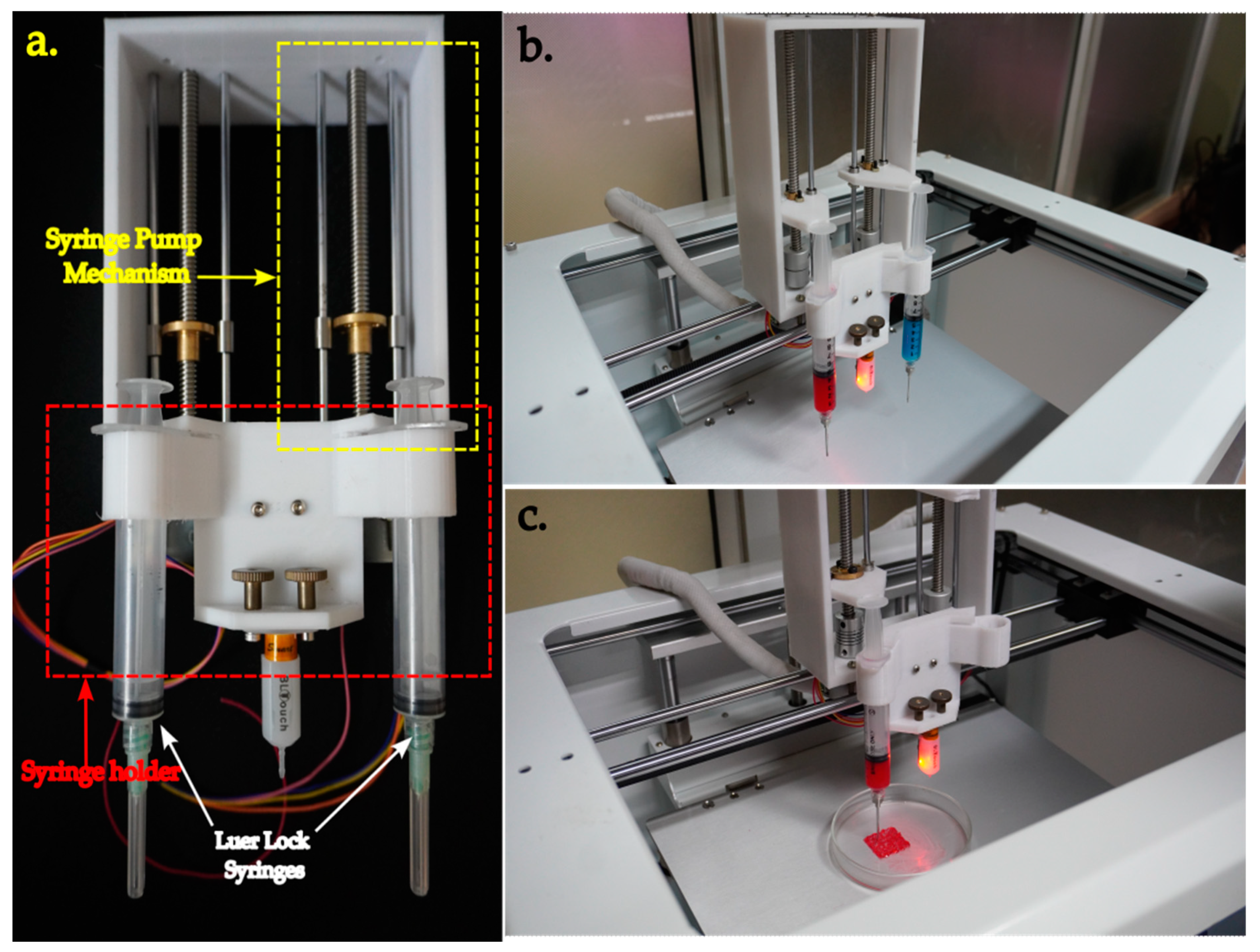
3.1. Replacement of Extruder with the Syringe Pump
3.2. Conversion of the Electronic Mainboard
3.3. Configuration of the Firmware
- #define X_DRIVER_TYPE TMC2209
- #define Y_DRIVER_TYPE TMC2209
- #define Z_DRIVER_TYPE TMC2209
- #define E0_DRIVER_TYPE TMC2209→//Driver for the first extruder
- #define E1_DRIVER_TYPE TMC2209→//Driver for the second extruder
- #define EXTRUDERS 2
- #define PREVENT_COLD_EXTRUSION
- #define EXTRUDE_MINTEMP 20
