Abstract
The use of alternative fuels remains an important factor in solving the problem of reducing harmful substances caused by vehicles and decarbonising transport. It is also important to ensure the energy efficiency of vehicle power plants when using different fuels at a sufficient level. The article presents the results of theoretical and experimental studies of the conversion of diesel engine to alternative fuels with hydrogen admixtures. Methanol is considered as an alternative fuel which is a cheaper alternative to commercial diesel fuel. The chemical essence of improving the calorific value of alternative methanol fuel was investigated. Studies showed that the energy effect of burning an alternative mixture with hydrogen additives exceeds the effect of burning the same amount of methanol fuel. The increase in combustion energy and engine power is achieved as a result of heat from efficient use of the engine exhaust gases and chemical conversion of methanol. An experimental installation was created to study the work of a converted diesel engine on hydrogen–methanol mixtures and thermochemical regeneration processes. Experimental studies of the energy and environmental parameters of diesel engine converted to work on an alternative fuel with hydrogen admixtures have shown that engine power increases by 10–14% and emissions of harmful substances decrease.
1. Introduction
As the global community’s concern about climate change and environmental sustainability grows, so does the demand for cleaner and greener transportation options. The most energy-intensive and environmentally dangerous element of a vehicle is its power plant. One of the ways to improve the energy efficiency and environmental performance of a vehicle power plant is the use of alternative types of fuel. Fuels such as hydrogen, natural gas, biofuels, ethanol, dimethyl ether, propane–butane mixtures, and electricity can significantly reduce greenhouse gas and air pollutants emissions compared to conventional fossil fuels. Alternative fuels can diversify the energy sources used for transportation, reducing dependence on imported oil and increasing energy security. This is especially relevant for countries that are largely dependent on oil imports for their transportation needs. The authors [1] investigated methods to evaluate the efficiency of vehicles with traditional and alternative power plants to ensure a rational choice of transport technology under given conditions. Despite the fact that there are problems, for example, the development of infrastructure for the production, distribution and refuelling of hydrogen, the authors proved that its application is a promising option for sustainable transport.
Hydrogen can be used as a main fuel and as an additive in gasoline, diesel, and gas engines and can form mixtures in different ways. One common way is to use a hydrogen generator. The engine will burn less fuel by adding hydrogen simultaneously, releasing fewer toxic components. A smaller amount of the toxic components is emitted with more complete fuel combustion [2]. The incomplete combustion of petroleum fuels is the main cause of HC and CO emissions in diesel and petrol engines.
The use of hydrogen in vehicle power plants as an additive to the main motor fuel is currently subject to a great deal of discussions. This is due to the different methods to obtain such fuel and its physical and chemical properties [3]. The fact is that the addition of hydrogen is not only able to replace the energy resource of the decreasing part of diesel or petrol fuel; the action of hydrogen is more interesting, as hydrogen has a high diffusion rate and therefore can form a homogeneous mixture in the combustion chambers of the engine in a shorter period [4].
It should also be noted [5] that the flame expansion rate (burning speed) of hydrogen is significantly higher than the flame expansion rate of similar mixtures based on diesel or petrol fuel. Based on the results obtained in [5], the characteristics of the studied fuels are presented (Table 1).

Table 1.
Fuel characteristics.
The combustion time of the fuel mixture is significantly reduced with minimal additions of hydrogen [6]. This is because hydrogen effectively ignites the fuel mixture in the entire cylinder volume, mixing with the entering their mixture. This is because hydrogen molecules can act as initiating centres during the combustion of hydrocarbon fuels [7]. When the engine is running, it means ignition can be delayed by adding hydrogen and the amount of the main fuel to be used can be reduced.
Hydrogen is the lightest gas; for example, it is 14.5 times lighter than air. If the mass of the molecules is less, it will have a higher speed at the same temperature. Thus, hydrogen molecules move faster than the molecules of any other gas and therefore can transfer heat from one body to another faster [8]. It follows from this that hydrogen has the highest thermal conductivity among gases and, for example, its thermal conductivity is approximately seven times higher than the thermal conductivity of atmospheric air.
Considering that the purpose of using hydrogen in road transport is to improve the economic and environmental performance of cars, the issues of the physical and chemical properties of hydrogen, and the methods for choosing economically profitable and environmentally safe production and storage methods of hydrogen will be extremely important [9].
The following methods of hydrogen storage are fundamentally possible at present [10]:
- In a gaseous state under pressure in containers;
- In the solid-phase bound state in metal hydrides;
- In a chemically bound state in liquid media;
- In a chemically liquid state in cryogenic tanks.
The following ways have been the most adopted [11]: in a liquefied state in cryogenic tanks, in the compressed gas in high-pressure cylinders, and in a bound state in metal hydride batteries. Based on the results [11], a unified approach to choosing an acceptable method of storing hydrogen in cars is determined (Table 2).

Table 2.
Modern ways of hydrogen storage in cars.
Despite the obvious advantages of hybrid tanks over metal cylinders, the major disadvantage of hybrid tanks is the significant cost of materials for the manufacture of such a tank and their still quite significant weight [12]. In addition, the slow release of hydrogen from the hydride presents the problem of obtaining the required amount of hydrogen at various engine operating modes. Another big problem for all methods of storing hydrogen on a vehicle board is the small number of hydrogen refuelling stations. However, the creation of a developed hydrogen infrastructure requires a huge amount of financial cost and is a complex technical task. Therefore, a promising direction is the development and use of technical solutions to produce hydrogen on a carboard.
Hydrogen practically does not occur in nature in a pure form and must be extracted from other compounds using various methods [13]. A huge number of substances containing hydrogen and a variety of methods for producing hydrogen are the two main advantages of hydrogen energy.
The most common ways to produce hydrogen are the following [14]:
- Electrolysis of water;
- Cracking of methane (catalytic decomposition);
- Auto thermal reforming (oxygen or air conversion);
- Steam conversion.
Steam methane conversion and its homologues are the main industrial method of producing hydrogen. The process is as follows: steam reacts with natural gas at low pressures and high temperatures and in the presence of a catalyst. Depending on further use, hydrogen is produced under different pressures: from 1.0 to 4.2 MPa. Using steam conversion, hydrogen can be produced in varying purities, typically 95–98%. The raw material (light oil fractions or natural gas) is heated to 350–400 °C in a heat exchanger or convection oven and enters the desulphurisation apparatus. After the stages of low-temperature and high-temperature conversion, the gas is supplied for the adsorption of CO2 and CO. The result is hydrogen of up to 98.5% purity containing 1–5% methane [15].
In case it is necessary to obtain especially pure hydrogen, an adsorption gas separation unit is used. The gas mixture containing CH4, CO2, H2O, and CO is cooled to remove water and sent to adsorption devices with zeolites. All impurities are adsorbed in one step at ambient temperature. The pressure of the produced hydrogen is 1.5–2.0 MPa. The result is hydrogen with a purity of 99.99% [16].
Currently, approximately half of the global hydrogen market is produced using this method. The inconvenience of using this method on a car is that the reactor to produce hydrogen is large and cannot be placed on a transport base [17]. But this method cannot be completely excluded from consideration for cars because in the future it is possible to develop a more compact reactor.
During autothermal reforming, a mixture of natural gas, steam, and oxygen is supplied to the reaction catalytic volume in proportions in which one part of the methane reacts with water vapor, and the other burns in oxygen, producing hydrogen and carbon oxides. The partial combustion of natural gas provides the high temperature required for the shift reaction [18].
Natural gas is heated to temperatures above 1000 °C in the process of methane cracking at which the process of decomposition of the methane molecule into carbon and hydrogen occurs. The process has a 2-fold lower yield of hydrogen from methane but with a high degree of methane decomposition; it allows hydrogen to be isolated at a lower cost. This process is promising because the reaction produces two valuable products: carbon and hydrogen. Unlike the oxidative transformations of methane, the resulting hydrogen does not require purification from impurities, in particular CO2 and CO [19].
The production of hydrogen via the electrolysis of water is the simplest method. Two electrodes are placed in water and a voltage is applied to them. Since pure water is a poor conductor of an electric current, electrolytes (potassium hydroxide KOH, etc.) are added to it. Oxygen is released at the anode during electrolysis and an equivalent amount of hydrogen is released at the cathode. The hydrogen produced using electrolysis is very pure, except for the admixture of small amounts of oxygen, which, if necessary, can be easily removed by passing the hydrogen over suitable catalysts, for example over palladium. However, in installations operating on this principle, 4–5 kWh of electricity is required to produce one cubic metre of hydrogen, which is quite expensive and difficult to provide on a carboard [20].
A significant number of automobiles, especially commercial ones, use diesel internal combustion engines. This requires the high cost of expensive diesel fuel which is characterised by a very significant toxicity of exhaust gases, in particular nitrogen oxides. It requires the use of increasingly complex and expensive equipment to reduce the toxicity of exhaust gases [21]. Modern diesel engines have practically exhausted their potential for improving environmental performance, as the cost of onboard automotive equipment to neutralise toxic components becomes unreasonably expensive.
In this regard, a significant number of car manufacturers have announced their plans to stop the production of cars with engines running on diesel fuel already in the coming years. Therefore, it is obvious that it is necessary to find ways to transfer piston engines from diesel fuel to other alternative types of fuel, the main characteristics of which should be better environmental characteristics and a cost that will be lower than traditional petroleum fuels [22]. It should also be noted that diesel engines have a large resource before major repairs. Therefore, even after the production of new cars with diesel engines is stopped, already-produced cars with diesel engines will continue to drive for a long time. Therefore, the problem of converting already-manufactured cars with diesel engines to other alternative fuel types of fuel will be an urgent issue.
The conversion of diesel-powered vehicles to gas fuels deserves special attention. Technologies for the conversion of diesel engines to methane or liquefied petroleum gas (propane–butane) are known [23]. However, these gases are a non-renewable resource that can be used in a limited way. This applies especially to such a resource as liquefied petroleum gas.
Several studies have been conducted on the benefits of introducing hydrogen in small quantities to diesel engines. In these studies, diesel fuel was injected as a pilot fuel in the usual way [24]. The results showed a decrease in toxic components in exhaust gases, with carbon emissions decreasing from 34 to 31.5 ppm and CO emissions decreasing from 0.09 to 0.045%. Furthermore, there was an increase in engine power by 29%. Another article [25] presents experimental data on the use of hydrogen as a fuel for a diesel engine. The study used different amounts of hydrogen supply in the range of 18–34% to diesel fuel. The results showed that CO2 emissions were reduced by up to 20%, NOx emissions were reduced by up to 50%, and smoke was reduced by up to 74% compared to monodiesel fuel. The higher calorific value and burning rate of hydrogen also provided an increase in power up to 27%.
The analysis shows that hydrogen and its mixtures are a promising type of automotive fuel but the methods of storing hydrogen or obtaining it on board the car are currently quite expensive and technically difficult. Therefore, the purpose of the proposed work is theoretical and experimental research on the conversion of diesel engines to alternative mixed hydrogen gas fuels is needed.
The tasks of this work are as follows:
- To theoretically investigate the possibilities of converting diesel engines to alternative mixed hydrogen gas fuels based on methanol with hydrogen generation on the carboard;
- To experimentally investigate the main energy and environmental characteristics of the D21 diesel engine converted to operate on alternative mixed hydrogen gas fuels based on methanol.
2. Materials and Methods
2.1. Thermochemical Generation in Automobile On-Board Hydrogen Reactors
In automotive transport, the research and use of alternative mixed hydrogen gas fuels with onboard hydrogen generation started relatively recently. However, some experience of such developments and research is already being gained [26,27]. In general, both liquid and gaseous hydrocarbons can be used to obtain hydrogen. Hydrogen gas can be obtained from crude oil and any petroleum products. From the point of view of providing better mass-dimensional characteristics of the car, liquids as a carrier of hydrogen are better. And, for example, for petrol the mass indicator of hydrogen accumulation is 14–15% [28]. But heavy petroleum products, for example, diesel fuel, have relatively little hydrogen and also have coking properties and high sulphur content [29]. This greatly complicates the production of hydrogen from diesel fuels. Significant limitations are imposed by the high reaction temperature (more than 1200 °C without the presence of catalysts). Therefore, it is better to use light gasoline fractions as raw materials in hydrogen production generators.
Several serious problems arise when creating onboard hydrogen generation systems, which hinders their implementation in automotive transport. The high temperature during the conversion of liquid hydrocarbons makes it necessary to find and provide additional thermal energy costs for onboard hydrogen generation systems. In addition, the presence of sulphur in diesel fuel almost excludes the possibility of using catalysts, which creates additional difficulties in the application of hydrogen generation systems [30].
The indicated difficulties associated with the organisation of the process of conversion of traditional diesel fuels make it necessary to find other sources of energy and raw materials for the production of mixed hydrogen gas fuels. Hydrocarbon compounds have a lower dissociation temperature and a simpler molecular structure in comparison to diesel fuels. At the same time, compounds with thermal effects and dissociation temperatures in endothermic decomposition reactions, which are commensurate with the energies and temperatures of exhaust gases at the exit from the automobile engine, are logical and appropriate for use in diesel engines [31].
In this case, there is an opportunity to organise conversion processes using the thermal energy of the exhaust gases of the automobile engine without involving an external energy source. These properties are characteristic of alcohols, one of which is methyl alcohol (methanol) [32]. Methyl alcohol is used in vehicle power plants in some countries as a partial replacement for traditional petroleum fuels. The use of liquid methyl alcohol as a hydrogen carrier in automobiles, according to the authors, is the most expedient because of the high content of hydrogen in methyl alcohol.
The molecular part of hydrogen in methanol is about 67%. The mass content of hydrogen in a unit volume of methyl alcohol is 1.5 times higher than the specific weight of liquid cryogenic hydrogen. The mass index of hydrogen in methanol is about 8.5 kg/kg. In other words, 8.5 kg of methyl alcohol contains 1 kg of hydrogen [33]. It is also important for the operating conditions of automotive equipment that the accumulation of hydrogen in a chemically bound state in liquid methanol provides higher safety in emergencies.
It is important to point out that the complete conversion of methyl alcohol can be achieved at conversion temperatures of 300 °C (Table 3) [34].

Table 3.
Conversion temperature of the fuels that can be used in engines.
This establishes the values of the minimum possible temperatures of exhaust gases (engine coolant), below which the fuel conversion process is impossible and there emerges a need to place the reactor near the exhaust manifold of the converted engine.
The conversion of methanol into a hydrogen mixture is possible in any type of automotive internal combustion engine using a small hydrogen reactor. The highest output of hydrogen in the fuel mixture is achieved at certain temperature regimes of the reactor and the engine and a certain energy balance between the hydrogen reactor and the exhaust system. The need to supply the heat of the exhaust gases intensively to the reaction zone of the hydrogen generator is due to the high endothermic effect of converting methanol into a hydrogen mixture [26].
Therefore, for the effective implementation of the conversion process, the heat carrier (waste gases) must have the necessary temperatures and thermal power levels. In addition to direct heat consumption for methanol conversion, additional thermal power is also required for the organisation of other stages of the hydrogen generator: for preheating methyl alcohol to its boiling point; for evaporation; and for raising the temperature of the steam to the level of the operating temperature of the hydrogen generator. The total cost of warm energy for the organisation of the completed conversion process of 1 litre of methyl alcohol reaches approximately 5.6 MJ.
An important feature of the onboard synthesis of a hydrogen mixture from methyl alcohol is a significant energy-saving effect. The essence of increasing the energy of the original methyl fuel is explained using the basic provisions of thermochemistry and thermodynamics. As a result of the endothermic transformation in the hydrogen reactor, the chemical energy of the methyl product is transformed about the original product into energy equal to the amount of energy obtained from the engine’s exhaust gases. The method of utilisation of thermal energy of engine exhaust gases is called thermochemical heat recovery. It is based on the thermochemical transformation of the energy of the original methyl fuel to a higher energy level of the hydrogen mixture using the heat of the exhaust gases [35,36].
This effect can be shown based on studying the effects of thermochemistry and thermodynamics from the combustion of methanol (CH3OH), which can be achieved using two fundamentally different methods.
For a correct comparison, the final and primary states of the system for both methods of methyl alcohol combustion coincide with initial—2 kmol of methyl alcohol, 3 kmol of oxygen; and final—4 kmol of water and 2 kmol of carbon dioxide. According to the first method without a hydrogen reactor, a portion of methyl alcohol is burnt in the combustion chamber of the engine [26]:
where QM is the thermal exothermic effect from the combustion of air–methanol fuel, QM = 1258.9 MJ.
As a result of (1) through the first method, 6 kmol of oxidation products are formed. Therefore, in the two-stage method of air–methanol fuel conversion, alcohol is decomposed at first [37]:
It produces 4 kmol H2 and 2 kmol CO with heat endothermic conversion QC.
Then, the 6 kmol obtained by the (2) of conversion products of air–methanol fuel is burned in the air:
Total thermal effect:
Q∑ = 963 + 565.2 = 1528.2 MJ.
Thermal effects for (3)–(4) are given by the data [38]. Then, according to Hess’s law, the thermal effects for different routes of air–methanol fuel oxidation must coincide.
1258.9 MJ = 1528.2 MJ − QC.
Therefore, the endothermic effect of the conversion (air–methanol fuel dissociation reaction) will be QC = 269.3 MJ/2 kmol CH3OH = 134. 65 MJ/kmol CH3OH.
Therefore, the calculation showed that the heat of combustion when creating a hydrogen mixture is greater in comparison with the heat of combustion of ordinary methanol of the same amount by 134.660 kJ per 1 kmol of CH3OH or 21.4%. That is, the synthesis of hydrogen on board the car in a hydrogen reactor and the subsequent use of the hydrogen mixture can significantly increase the efficiency of fuel combustion in the engine.
For methanol, the stoichiometric air/fuel ratio is 6.45/1. The obtained hydrogen mixture is a product of methanol conversion. The oxidation of two molecules of methanol requires three molecules of oxygen. When two molecules of methanol are converted, four molecules of hydrogen and two molecules of carbon monoxide are obtained. The oxidation of two molecules of carbon monoxide requires one molecule of oxygen, and the oxidation of four molecules of hydrogen requires two molecules of oxygen. That is, the same three molecules of oxygen are together, so it was decided to leave the same stoichiometric air/fuel ratio of 6.45/1 for the convertible gas mixture.
The theoretical basis of the use of automotive on-board hydrogen reactors in modern research remains poorly studied. Therefore, they are of important interest in assessing the potential for increasing the energy efficiency of engines on alternative fuels, in particular for available diesel engines that will be remade to run on hydrogen mixtures.
The implementation of the specified method of heat utilisation for hydrogen reactors of spark-ignition engines is possible if the fuel for the hydrogen reactor is compounded with a low temperature of conversion reactions (various ethers, alcohols, and other compounds). An important possibility is the possibility of implementing automotive on-board hydrogen reactors in the power systems of diesel engines that have been converted to work on gas [38].
2.2. Experimental On-Board Hydrogen Reactors of Diesel Engine
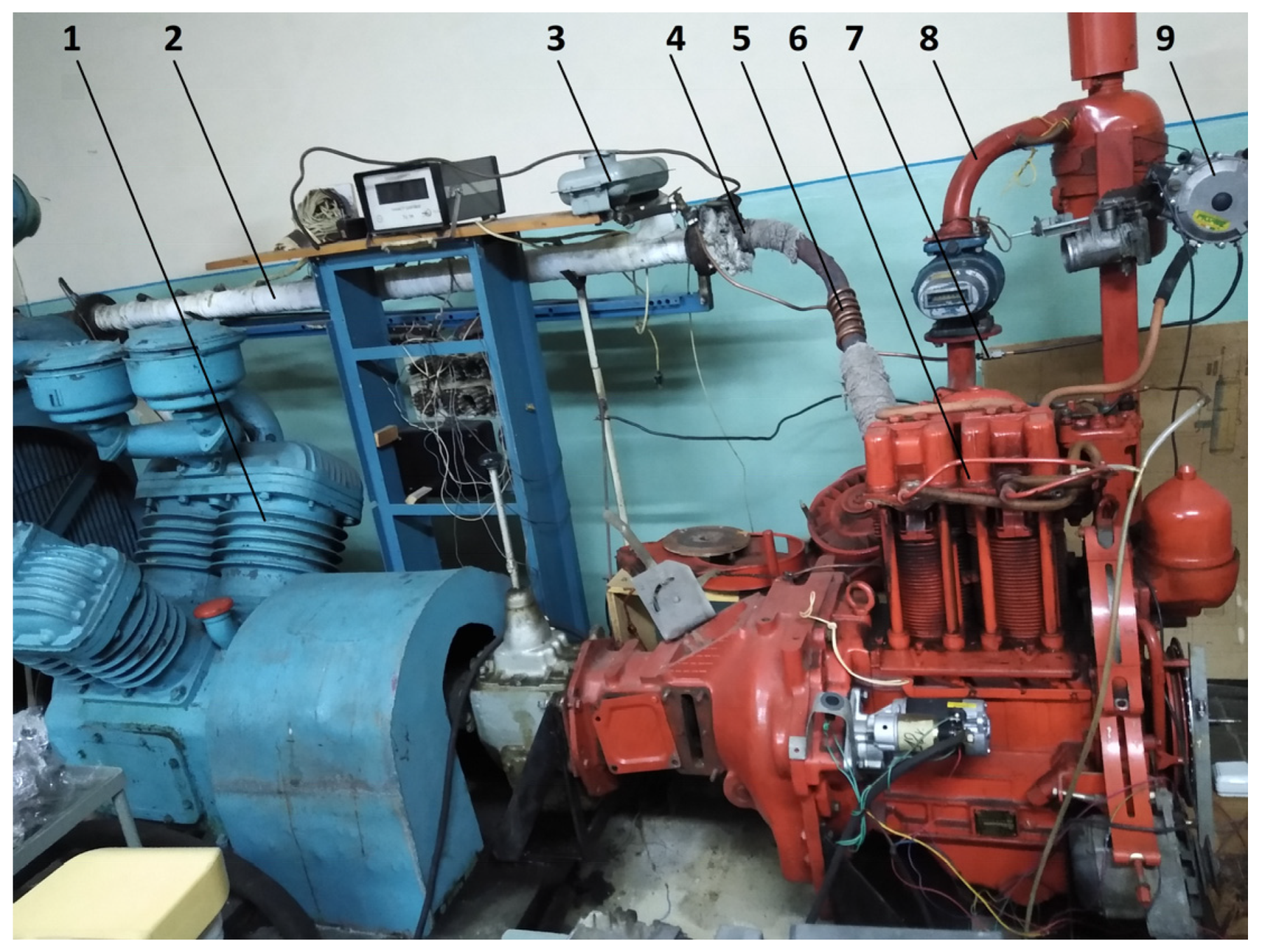
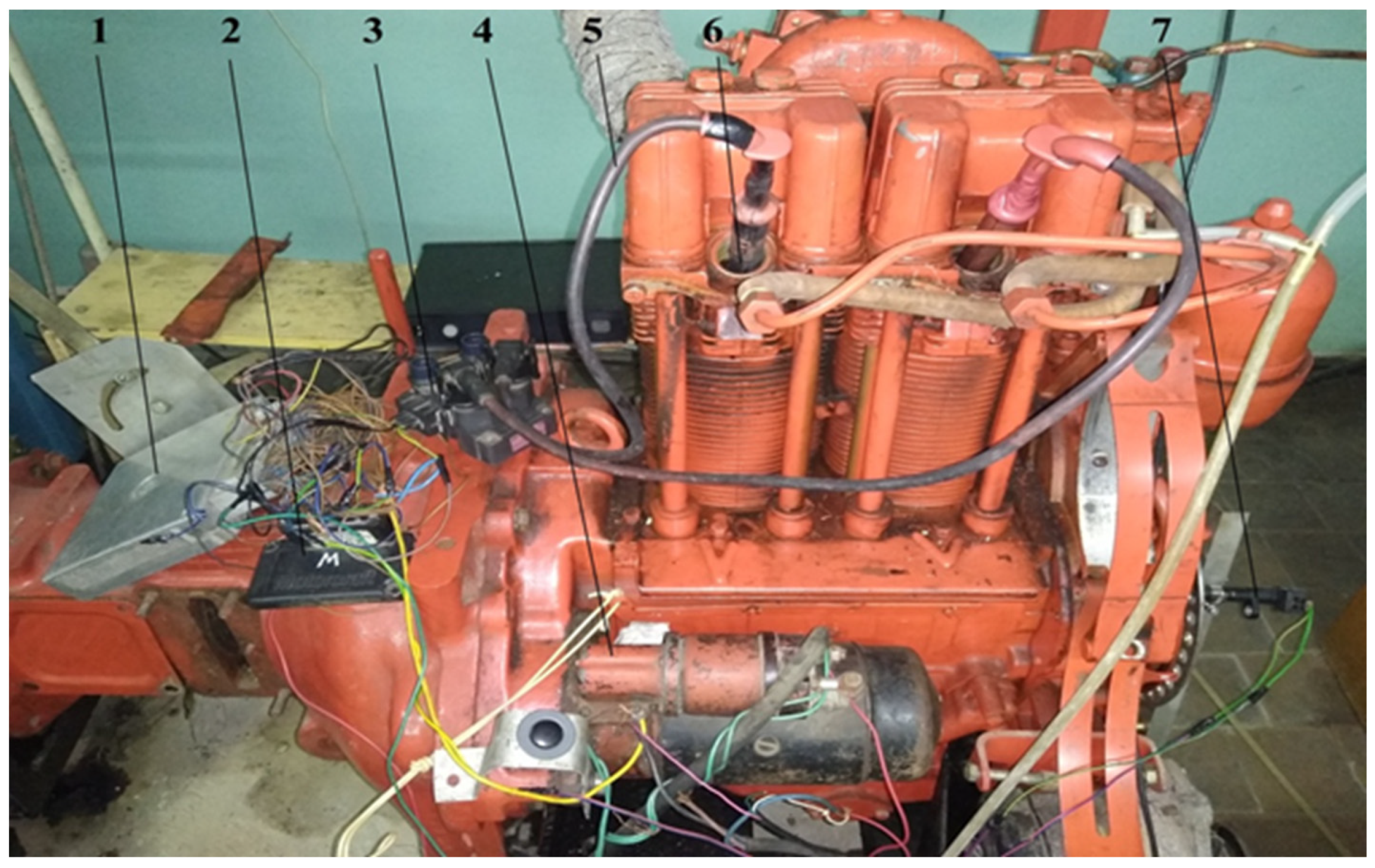
The purpose of experimental research was to determine the main fuel-economic, power, and environmental parameters of the converted diesel engine D21 during its operation using alternative hydrogen fuel mixtures and diesel fuel to evaluate the energy-saving effect. Experimental studies were performed on the diesel motor stand of the D21 engine (Figure 1 and Figure 2). Thermal losses were minimised during test studies by using highly efficient heat insulation of the exhaust pipeline and hydrogen reactor based on an asbestos sheet (in Figure 1, for clarity, the insulator is partially removed).