- #define SENSORLESS_HOMING
- #define X_STALL_SENSITIVITY 100
- #define Y_STALL_SENSITIVITY 100
- #define Z_STALL_SENSITIVITY 100
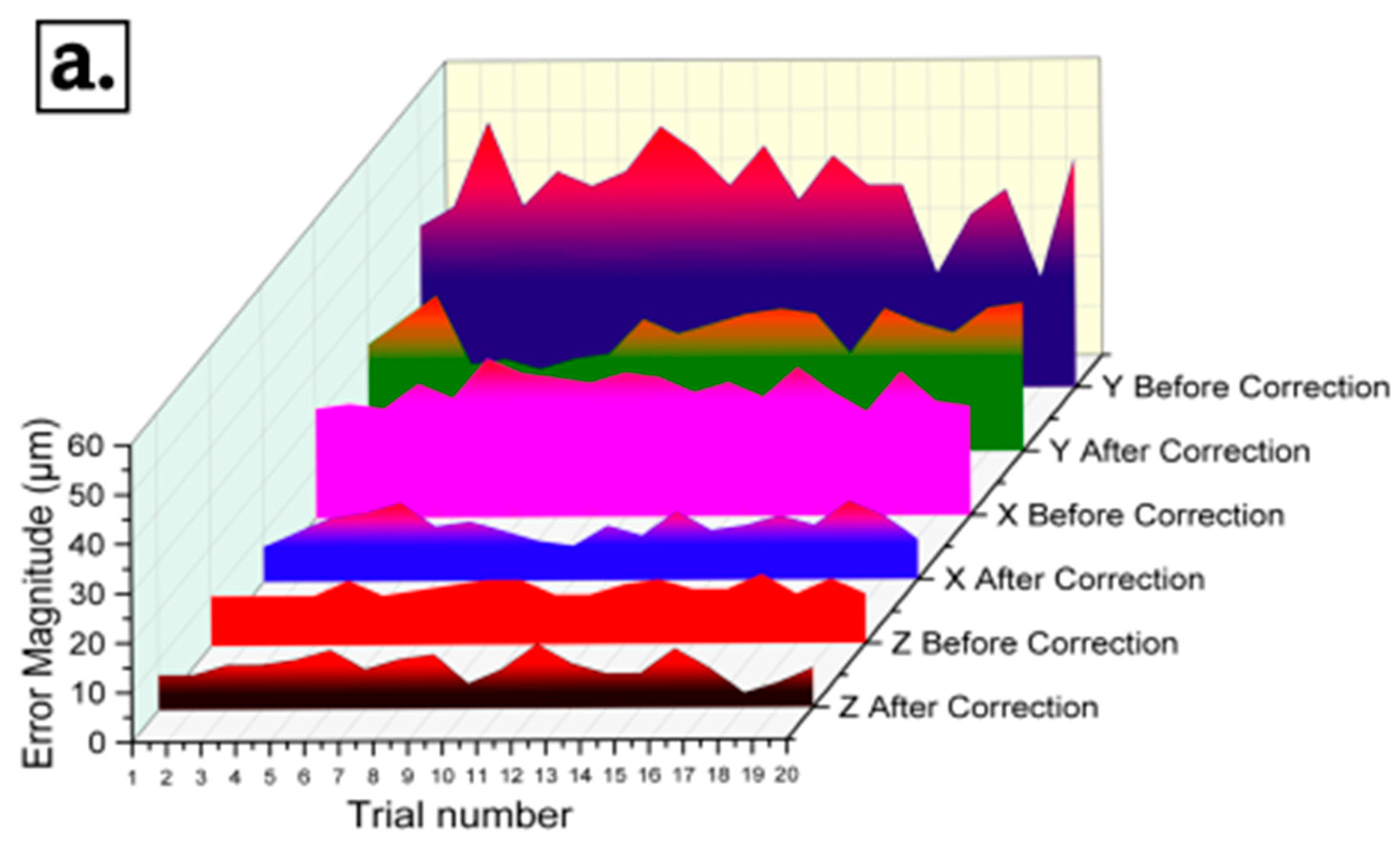
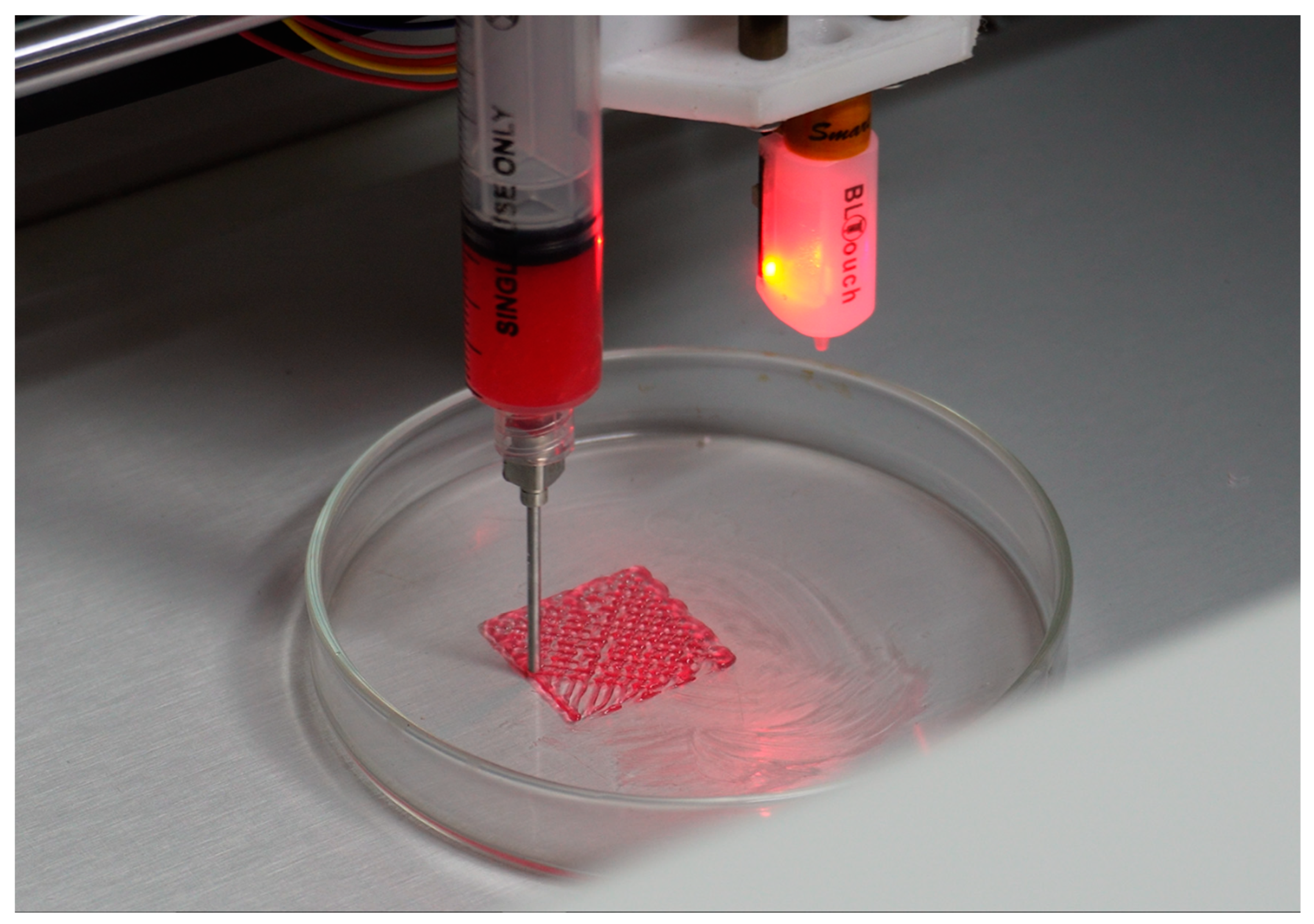
3.4. Testing of the Bioprinter
4. Discussion
5. Conclusions
- With adjustments made, the adapted printer can now print with incredibly high precision—resolutions of less than one micron. This level of accuracy is essential for complex bioprinting applications.
- The developed printer exhibits performance comparable to high-end, more-expensive commercial bioprinters, thanks to modifications made to an easily accessible machine and the use of open-sourced firmware.
- The ability to increase bioprinting’s accessibility is among the work’s most important ramifications. Expensive prices frequently serve as obstacles, but, by making changes to current equipment, we can remove these obstacles and democratize access.