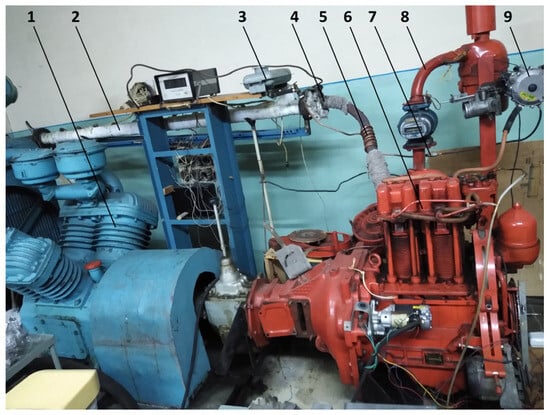
Figure 1.
Appearance of the hydrogen reactor system for the study of the stand based on the diesel/hydrogen engine D21: 1—loading compressor of the engine D21; 2—exhaust manifold; 3—tank for methanol (liquefied); 4—thermal insulation of the exhaust collector; 5—thermochemical hydrogen reactor (thermal insulation of the reactor is partially dismantled); 6—diesel/hydrogen engine D21; 7—window in the supply tube of alternative gaseous fuel mixtures; 8—intake manifold; 9—alternative gaseous mixtures reducer.
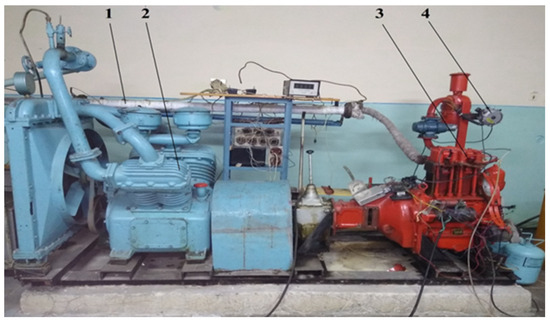
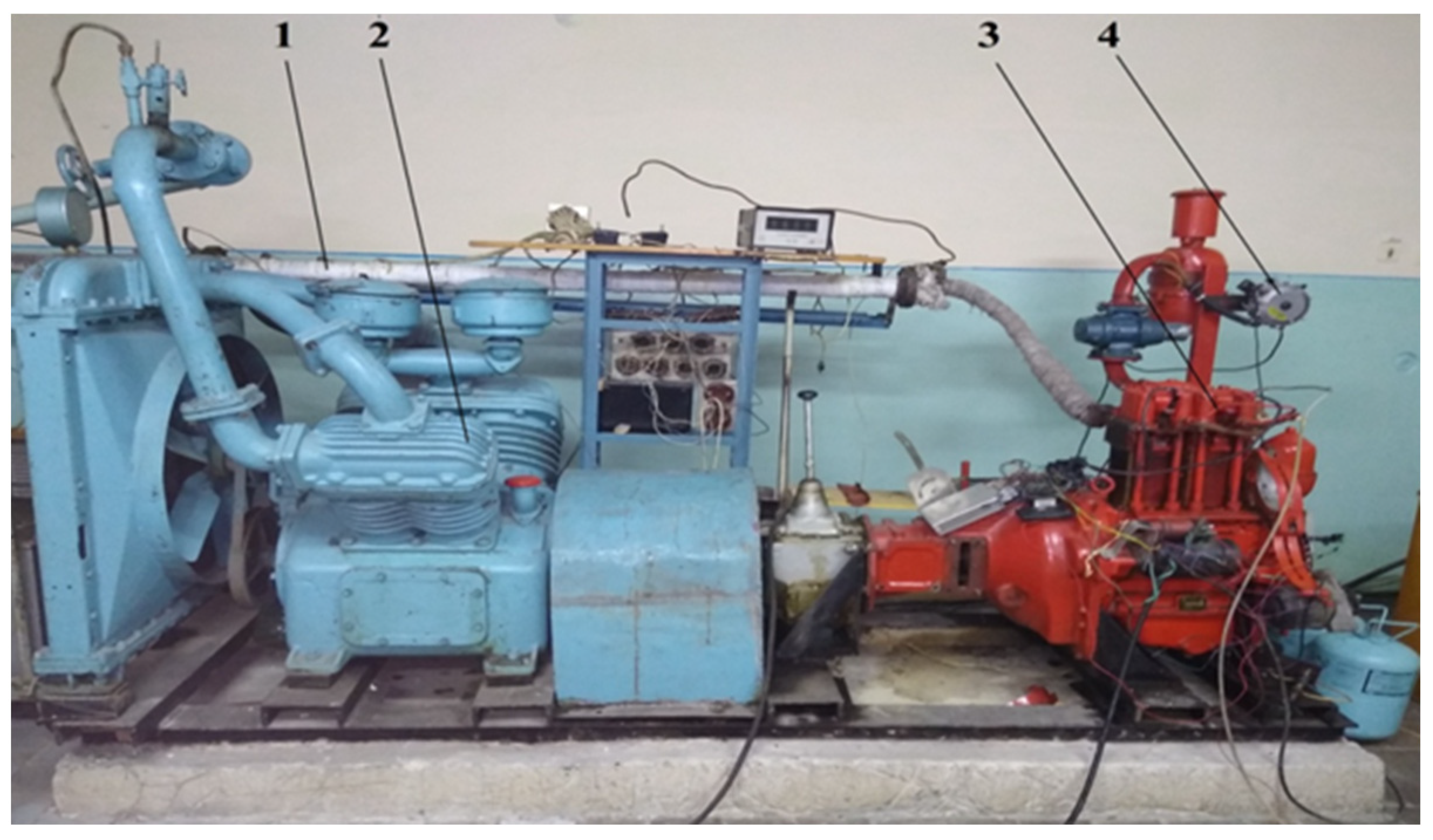
Figure 2.
Appearance of the experimental diesel engine D21 for the study of characteristics on alternative hydrogen fuel mixtures. 1—thermochemical hydrogen reactor; 2—loading device of the engine D21; 3—diesel engine; 4—mixtures gas reducer.
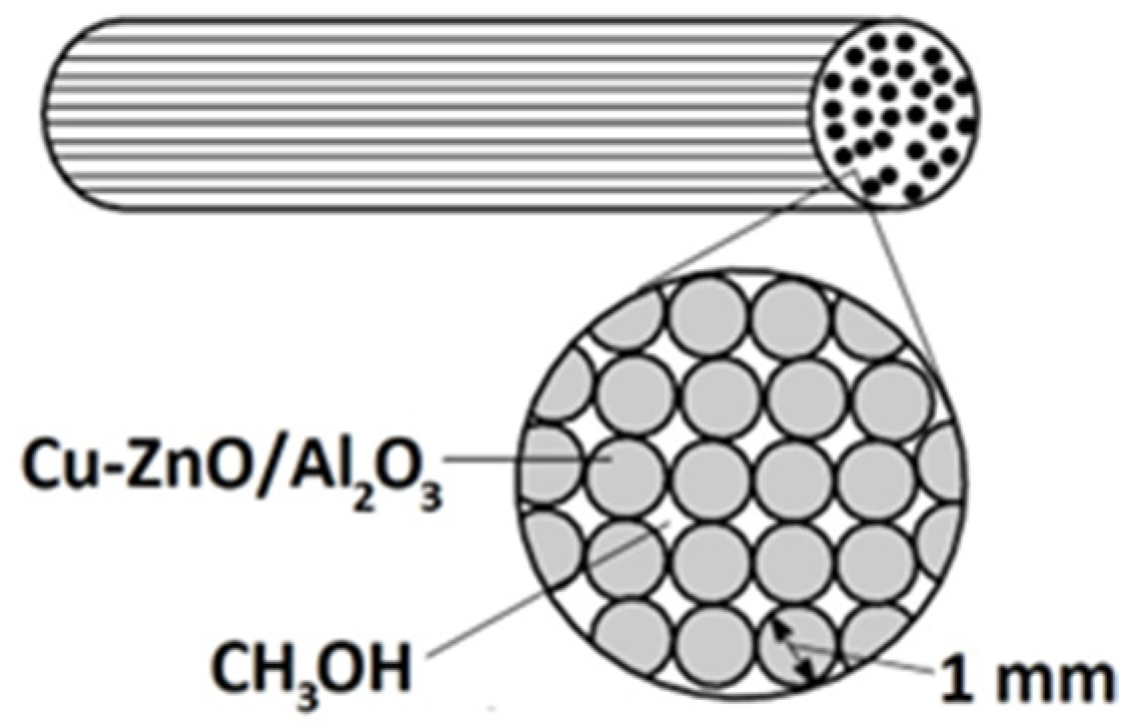
Cu-ZnO/Al2O3 was used as a catalyst in the reactor (Figure 3). The reactor, with an internal diameter of 16 mm and a length of 520 mm, was filled with aluminium rods with a diameter of 1 mm and with a catalyst of copper and zinc oxide deposited on them.
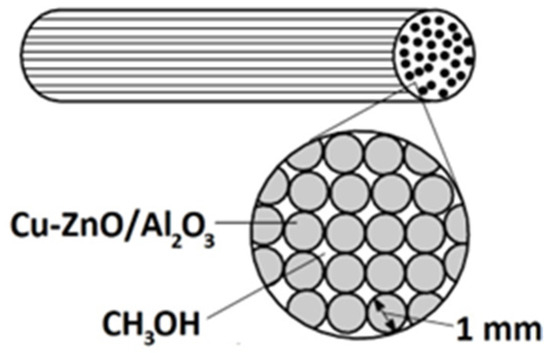
Figure 3.
Scheme of the catalytic reactor.
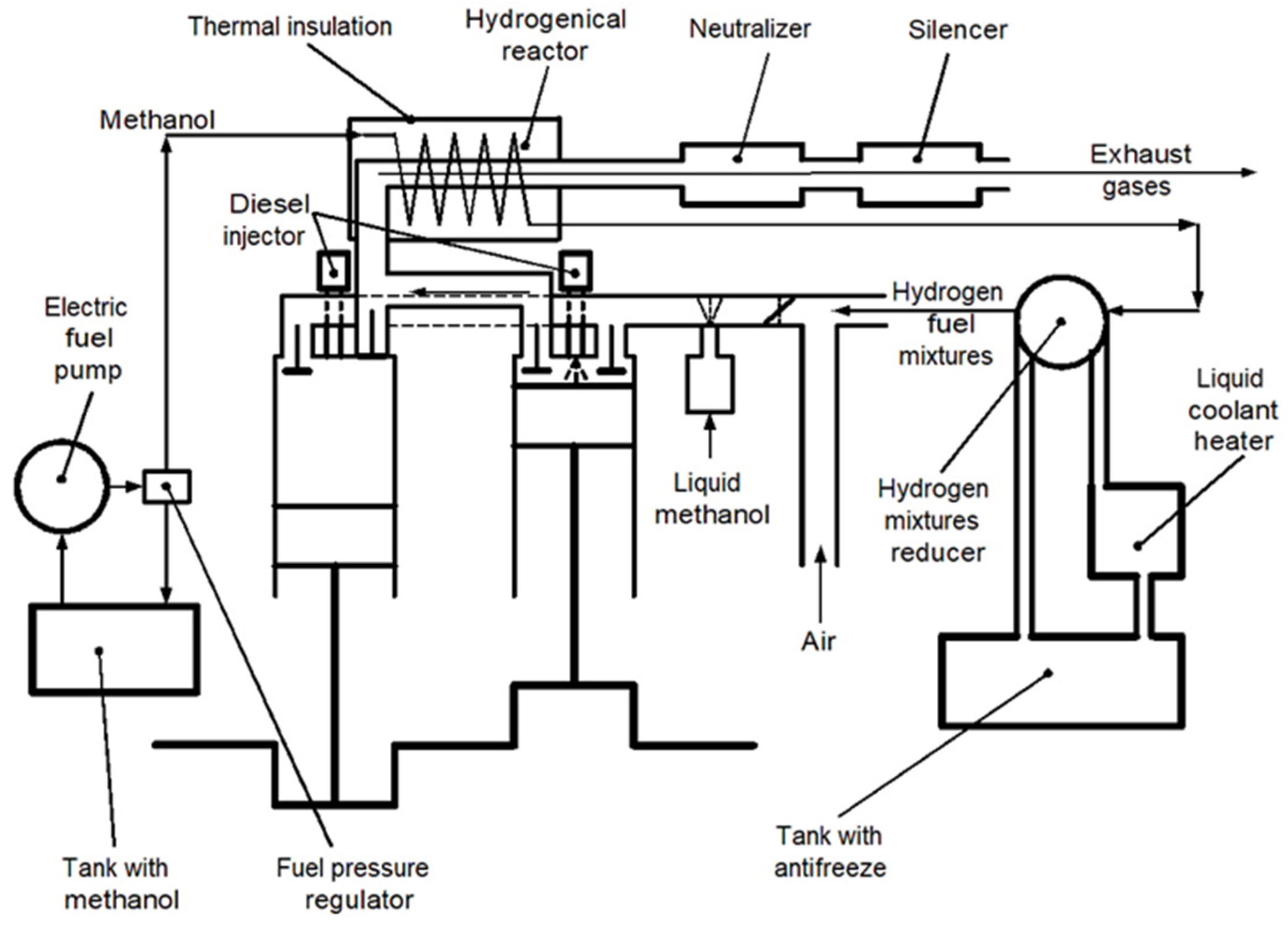
A scheme of the stand based on the diesel/hydrogen engine D21 for the research of its work to products of alternative hydrogen fuel mixtures is shown in Figure 4.