- The use of Venturi-effect-based cell and medium mixing during extrusion may be investigated in the future. This procedure might be able to solve the present problem of pressure-induced cell death during printing, which is common in extrusion systems. Increasing operating efficiency could be achieved by another design enhancement that makes it easier for print heads to load and release quickly. Furthermore, as other study publications have indicated, including the FRESH bioprinting method may greatly enhance the printer’s performance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and Its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and Bioprinting: A Focused Review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Yu, F.; Choudhury, D. Microfluidic Bioprinting for Organ-on-a-Chip Models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Antill-O’Brien, N.; Bourke, J.; O’Connell, C.D. Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models. Materials 2019, 12, 3218. [Google Scholar] [CrossRef]
- Ma, H.; Xing, F.; Yu, P.; Xu, J.; Wu, X.; Luo, R.; Xiang, Z.; Maria Rommens, P.; Duan, X.; Ritz, U. Integrated Design and Fabrication Strategies Based on Bioprinting for Skeletal Muscle Regeneration: Current Status and Future Perspectives. Mater. Des. 2023, 225, 111591. [Google Scholar] [CrossRef]
- Banda Sánchez, C.; Cubo Mateo, N.; Saldaña, L.; Valdivieso, A.; Earl, J.; González Gómez, I.; Rodríguez-Lorenzo, L.M. Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling. Polymers 2023, 15, 3196. [Google Scholar] [CrossRef]
- Moore, C.A.; Siddiqui, Z.; Carney, G.J.; Naaldijk, Y.; Guiro, K.; Ferrer, A.I.; Sherman, L.S.; Guvendiren, M.; Kumar, V.A.; Rameshwar, P. A 3D Bioprinted Material That Recapitulates the Perivascular Bone Marrow Structure for Sustained Hematopoietic and Cancer Models. Polymers 2021, 13, 480. [Google Scholar] [CrossRef]
- Ioannidis, K.; Danalatos, R.I.; Champeris Tsaniras, S.; Kaplani, K.; Lokka, G.; Kanellou, A.; Papachristou, D.J.; Bokias, G.; Lygerou, Z.; Taraviras, S. A Custom Ultra-Low-Cost 3D Bioprinter Supports Cell Growth and Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 1279. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Li, L.; Lu, P.; Liu, Y.; Yang, J.; Li, S. Three-Dimensional-Bioprinted Bioactive Glass/Cellulose Composite Scaffolds with Porous Structure towards Bone Tissue Engineering. Polymers 2023, 15, 2226. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, C.; Soloperto, A.; Ruocco, G.; Cidonio, G. Bioprinting Stem Cells: Building Physiological Tissues One Cell at a Time. Am. J. Physiol. Cell Physiol. 2020, 319, C465–C480. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of Bioink Properties on Printability and Cell Viability for 3D Bioplotting of Embryonic Stem Cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser Bioprinting of Human Induced Pluripotent Stem Cells—The Effect of Printing and Biomaterials on Cell Survival, Pluripotency, and Differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-W.; Lee, K.-W.; Park, J.-H.; Lee, J.; Jung, C.-R.; Yu, J.; Kim, H.-Y.; Kim, D.-H. 3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, Z.; Yao, B.; Enhe, J.; Song, W.; Zhang, C.; Zhu, P.; Huang, S. Extrusion Bioprinting of Cellular Aggregates Improves Mesenchymal Stem Cell Proliferation and Differentiation. Biomater. Adv. 2023, 149, 213369. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S. 3D Bioprinting of Neural Stem Cell-Laden Thermoresponsive Biodegradable Polyurethane Hydrogel and Potential in Central Nervous System Repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Tao, J.; Zhu, S.; Liao, X.; Wang, Y.; Zhou, N.; Li, Z.; Wan, H.; Tang, Y.; Yang, S.; Du, T.; et al. DLP-Based Bioprinting of Void-Forming Hydrogels for Enhanced Stem-Cell-Mediated Bone Regeneration. Mater. Today Bio 2022, 17, 100487. [Google Scholar] [CrossRef]
- Yun, Y.E.; Jung, Y.J.; Choi, Y.J.; Choi, J.S.; Cho, Y.W. Artificial Skin Models for Animal-Free Testing. J. Pharm. Investig. 2018, 48, 215–223. [Google Scholar] [CrossRef]
- Frankowski, J.; Kurzątkowska, M.; Sobczak, M.; Piotrowska, U. Utilization of 3D Bioprinting Technology in Creating Human Tissue and Organoid Models for Preclinical Drug Research—State-of-the-Art. Int. J. Pharm. 2023, 644, 123313. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, J.; Gazdowicz, G.; Kramarz, J.; Łątka, P.; Krzykawski, M.; Miroszewski, A.; Pieczarko, P.; Szczelina, R.; Warchoł, P.; Wróbel, S. A Prototype of a 3D Bioprinter. Solid State Phenom. 2015, 237, 221–226. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.A.; Epstein, C.J.; Schwartz, J.; Krush, A.; Lagalante, D.J.; Mercadante, K.P.; Zeltsman, D.; Smith, L.P.; Grande, D.A. Feasibility of Bioprinting with a Modified Desktop 3D Printer. Tissue Eng. Part C Methods 2016, 22, 1071–1076. [Google Scholar] [CrossRef]
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible Bioprinting: Adaptation of a Low-Cost 3D-Printer for Precise Cell Placement and Stem Cell Differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef]
- Roehm, K.D.; Madihally, S.V. Bioprinted Chitosan-Gelatin Thermosensitive Hydrogels Using an Inexpensive 3D Printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef] [PubMed]
- Bessler, N.; Ogiermann, D.; Buchholz, M.-B.; Santel, A.; Heidenreich, J.; Ahmmed, R.; Zaehres, H.; Brand-Saberi, B. Nydus One Syringe Extruder (NOSE): A Prusa I3 3D Printer Conversion for Bioprinting Applications Utilizing the FRESH-Method. HardwareX 2019, 6, e00069. [Google Scholar] [CrossRef]