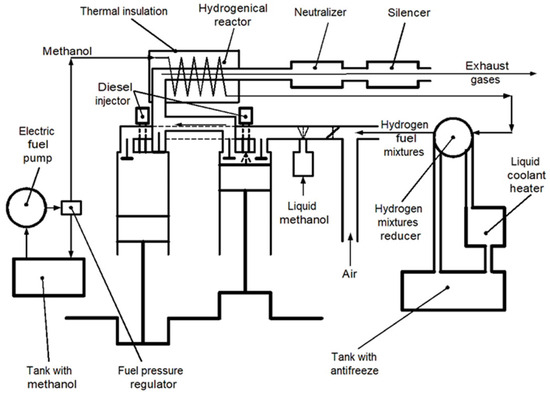
Figure 4.
Scheme for study parameters of the stand based on the test diesel-hydrogen engine D21.
A brief technical description of the hydrogen fuel mixtures engine is shown in Table 4.

Table 4.
Technical parameters of the experimental hydrogen fuel mixtures engine.
The fuel-air hydrogen mixture was ignited using an ignition system of our own design. The developed ignition system consists of an electronic control unit 1 (Figure 5), which changes the angle of ignition depending on the operation of the experimental engine.
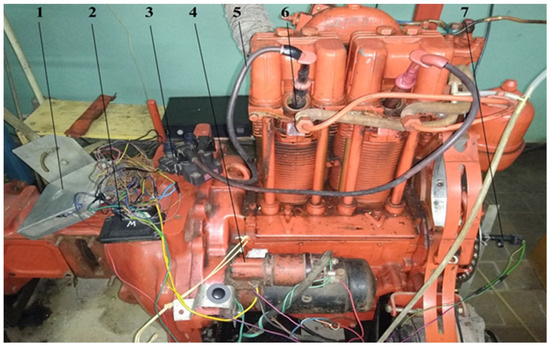
Figure 5.
Ignition system of the experimental diesel/hydrogen mixture engine D21: 1—control unit; 2—ignition amplifier; 3—ignition DIS-module; 4—starter of engine; 5—spark wires; 6—high-voltage plugs; 7—sensor of crankshaft.
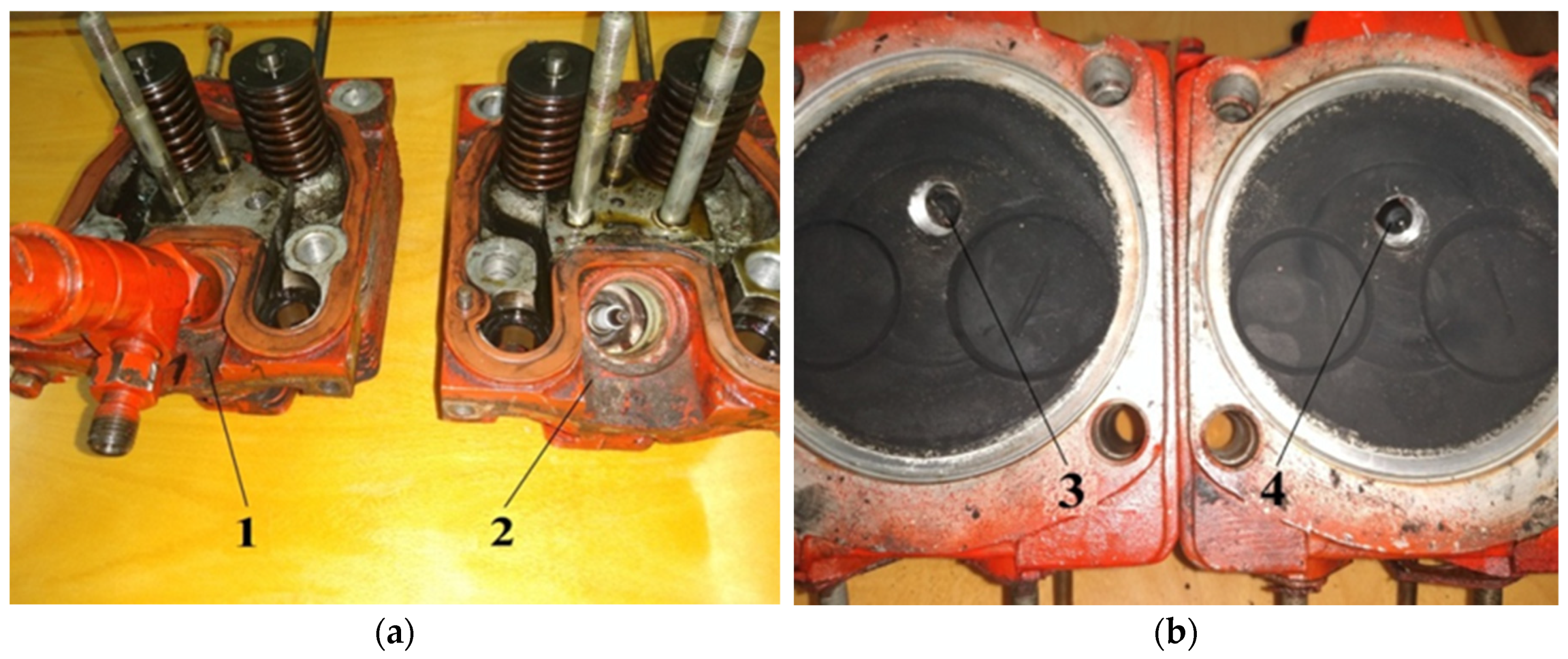
The experimental diesel engine was converted to work on the hydrogen gas mixture. For this reason, diesel fuel injectors were dismantled from the unit heads of the engine, additional carvings were cut in the injector channels, and high-voltage plugs were installed. The experimental engine with the dismantled heads is shown in Figure 6.

Figure 6.
The experimental diesel engine with dismantled heads of the block.
The design of the experimental engine head is redesigned, so that instead of using high-voltage plugs, the diesel fuel injectors can be screwed back. This conversion of the D21 engine allows the switching of a hydrogen gas mixture or diesel fuel for 10–15 min through the replacement of high-voltage plugs for diesel fuel injectors. The heads of the engine block converted from a diesel to hydrogen gas mixture are shown in Figure 7.
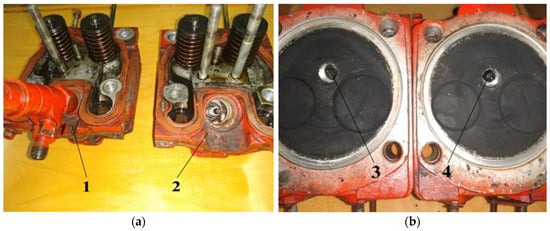
Figure 7.
Diesel fuel to hydrogen gas mixture converted heads of the block: (a) View from the timing valves heads block; (b) view from the side of the combustion chamber heads. 1—the head of the engine before re-equipment with the established diesel fuel injector; 2—the head of the engine after re-equipment with the established high-voltage plug and the dismantled fuel injector; 3—high-voltage plug; 4—diesel fuel injector.
Measurements were carried out at stationary engine operating modes. The preliminary determination of the amount of toxic components in the exhaust gases was carried out using the Autotest 02.03P gas analyser (Meta, Russia) by direct measurement. The graphing and final determination of the amount of toxic components in exhaust gases was carried out with a NeoCHROM Class C (NeoCHROM, Zaporizhzhia, Ukraine) using gas chromatography. The measurement of the amount of toxic components in exhaust gases was carried out by taking samples into sampling bags. A heated flame ionisation detector was used for hydrocarbon analysis. An adsorption-type infrared non-dispersive detector was used for the analysis of carbon oxides. A chemiluminescence detector was used to analyse nitrogen oxides.
3. Results
The research of the hydrogen reactor operation for methanol conversion included determining the zone of stable operation in terms of a hydrogen gas mixture, checking its performance, assessing the productivity of the main components of the synthesised mixture, and taking the main characteristics that prove the relationship between the set of the products obtained in the hydrogen reactor, the degree of hydrogen conversion, and the temperature regime of the hydrogen conversion process.
One of the largest problems for the normal functioning of the hydrogen conversion system is providing it with the correct amount of energy and a given level of temperatures in the hydrogen reaction space to achieve the most in-depth hydrogen thermochemical conversion process and the maximum rate of methanol conversion (Methanol Conversion—MC). At MC < 100%, methanol products at their outlet from the hydrogen reactor, along with H2 and CO, contain vapours of unreacted liquefied methanol.
Table 5 shows the temperature area of the experimental hydrogen reactor.

Table 5.
Hydrogen power for different engine modes.
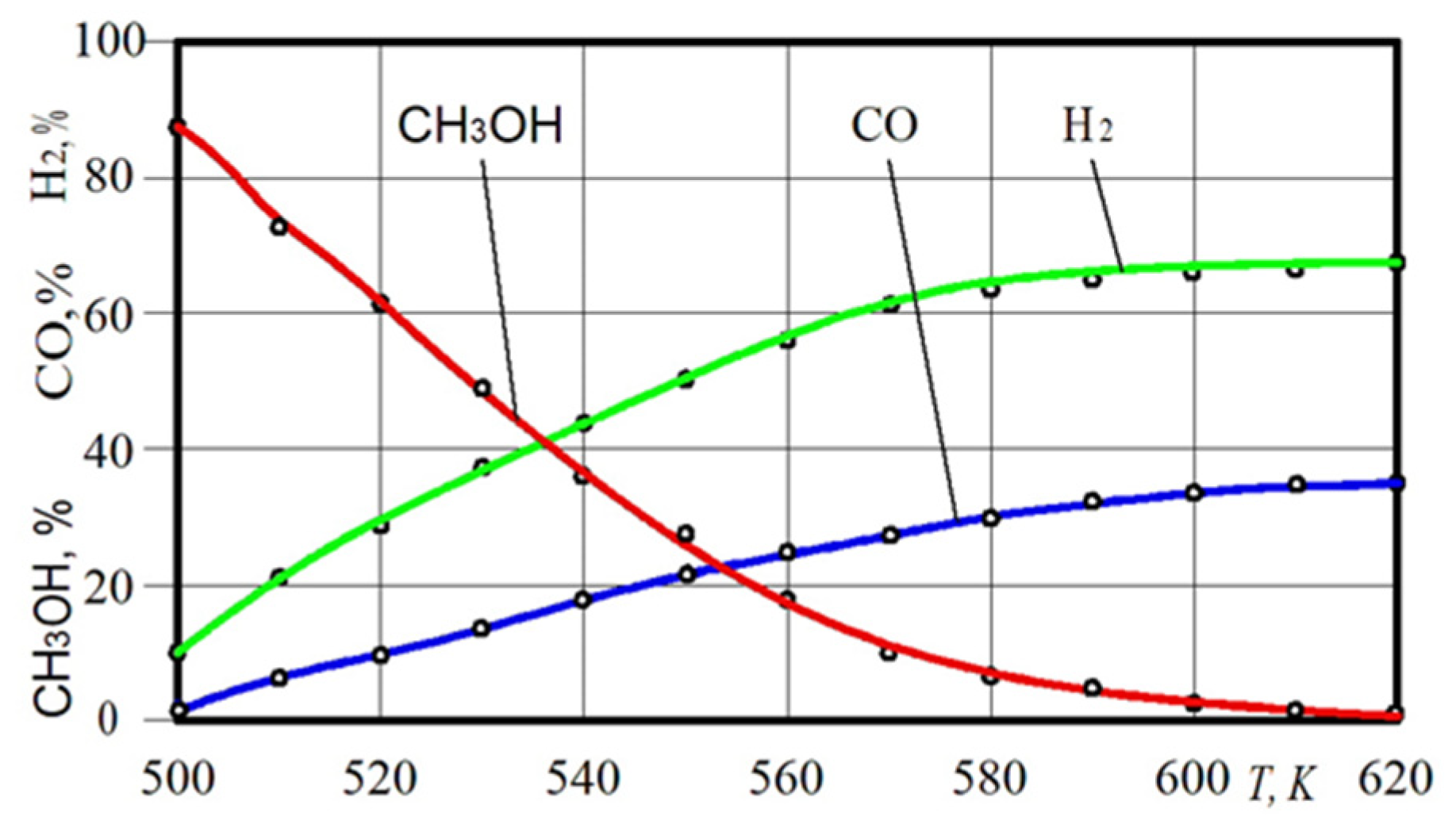
The important parameters of a hydrogen conversion reactor include the dependence of the rate of conversion on the temperature in the catalytic reactor. Such characteristics can only be reached on the basis of the experiment (Figure 8). It was experimentally found that almost full conversion (MC > 80%) of methanol is achieved in the hydrogen reactor at 545–550 K.