- Kahl, M.; Gertig, M.; Hoyer, P.; Friedrich, O.; Gilbert, D.F. Ultra-Low-Cost 3D Bioprinting: Modification and Application of an Off-the-Shelf Desktop 3D-Printer for Biofabrication. Front. Bioeng. Biotechnol. 2019, 7, 184. [Google Scholar] [CrossRef]
- Yenilmez, B.; Temirel, M.; Knowlton, S.; Lepowsky, E.; Tasoglu, S. Development and Characterization of a Low-Cost 3D Bioprinter. Bioprinting 2019, 13, e00044. [Google Scholar] [CrossRef]
- Sanz-Garcia, A.; Sodupe-Ortega, E.; Pernía-Espinoza, A.; Shimizu, T.; Escobedo-Lucea, C. A Versatile Open-Source Printhead for Low-Cost 3D Microextrusion-Based Bioprinting. Polymers 2020, 12, 2346. [Google Scholar] [CrossRef] [PubMed]
- Tashman, J.W.; Shiwarski, D.J.; Feinberg, A.W. Development of a High-Performance Open-Source 3D Bioprinter. Sci. Rep. 2022, 12, 22652. [Google Scholar] [CrossRef] [PubMed]
- Lei, I.M.; Sheng, Y.; Lei, C.L.; Leow, C.; Huang, Y.Y.S. A Hackable, Multi-Functional, and Modular Extrusion 3D Printer for Soft Materials. Sci. Rep. 2022, 12, 12294. [Google Scholar] [CrossRef] [PubMed]
- Breideband, L.; Wächtershäuser, K.N.; Hafa, L.; Wieland, K.; Frangakis, A.S.; Stelzer, E.H.K.; Pampaloni, F. Upgrading a Consumer Stereolithographic 3D Printer to Produce a Physiologically Relevant Model with Human Liver Cancer Organoids. Adv. Mater. Technol. 2022, 7, 2200029. [Google Scholar] [CrossRef]
- D’Atanasio, P.; Fiaschini, N.; Rinaldi, A.; Zambotti, A.; Cantini, L.; Mancuso, M.; Antonelli, F. Design and Implementation of an Accessible 3D Bioprinter: Benchmarking the Performance of a Home-Made Bioprinter against a Professional Bioprinter. Appl. Sci. 2023, 13, 10213. [Google Scholar] [CrossRef]
- Yilmaz, B.; Al Rashid, A.; Mou, Y.A.; Evis, Z.; Koç, M. Bioprinting: A Review of Processes, Materials and Applications. Bioprinting 2021, 23, e00148. [Google Scholar] [CrossRef]
- Betancourt, N.; Chen, X. Review of Extrusion-Based Multi-Material Bioprinting Processes. Bioprinting 2022, 25, e00189. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat Polymerization-Based Bioprinting—Process, Materials, Applications and Regulatory Challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhang, X.; Zhang, Y.; Hou, D. Recent Progress of the Vat Photopolymerization Technique in Tissue Engineering: A Brief Review of Mechanisms, Methods, Materials, and Applications. Polymers 2023, 15, 3940. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A Brief Review of Extrusion-based Tissue Scaffold Bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Yang, B.; Mohabatpour, F.; Betancourt, N.; Sarker, M.; Papagerakis, P.; Chen, X. Process-Induced Cell Damage: Pneumatic versus Screw-Driven Bioprinting. Biofabrication 2020, 12, 025011. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pradas, J.M.; Colina, M.; Serra, P.; Domínguez, J.; Morenza, J.L. Laser-Induced Forward Transfer of Biomolecules. Thin Solid Film. 2004, 453–454, 27–30. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In Situ Printing of Mesenchymal Stromal Cells, by Laser-Assisted Bioprinting, for in Vivo Bone Regeneration Applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [PubMed]