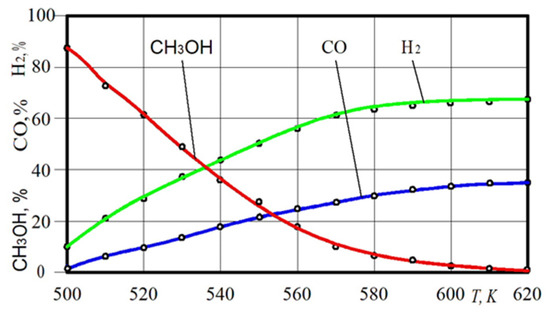
Figure 8.
Dependence of the receiving of CH3OH, H2 and CO on the temperature T in the hydrogen reactor.
The chosen medium contributes to the process of methanol transformation already at a temperature of 505–510 K, making it possible to convert methyl alcohol with the release of a hydrogen gas in small load modes of the engine with a temperature shortfall. Figure 8 shows the dependence of the H2 and CO receiving on the temperature T in the hydrogen reactor. Experiments have shown that the maximum CO content is 35% and the maximum possible H2 content is close to 65%. In general, analysing the results of an experimental study of the parameters of the hydrogen conversion reactor, the temperature level, above which the CH3OH decomposition reaction can be considered complete, corresponds to 580–590 K.
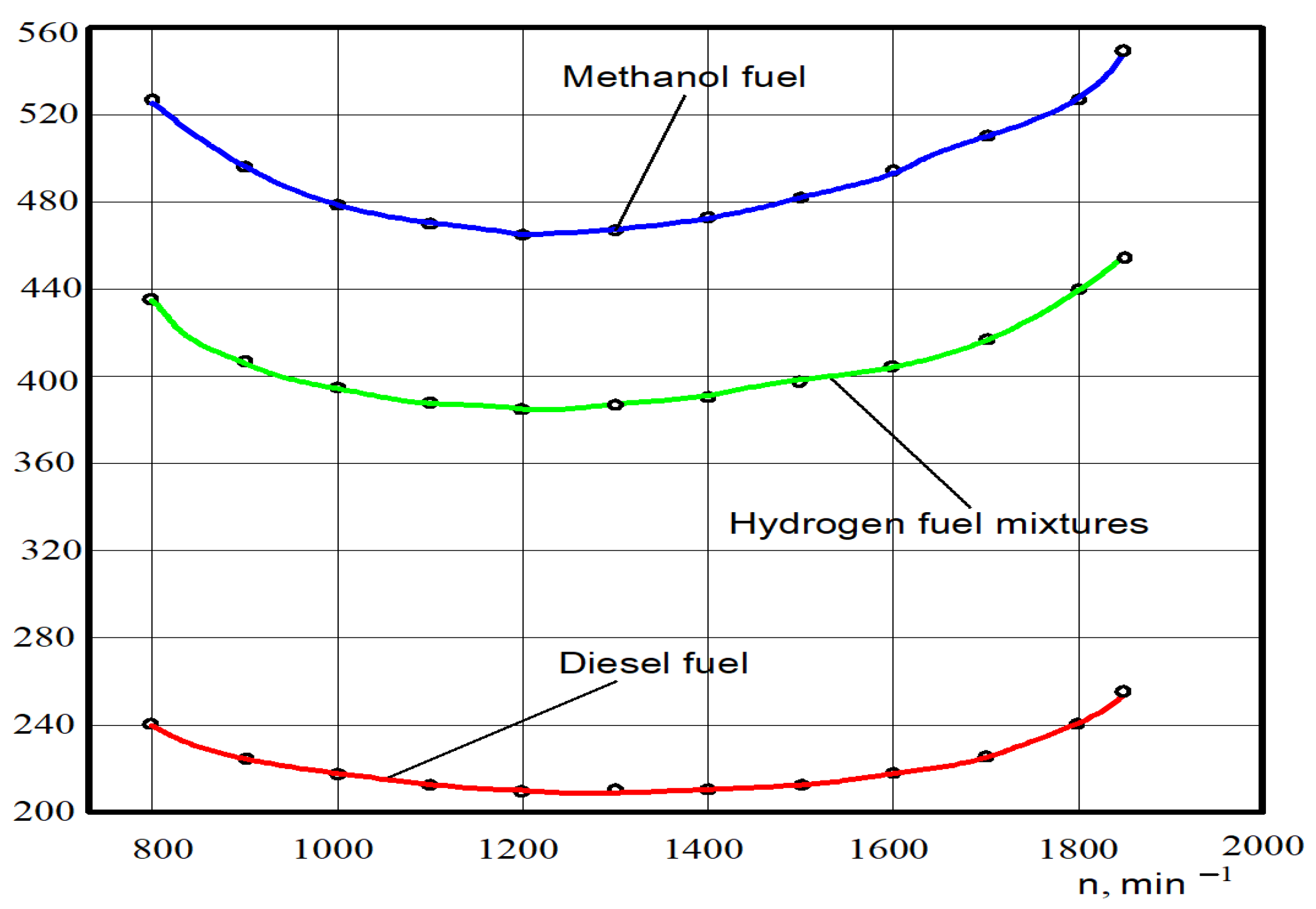
The test dependences of specific fuel consumption ge on the crankshaft rotation n of the experimental engine D21 working on diesel fuel, hydrogen fuel mixtures, and methanol fuel are shown in Figure 9.
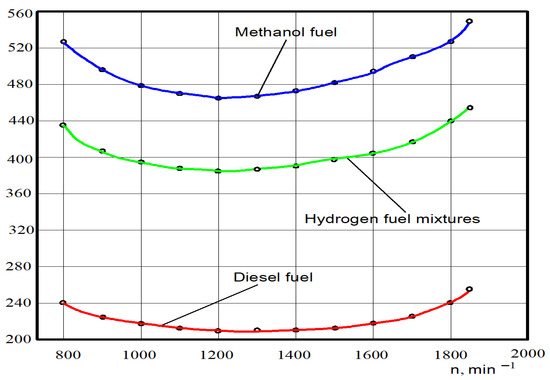
Figure 9.
Test dependences of the specific fuel consumption ge on the crankshaft rotation n for different engine fuels.
It was found through analysing the tested power that for diesel fuel the minimal specific fuel consumption at crankshaft rotation n = 1250 min−1 was 214 g/(kWh); specific fuel consumption at the nominal crankshaft rotation n = 1850 min−1 was 252 g/(kWh). The minimum specific fuel consumption at crankshaft rotation n = 1250 min−1 was 464 g/(kWh) for methanol; specific fuel consumption at nominal crankshaft rotation was 552 g/(kWh). The minimum consumption of specific hydrogen fuel mixtures was 384 g/(kWh); specific hydrogen fuel mixtures consumption at nominal crankshaft rotation was 452 g/(kWh).
The specific fuel consumption of the crankshaft rotation in the entire range of the crankshaft rotation compared to diesel working on 100% of hydrogen fuel mixtures increased by 78–82%. Specific fuel consumption from the crankshaft rotation on average in all ranges of the crankshaft rotation, compared to diesel working on 100% of methanol, increased by 110–115%.
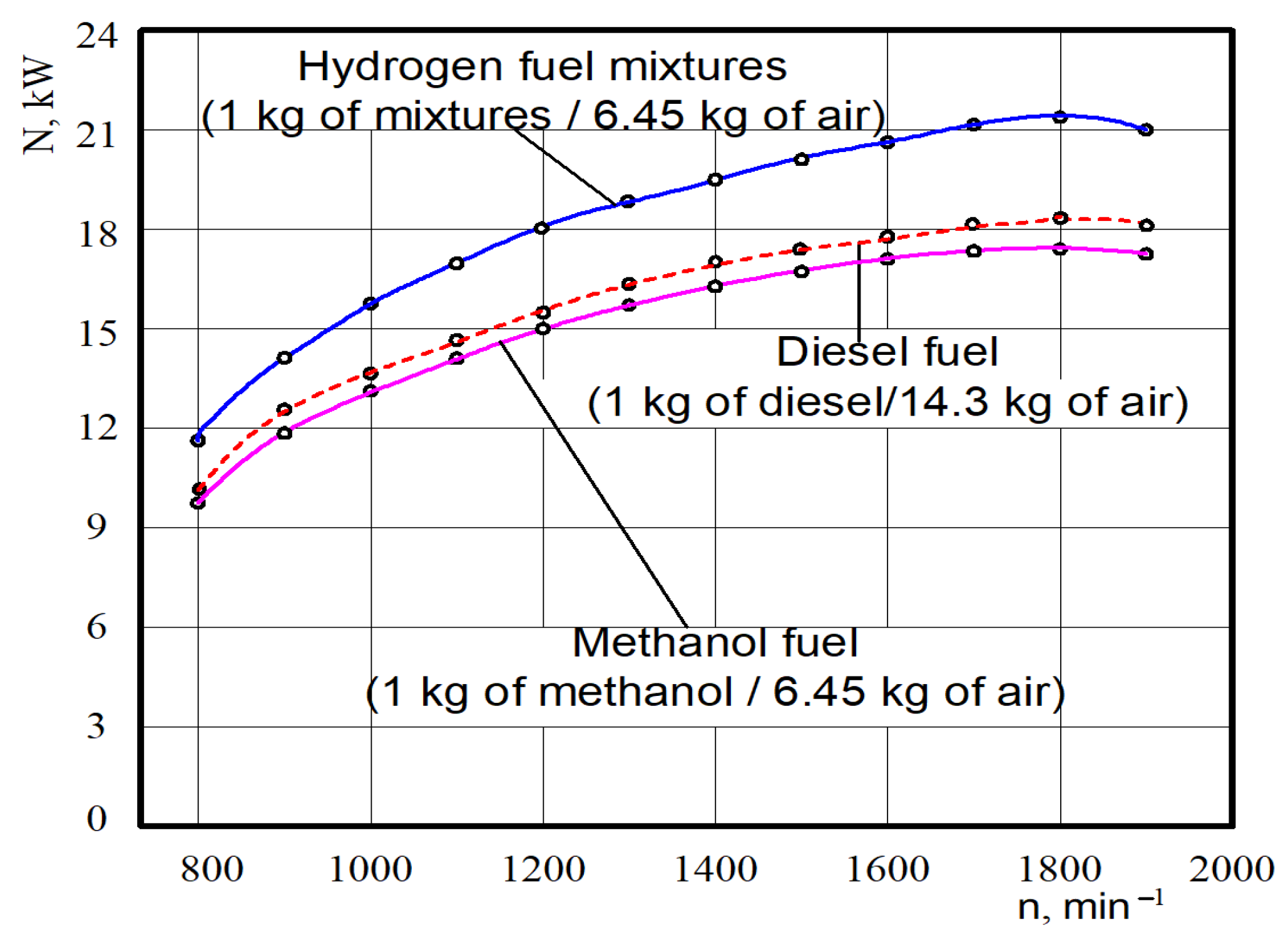
The test dependences of the power parameters of the experimental engine D21 converted for hydrogen fuel mixtures, diesel and methanol fuel are shown in Figure 10. It was found through analysing tested power that at crankshaft rotation n = 1850 min−1 the nominal effective power for the diesel engine was 18.2 kW.
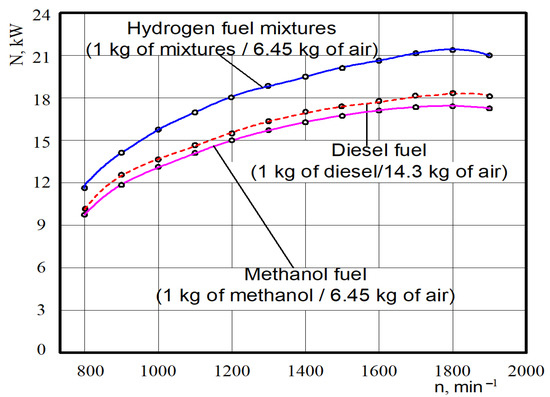
Figure 10.
Test dependences of the nominal effective power N on the hydrogen fuel mixtures crankshaft rotation n for different engine fuels.
When analysing the test power, it was found that at crankshaft rotation n = 1850 min−1 the nominal effective power for methanol (6.45 kg of air on 1 kg of CH3OH) was 17.2 kW, but on the hydrogen fuel mixtures (6.45 kg of air on 1 kg of mixtures), it was equal to 21.3 kW. The value of the average effective power of the engine in the whole range of crankshaft rotation in comparison to the diesel working on methanol (6.45 kg of air in 1 kg of CH3OH) decreased by 5.5%, and for hydrogen fuel mixtures (6.45 kg of air on 1 kg of mixtures) it increased by 17.0%.
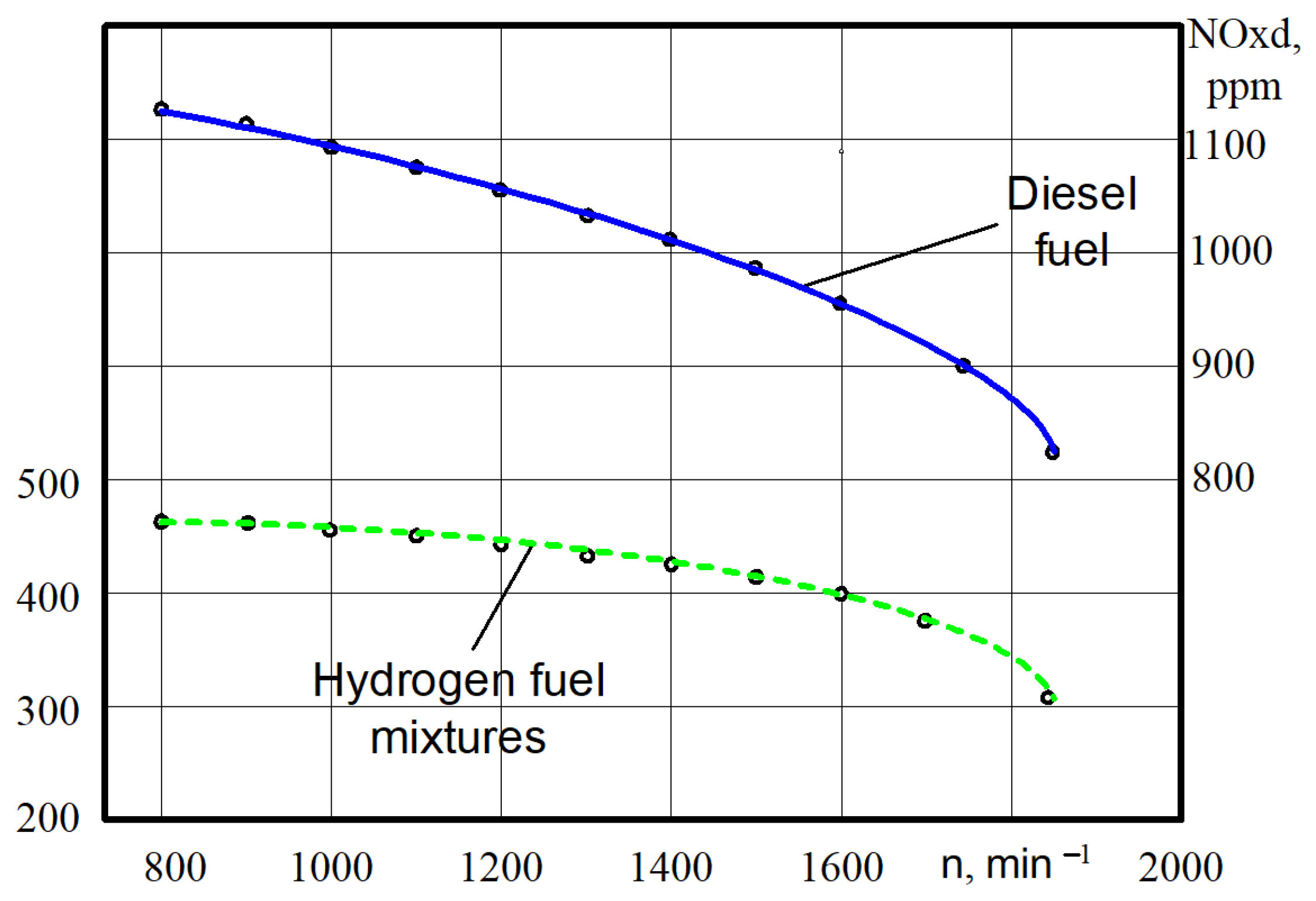
Analysing the change in toxic components in the exhaust gases engine during the transition from diesel to hydrogen fuel mixtures (6.45 kg of air on 1 kg of mixtures) the following can be noted. There is a large decrease in nitrogen oxides in all ranges of the rotation of the crankshaft (Figure 11). Thus, at n = 790–810 min−1 of the crankshaft rotation, the nitrogen oxides decreased from 1140 ppm (8.4 g/kW·h) when the engine was on diesel to 455 ppm (3.4 g/kW·h) when the engine was on the hydrogen fuel mixtures. The decrease in the nitrogen oxides was 60.1%. At n = 1840–1860 min−1 the nitrogen oxides decreased from 830 ppm (6.1 g/kW·h) when the engine was on diesel to 310 ppm (2.3 g/kW·h) when the engine was on the hydrogen fuel mixtures. That is, the decrease in the nitrogen oxides was 62.6%. The decrease in the nitrogen oxides during the engine on diesel in comparison with the engine on the hydrogen fuel mixtures is explained through lower thermal dissipation and a lower increase in the combustion pressure of the engine.
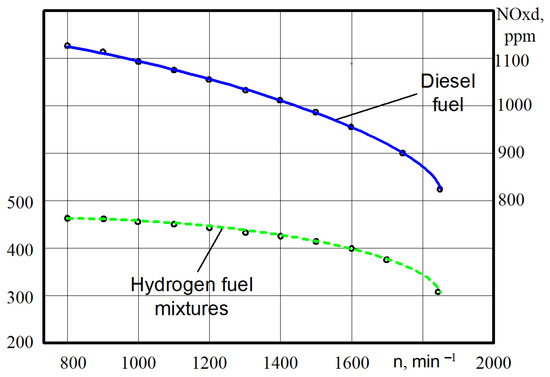
Figure 11.
Test dependences of the nitrogen oxides NOx in exhaust gases on the crankshaft rotation n for different engine fuels.
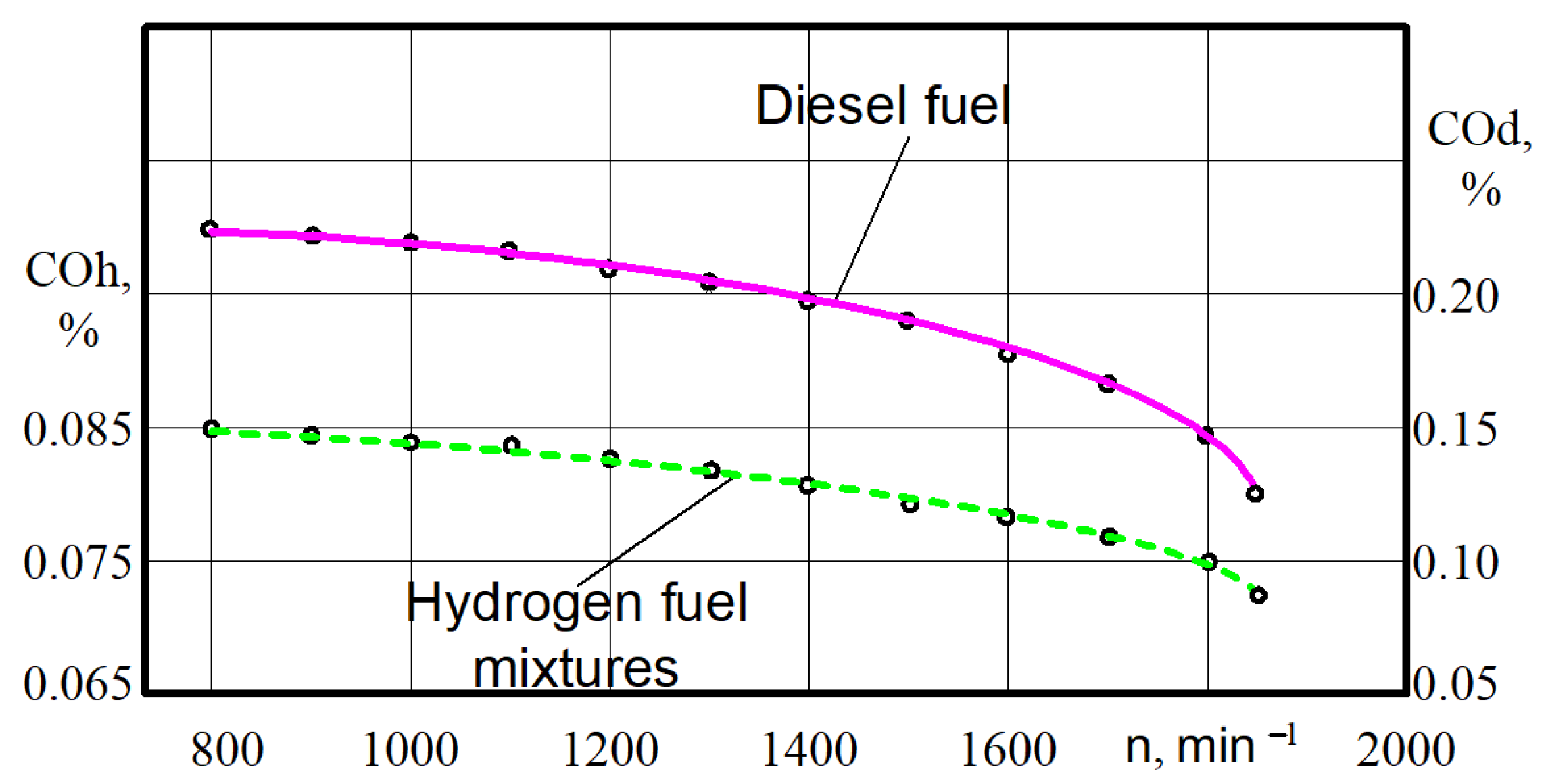
There is a large decrease in CO in all ranges of the crankshaft rotation (Figure 12).
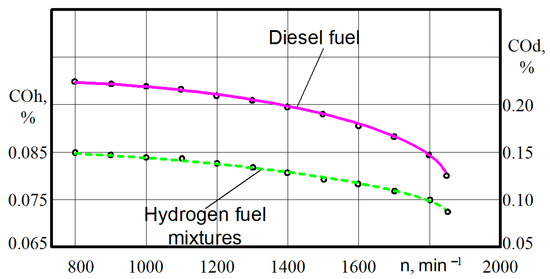
Figure 12.
Test dependences of the carbon monoxide CO on the crankshaft rotation n for different engine fuels.
Thus, at the crankshaft rotation n = 790–810 min−1, the carbon monoxide decreased from 0.228% when the engine was on diesel to 0.085% when the engine was running on the hydrogen fuel mixtures. At crankshaft rotation n = 1840–1860 min−1, the carbon monoxide decreased from 0.122% when the engine was on diesel to 0.072% when the engine wa on hydrogen fuel mixtures. The decrease in CO content occurs in the range of 51.6–61.8%.
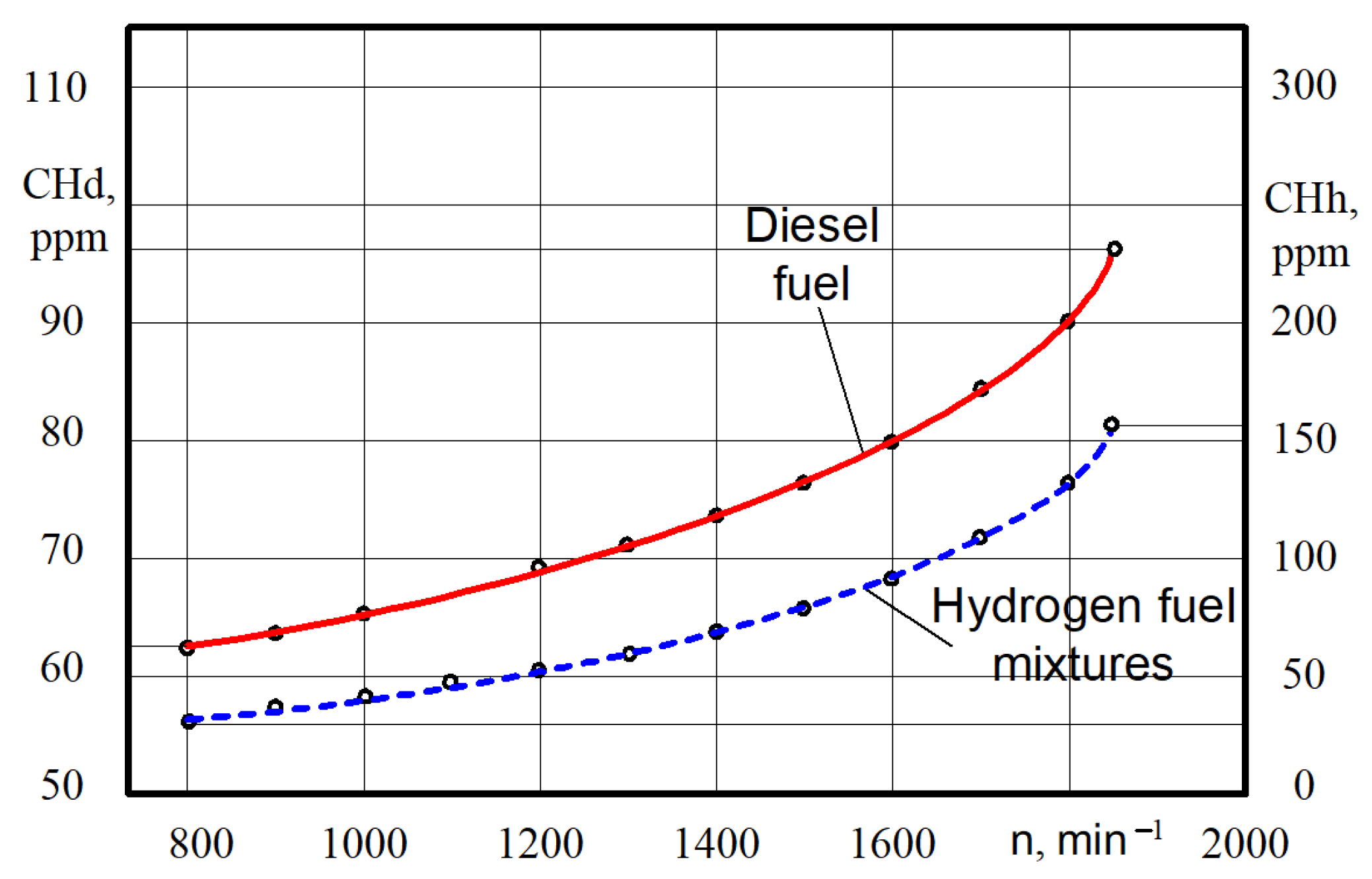
The hydrocarbon CnHm content increases some in all the ranges of crankshaft rotation (Figure 13).