- Wijshoff, H. The Dynamics of the Piezo Inkjet Printhead Operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Kamisuki, S.; Hagata, T.; Tezuka, C.; Nose, Y.; Fujii, M.; Atobe, M. A Low Power, Small, Electrostatically-Driven Commercial Inkjet Head. In Proceedings of the MEMS 98. IEEE. Eleventh Annual International Workshop on Micro Electro Mechanical Systems. An Investigation of Micro Structures, Sensors, Actuators, Machines and Systems, Cat. No.98CH36176, Heidelberg, Germany, 25–29 January 1998; IEEE: Piscataway, NJ, USA, 1998; pp. 63–68. [Google Scholar]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Chan Choi, Y.; Cho, Y.W. Piezoelectric Inkjet Printing of Polymers: Stem Cell Patterning on Polymer Substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kim, G.J.; Kim, H.W.; Lee, J.; Zhang, X.; Kang, M.G.; Seo, J.W.; Cha, J.M.; Park, H.J.; Lee, M.-Y.; et al. Kappa-Carrageenan-Based Dual Crosslinkable Bioink for Extrusion Type Bioprinting. Polymers 2020, 12, 2377. [Google Scholar] [CrossRef]
- Khoshnood, N.; Zamanian, A. A Comprehensive Review on Scaffold-Free Bioinks for Bioprinting. Bioprinting 2020, 19, e00088. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to Assess Printability of Bioinks for Extrusion-Based Bioprinting and Evaluation of Rheological Properties Governing Bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Pepelnjak, T.; Stojšić, J.; Sevšek, L.; Movrin, D.; Milutinović, M. Influence of Process Parameters on the Characteristics of Additively Manufactured Parts Made from Advanced Biopolymers. Polymers 2023, 15, 716. [Google Scholar] [CrossRef] [PubMed]
- Suntornnond, R.; Tan, E.; An, J.; Chua, C. A Mathematical Model on the Resolution of Extrusion Bioprinting for the Development of New Bioinks. Materials 2016, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2021, 21, e2000280. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Adv. Healthc. Mater. 2020, 9, e2000156. [Google Scholar] [CrossRef] [PubMed]
- Sameni, F.; Ozkan, B.; Zarezadeh, H.; Karmel, S.; Engstrøm, D.S.; Sabet, E. Hot Lithography Vat Photopolymerisation 3D Printing: Vat Temperature vs. Mixture Design. Polymers 2022, 14, 2988. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink Properties before, during and after 3D Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Mandujano-Tinoco, E.A.; Krötzsch, E. Tailoring of the Rheological Properties of Bioinks to Improve Bioprinting and Bioassembly for Tissue Replacement. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129782. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Damiati, L.A.; El-Yaagoubi, M.; Damiati, S.A.; Kodzius, R.; Sefat, F.; Damiati, S. Role of Polymers in Microfluidic Devices. Polymers 2022, 14, 5132. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, e1902026. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of Bioprinter Technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Tröndle, K.; Zengerle, R.; Zimmermann, S.; Koltay, P. Open-Source Hybrid 3D-Bioprinter for Simultaneous Printing of Thermoplastics and Hydrogels. HardwareX 2021, 10, e00230. [Google Scholar] [CrossRef]
- Lobo, D.A.; Ginestra, P. Cell Bioprinting: The 3D-BioplotterTM Case. Materials 2019, 12, 4005. [Google Scholar] [CrossRef]
- Krige, A.; Haluška, J.; Rova, U.; Christakopoulos, P. Design and Implementation of a Low Cost Bio-Printer Modification, Allowing for Switching between Plastic and Gel Extrusion. HardwareX 2021, 9, e00186. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, H.; Lewinski, N. Key Parameters and Applications of Extrusion-Based Bioprinting. Bioprinting 2021, 23, e00156. [Google Scholar] [CrossRef]




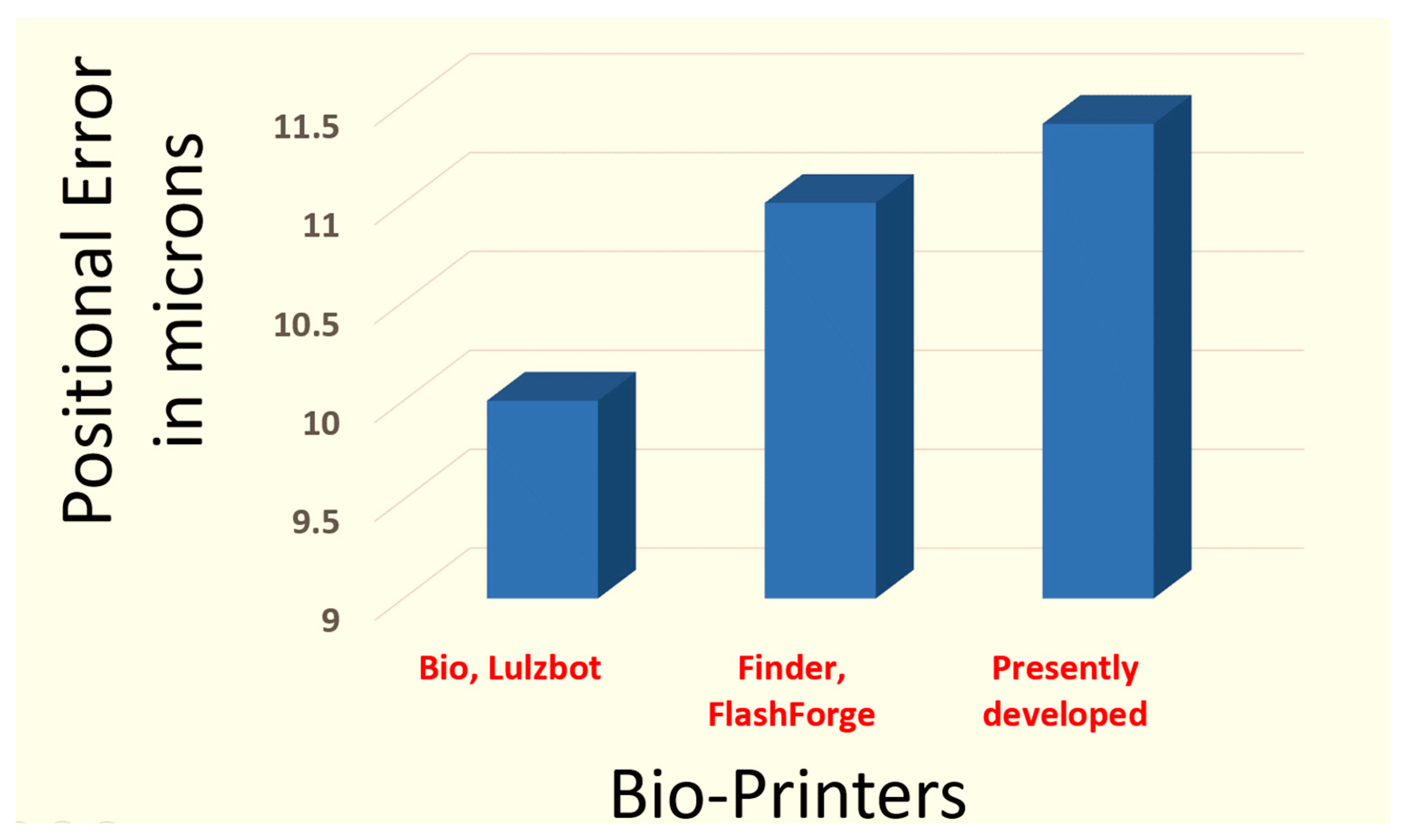

| Printer | Manufacturer | Resolution in the X and Y Direction (μm) | Resolution in the Z Direction (μm) |
|---|---|---|---|
| 3D Bioplotter | EnvisionTEC | 1 | 1 |
| Allevi 2 | Allevi 3D | 5 | 1 |
| Bio | Lulzbot | 10 | 5 |
| BioX | Cellink | 1 | 1 |
| Finder | FlashForge | 11 | 2.5 |
| Customized Bioprinter | - | 0.78 | 0.08 |
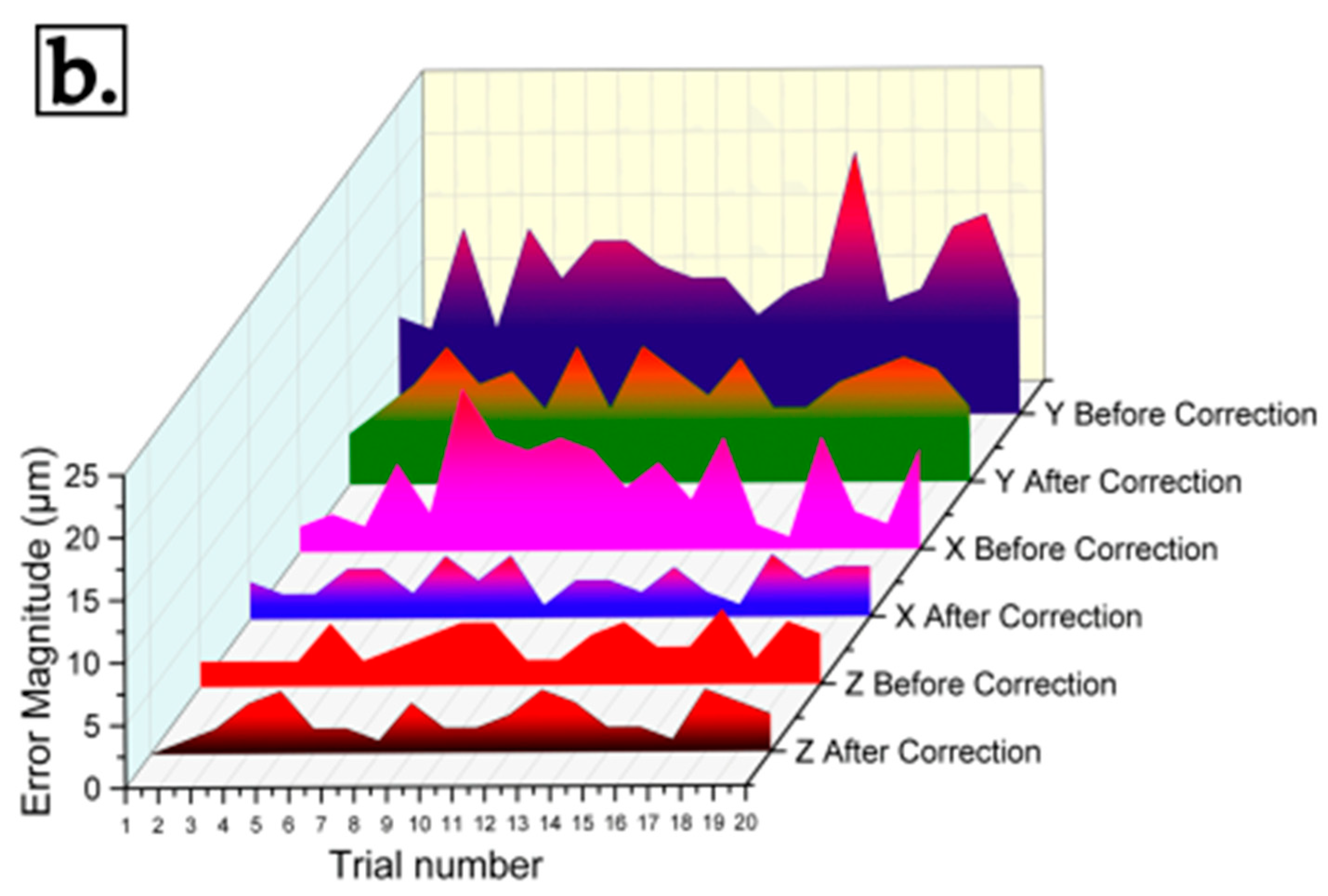
| Axis | Positional Error (μm) | Repeatability (μm) | ||
|---|---|---|---|---|
| Before Correction | After Correction | Before Correction | After Correction | |
| X | 26.4 | 11.2 | 5.7 | 3.1 |
| Y | 40.2 | 24.7 | 11.7 | 8 |
| Z | 11.0 | 8.4 | 3.4 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmaraj, J.J.J.; Navasingh, R.J.H.; Krolczyk, G.; Pitchumani, S.V. Extrusion-Based Bioprinting in a Cost-Effective Bioprinter. Machines 2024, 12, 518. https://doi.org/10.3390/machines12080518
Dharmaraj JJJ, Navasingh RJH, Krolczyk G, Pitchumani SV. Extrusion-Based Bioprinting in a Cost-Effective Bioprinter. Machines. 2024; 12(8):518. https://doi.org/10.3390/machines12080518
Chicago/Turabian StyleDharmaraj, Jones Joseph Jebaraj, Rajesh Jesudoss Hynes Navasingh, Grzegorz Krolczyk, and Shenbaga Velu Pitchumani. 2024. "Extrusion-Based Bioprinting in a Cost-Effective Bioprinter" Machines 12, no. 8: 518. https://doi.org/10.3390/machines12080518
APA StyleDharmaraj, J. J. J., Navasingh, R. J. H., Krolczyk, G., & Pitchumani, S. V. (2024). Extrusion-Based Bioprinting in a Cost-Effective Bioprinter. Machines, 12(8), 518. https://doi.org/10.3390/machines12080518