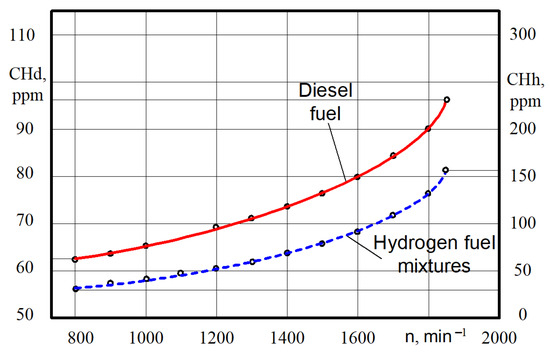
Figure 13.
Test dependences of the CnHm content on the crankshaft rotation n for different engine fuels.
Thus, at the crankshaft rotation n = 790–810 min−1, the CnHm content varies from 62 ppm when the engine is on diesel to 32 ppm when the engine is on hydrogen fuel mixtures. That is, the decrease in CnHm content was 30 ppm. At crankshaft rotation n = 1840–1860 min−1, the CnHm content increases from 96 pm when the engine is on diesel to 155 ppm when the engine is on hydrogen fuel mixtures. That is, the increase in hydrocarbons was 1.6 times greater.
The experiments showed that the CnHm content for the diesel engine was lower in most modes. However, for this particular engine at close to idle speeds, the CnHm content for the diesel engine was higher. In our opinion, the higher level of CnHm emissions from a diesel engine with a low crankshaft rotation is caused by the incomplete evaporation and combustion of the D21 diesel engine with a mechanical injection system of diesel fuel. Hydrogen fuel mixtures arrive already in a gaseous state, which improves the combustion process.
4. Discussion
The experiments showed that the fuel-economy indicators during the engine run with a catalytic reactor on a hydrogen mixture at all the modes of engine crankshaft rotation frequency were higher (on average by 15–21%) than when the engine operated on pure methanol without a hydrogen reactor. At the same time, at low crankshaft rotation frequencies (800 to 1000 min−1), the specific fuel consumption when the engine was running on a hydrogen mixture with a catalytic reactor differed less than the specific fuel consumption when the engine was running on pure methanol. This is obviously explained by the relatively low temperature levels of exhaust gases when the engine is operating at low loads, which is in good agreement with the work of [39]. The low temperatures of the exhaust gases, in turn, lead to a somewhat lower productivity of the reactor in terms of hydrogen output.
The BSFC of the obtained mixtures has a high value. This is due to the relatively low heat of the combustion of methanol, which is 22.5 MJ/kg compared to 42.5 MJ/kg for diesel fuel. However, at an air/fuel ratio of 6.45/1 for methanol, the calorific value of the converted methanol mixture will be approximately 3950 kJ/m3, compared to 3400 kJ/m3 for the diesel mixture. Therefore, the power of the experimental engine when operating on a mixture of converted methanol during the experiments was higher than when the engine was operating on diesel fuel.
The greatest increase in thermal efficiency and decrease in specific fuel consumption (by 20–21% compared to operation on pure methanol) was observed in the range of engine crankshaft rotation frequency from 1250 to 1300 min−1 (at exhaust gas temperatures in the range of 450–500 °C). This is explained by the fact that in this range of exhaust gas temperatures, the consumed thermal energy of the reactor and the thermal energy of the exhaust gases already become practically the same. Experimental studies have shown that in the regimes of crankshaft revolutions and engine loads, when the temperature of exhaust gases at the entrance to the reactor exceeds 450 °C, the performance of the reactor, taking into account the hydrogen component, reaches the highest value, which maximally reduces the specific fuel consumption.
As confirmed by the authors [40], the improvement in the fuel economic parameters of the experimental engine with the proposed method of hydrogen fuel formation is due to the effect of two features: the effect of utilisation of waste gas heat and the improvement of kinetic combustion in the fuel due to the presence of hydrogen.
Compared to diesel fuel, the specific fuel consumption of an engine running on hydrogen methanol conversion mixtures has increased by 82%. Since the price of methanol is, on average, 30–35% of the cost of diesel fuel (Table 6), the conversion of diesel engines to work on hydrogen mixtures of methanol conversion is very profitable. The average cost of diesel in the EU ranges from 1.3 to 1.8 euro/L [41].

Table 6.
Cost of the main alternative fuels in the EU that can be used in car engines.
Methanol is currently identified as a transition fuel for industrial decarbonisation; among potential fuel alternatives (with significantly lower carbon content compared to diesel or gasoline), methanol is considered a short- to medium-term solution for decarbonisation [42]. However, the main powerful advantage of using a hydrogen mixture based on converted methanol in engines is the reduction in CO2 emissions during the life cycle of methanol. This is because technologies for obtaining methanol directly from CO2 have been developed today [43,44]. CO2, which is formed during the combustion of methanol in engines, can then be used as a raw material for the production of methanol. The obtained cost of methanol production was USD 565 per ton of produced methanol [44].
The total cost of engine conversion and the conditions in Ukraine amounted to 1.750 euros. If we take the fuel consumption of a diesel engine in a passenger car of 5 L per 100 km of mileage, savings from using a hydrogen mixture, based on methanol, is 2.5–5 euros per 100 km of mileage. The payback of the system will be from 35 to 70 thousand km. Payback will be faster for trucks with higher diesel fuel consumption.
The decrease in the cost of consumption of alternative hydrogen fuel was accompanied by an improvement in the power and environmental parameters of a diesel engine operating with an on-board hydrogen reactor.
As noted in [45], the presence of a supplementary amount of H2 in the influence zone leads to a decrease in NOx output, since during the combustion of a hydrogen fuel mixture, in addition to fuel oxidation reactions, nitrogen recovery, etc., occurs.
The experiments confirmed that depending on the frequency of rotation of the crankshaft and the load on the engine, the formation of NOx in the exhaust gases decreased by 54–61%, and the reduction in carbon monoxide takes place in the range of 52–62%.
In terms of NOx emissions, their reduction is extremely relevant, especially for diesel engines. The values obtained by us are close to the results of other engines that operated on methanol fuel. For example, the reduction in NOx emissions is recorded in the works [42,46]. In our opinion, the reduction in NOx emissions is due to significantly less oxygen in the air/methanol fuel mixture compared to the air/diesel fuel mixture. The air/methanol ratio is 6.45/1 and the air/diesel stoichiometric ratio is 14.7/1, but also, diesel engines also operate with an excess air factor λ greater than unity. Along with the positive result of reducing NOx emissions, the authors note that for a specific experimental engine for different operating modes, NOx reduction occurred at values of 2.3–3.4 g/kW·h. The obtained values correspond only approximately to Euro IV requirements for diesel engines, and, therefore, cannot be recommended for immediate implementation but require research to further reduce NOx emissions to current requirements.
At the same time, as stated in [47], the presence of hydrogen in fuel combustion counteracts the process of soot release. Hydrogen affects the release of soot at all phases of the formation and combustion of carbon particles. Hydrogen intensifies the processes of burning soot at the expense of water, which acts as an oxidiser. Reducing soot emissions in fuel combustion processes helps reduce heat loss and correspondingly increases engine power.
The experiments confirmed that the engine power, depending on the frequency of rotation of the crankshaft and the engine load, increased by 10–14% on the hydrogen mixture compared to the operation of the experimental engine on diesel fuel.
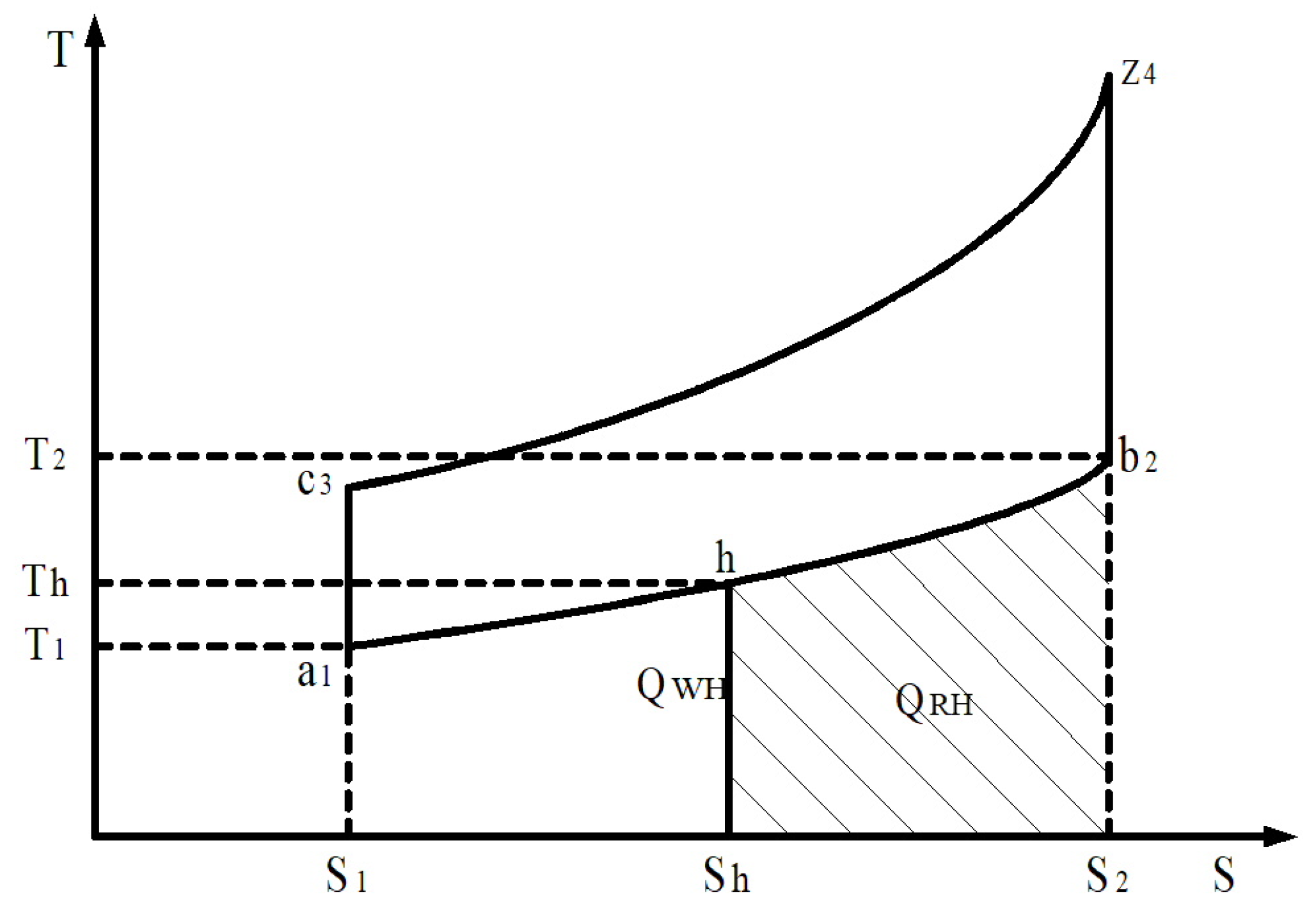
To ensure the operation of the hydrogen reactor on board the car, it is necessary to remove excess heat, for example, from the exhaust gases of the engine or the cooling system. In our opinion, this energy is most rationally obtained by utilising the thermal energy of exhaust gases. In gasoline and gas engines, energy losses in the overall heat balance reach 30–40% and in diesel engines—25–35%. This corresponds to 12–21 MJ of thermal energy of exhaust gases per 1 kg of fuel consumed by the engine. At the same time, the temperature of the engine exhaust manifold reaches from 750 to 900 K at idling speed to 1100–1300 K at maximum speed and loads. The thermodynamic cycle (Otto cycle) for engines converted from diesel fuel to a hydrogen mixture with spark ignition and heat recovery from exhaust gases can be depicted using a T-S diagram (Figure 14).
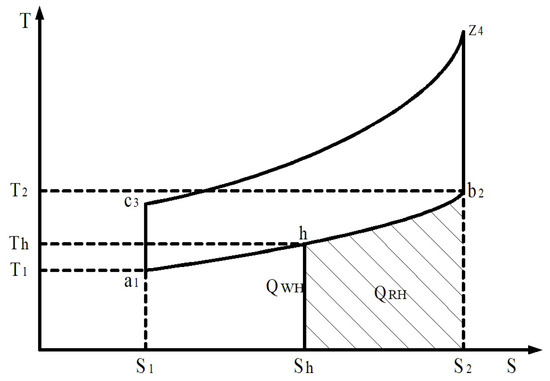
Figure 14.
Thermodynamic cycle for engines converted to gaseous products of hydrogen with heat regeneration. S1-c3-z4-S2—the supplied heat QSH; S1-a1-b2-S2—the waste heat QWH; Sh-h-b2-S2—the heat for regeneration QRH.
In Figure 14, heat suitable for regeneration is expressed as a share of the waste heat QWH, i.e., the degree of regeneration in the thermodynamic cycle is equal to
where QWH is the heat waste removed in the thermodynamic cycle.
Heat quantity removed per cycle QWH (Figure 14) is determined from the expression
where Cμ.νol is the average molar heat capacity of combustion products at constant volume and Mpr.com is the number of combustion products at a constant volume.
Endothermic heat conversion quantity QRH is determined from (8) and from the expression
In other words, the regeneration degree depends on the conversion process temperature Th and it increases with the decrease in Th and this can be determined from the expression.
The regeneration of the hydrogen degree depends on the conversion process temperature in the thermodynamic cycle Th; it increases with the decrease in Th in the cycle and it can be determined from
The total thermal energy consumption for the fully completed conversion of 1 kg of methanol reaches 6.9 MJ. The heat removed with exhaust gases for the D21 engine is, on average, 30–40 kJ/s, depending on the operating mode.
If the composition of the conversion products corresponds to the conditions of thermodynamic equilibrium (the complete completion of the conversion process), the conditions for achieving the maximum possible degree of regeneration will be realised. If the endothermic effect of the conversion reaction corresponds to the supply of an equivalent amount of heat into the reaction space from an external source (a heating coolant), which in this case is the engine exhaust gas, these conditions will be realised.
It is obvious, that the requirements for engine exhaust gas temperatures will not be met in all engine operating modes. For example, for an unheated engine, the efficiency of the hydrogen reactor will be reduced [48]. However, the operating time of the vehicle engines in the warm-up mode is relatively short.
In addition, catalysts make it possible to ensure the operation of on-board hydrogen reactors at lower operating temperatures. For example, the conversion of methanol in a hydrogen reactor will take place at temperatures of 300–400 °C [49] and these values will determine the minimum possible temperature regimes for the operation of on-board hydrogen reactors. Therefore, we can speak about the possibility of constant operation of on-board hydrogen reactors due to heat recovery of engine exhaust gases.
5. Conclusions
The use of the conversion reactor for hydrogen fuel mixtures of the vehicle power plants seems to be really promising. Due to the technical simplicity, its realisation does not require exclusive financial investments and technological developments. The basic element of the catalytic conversion system, the hydrogen reactor, has an ordinary design of a thermal exchanger. The weight and general characteristics of the reactor are close to standard mufflers. Therefore, the hydrogen reactor is useful to install in the exhaust pipeline.
In addition, this method has the properties of multifunctionality. The proposed method makes it possible at the same time to improve the ecological properties of the car and partially replace oil fuels with alternative fuels from raw renewable energy. Initially, the solution was aimed at solving the problem of transporting hydrogen fuels using motor vehicles and improving the combustion fuel process.
Hydrogen fuel mixtures of methanol conversion usage in vehicle power plants make it possible to solve an urgent task—improving their economic and environmental qualities. Biomass that accumulates solar energy is a practically unlimited raw material for obtaining environmentally friendly engine fuel. The automotive industry has the opportunity to develop a solution to the energy and fuel problem based on the replacement of oil fuels with source materials of plant origin, including alcohol methanol.
Author Contributions
Conceptualisation, S.K. and V.M.; methodology, L.K. and N.K.; software, M.S., N.K. and N.M.; validation, S.K. and V.M.; formal analysis, S.K. and J.M.; investigation, V.M. and M.S.; resources, M.S., J.M. and N.M.; data curation, M.S. and N.M.; writing—original draft preparation, S.K., V.M. and L.K.; writing—review and editing, L.K., N.K. and J.M.; visualisation, L.K., N.K. and N.M.; supervision, S.K. and V.M.; project administration, L.K. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mateichyk, V.; Kostian, N.; Smieszek, M.; Gritsuk, I.; Verbovskyi, V. Review of Methods for Evaluating the Energy Efficiency of Vehicles with Conventional and Alternative Power Plants. Energies 2023, 16, 6331. [Google Scholar] [CrossRef]
- Boichenko, S.; Tarasiuk, O. World Practicies and Prospects of Using Hydrogen As a Motor Fuel. In Proceedings of the 2022 IEEE 8th International Conference on Energy Smart Systems, ESS 2022—Proceedings, Kyiv, Ukraine, 12–14 October 2022; pp. 127–132. [Google Scholar] [CrossRef]
- Tsiuman, M.; Mateichyk, V.; Smieszek, M.; Sadovnyk, I.; Artemenko, R.; Tsiuman, Y.; Gritsuk, I.; Koval, A. The System for Adding Hydrogen-containing Gas to the Air Charge of the Spark Ignition Engine Using a Thermoelectric Generator. SAE Tech. Pap. 2020, 1, 2142. [Google Scholar] [CrossRef]
- Korohodskyi, V.; Leontiev, D.; Rogovyi, A.; Voronkov, O.; Prokopiuk, D. Research of Spark Ignition Engine and Internal Mixture Formation Using Single-Zone, Two-Zone and Three-Zone Calculation Model of It Working Process. SAE Tech. Pap. 2022, 1, 1000. [Google Scholar] [CrossRef]
- Panchuk, M.; Kryshtopa, S.; Panchuk, A. Innovative Technologies for the Creation of a New Sustainable, Environmentally Neutral Energy Production in Ukraine. In Proceedings of the 2020 International Conference on Decision Aid Sciences and Application, DASA, Sakheer, Bahrain, 8–9 November 2020; pp. 732–737. [Google Scholar] [CrossRef]
- Ribun, V.; Boichenko, S.; Kale, U. Advances in gas-to-liquid technology for environmentally friendly fuel synthesis: Analytical review of world achievements. Energy Rep. 2023, 9, 5500–5508. [Google Scholar] [CrossRef]
- Gorski, K.; Smigins, R. Impact of ether/ethanol and biodiesel blends on combustion process of compression ignition engine. In Proceedings of the 10th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 26–27 May 2021; Volume 10, pp. 260–265. Available online: https://www.tf.lbtu.lv/conference/proceedings2011/Papers/048_Gorski.pdf (accessed on 1 April 2023).
- Liu, J.; Liang, W.; Ma, H.; Ji, Q.; Xiang, P.; Sun, P.; Wang, P.; Wei, M.; Ma, H. Effects of integrated aftertreatment system on regulated and unregulated emission characteristics of non-road methanol/diesel dual-fuel engine. Energy 2023, 282, 128819. [Google Scholar] [CrossRef]
- Panchuk, M.; Kryshtopa, S.; Sladkowski, A.; Panchuk, A.; Mandryk, I. Efficiency of production of motor biofuels for water and land transport. Nase More 2019, 66, 6–12. [Google Scholar] [CrossRef]
- Marocco, P.; Ferrero, D.; Gandiglio, M.; Ortiz, M.; Sundseth, K.; Lanzini, A.; Santarelli, M. A study of the techno-economic feasibility of H2-based energy storage systems in remote areas. Energy Convers. Manag. 2020, 211, 112768. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Górski, K.; Longwic, R.; Smigins, R.; Kryshtopa, L.; Matijošius, J. Using Hydrogen Reactors to Improve the Diesel Engine Performance. Energies 2022, 15, 3024. [Google Scholar] [CrossRef]
- Modi, P.; Aguey-Zinsou, K. Titanium-iron-manganese (TiFe0.85Mn0.15) alloy for hydrogen storage: Reactivation upon oxidation. Int. J. Hydrog. Energy 2019, 44, 16757–16764. [Google Scholar] [CrossRef]
- Lázár, M.; Mihálik, I.; Brestovič, T.; Jasminská, N.; Tóth, L.; Dobáková, R.; Duda, F.; Kmeťová, Ľ.; Hudák, Š. A Newly Proposed Method for Hydrogen Storage in a Metal Hydride Storage Tank Intended for Maritime and Inland Shipping. J. Mar. Sci. Eng. 2023, 11, 1643. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.; Broom, D.; Zlotea, C. Materials for hydrogen-based energy storage—past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Comanescu, C. Graphene Supports for Metal Hydride and Energy Storage Applications. Crystals 2023, 13, 878. [Google Scholar] [CrossRef]
- Zhang, T.; Uratani, J.; Huang, Y.; Xu, L.; Griffiths, S.; Ding, Y. Hydrogen liquefaction and storage: Recent progress and perspectives. Renew. Sustain. Energy Rev. 2023, 176, 113204. [Google Scholar] [CrossRef]
- Beasy, K.; Emery, S.; Pryor, K.; Vo, T.A. Skilling the green hydrogen economy: A case study from Australia. Int. J. Hydrog. Energy 2023, 48, 19811–19820. [Google Scholar] [CrossRef]
- Stenina, I.; Yaroslavtsev, A. Modern Technologies of Hydrogen Production. Processes 2023, 11, 56. [Google Scholar] [CrossRef]
- Van, L.P.; Chi, K.D.; Duc, T.N. Review of hydrogen technologies based microgrid: Energy management systems, challenges and future recommendations. Int. J. Hydrog. Energy 2023, 48, 14127–14148. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Panchuk, M.; Kozak, F.; Dolishnii, B.; Mykytii, I.; Skalatska, O. Fuel economy raising of alternative fuel converted diesel engines. East. Eur. J. Enterp. Technol. 2018, 4/8, 6–13. [Google Scholar] [CrossRef]
- Górski, K.; Smigins, R.; Matijošius, J.; Rimkus, A.; Longwic, R. Physicochemical Properties of Diethyl Ether—Sunflower Oil Blends and Their Impact on Diesel Engine Emissions. Energies 2022, 15, 4133. [Google Scholar] [CrossRef]
- Smigins, R. Ecological Impact of CNG/gasoline bi-fuelled vehicles. Proc. Conf. Eng. Rural. Dev. 2017, 16, 128–133. [Google Scholar] [CrossRef]
- Boopathi, D.; Sonthalia, A.; Devanand, S. Experimental investigations on the effect of hydrogen induction on performance and emission behaviour of a single cylinder diesel engine fuelled with palm oil methyl ester and its blend with diesel. J. Eng. Sci. Technol. 2017, 12, 1972–1987. [Google Scholar]
- Cernat, A.; Pana, C.; Negurescu, N.; Nutu, C.; Fuiorescu, D.; Lazaroiu, G. Aspects of an experimental study of hydrogen use at automotive diesel engine. Heliyon 2023, 9, e13889. [Google Scholar] [CrossRef]
- Cherednichenko, O. Efficiency Analysis of Methanol Usage for Marine Turbine Power Plant Operation Based on Waste Heat Chemical Regeneration. Probl. Energeticii Reg. 2019, 1, 102–111. [Google Scholar] [CrossRef]
- Rudbahs, R.; Smigins, R. Experimental research on biodiesel compatibility with fuel system elastomers. Proc. Conf. Eng. Rural. Dev. 2014, 13, 278–282. [Google Scholar]
- Gritsuk, I.; Pohorletskyi, D.; Mateichyk, V.; Ahieiev, M.; Sadovnyk, I. Improving the Processes of Thermal Preparation of an Automobile Engine with Petrol and Gas Supply Systems (Vehicle Engine with Petrol and LPG Supplying Systems). SAE Tech. Pap. 2020, 1, 2031. [Google Scholar] [CrossRef]
- Smigins, R. Perspectives of low level ethanol and biodiesel/diesel fuel blends on diesel engine emission reduction. In Proceedings of the Transport Means-Proceedings of the International Conference, Juodkrante, Lithuania, 5 October 2016; pp. 337–341. [Google Scholar]
- Boretti, A. Advantages and Disadvantages of Diesel Single and Dual-Fuel Engines. Front. Mech. Eng. 2019, 5, 64. [Google Scholar] [CrossRef]
- Afanasev, A.; Tretyakov, A. Simulation of diesel engine energy conversion processes. J. Min. Inst. 2016, 222, 839–852. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Panchuk, M.; Dolishnii, B.; Hnyp, M.; Skalatska, O. Research into emissions of nitrogen oxides when converting the diesel engines to alternative fuels. East. Eur. J. Enterp. Technol. 2018, 1, 16–22. [Google Scholar] [CrossRef]
- Mäyrä, O.; Leiviskä, K. Modeling in Methanol Synthesis. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 475–492. [Google Scholar] [CrossRef]
- Liu, Z. Economic Analysis of Methanol Production from Coal/Biomass Upgrading. Energy Sources Part B-Econ. Plan. Policy 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Tartakovsky, L.; Baibikov, V.; Veinblat, M. Modeling Methanol Steam Reforming for Internal Combustion Engine. Energy Power 2014, 4, 50–56. [Google Scholar] [CrossRef]
- Shamsul, N.; Kamarudin, S.; Rahman, N.; Kofli, N. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- He, L.; Fu, Y.; Lidstrom, M. Quantifying Methane and Methanol Metabolism of “Methylotuvimicrobium buryatense” 5GB1C under Substrate Limitation. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Wargula, Ł.; Waluś, J.K.; Krawiec, P. The problems of measuring the temperature of the small engines (SI) on the example of a drive for non-road mobile machines. MATEC Web Conf. 2019, 254, 04004. [Google Scholar] [CrossRef]
- Tang, G.; Wang, S.; Zhang, L.; Shang, H. Diagnosis and Improvement of Combustion Characteristics of Methanol Miniature Reciprocating Piston Internal Combustion Engine. Micromachines 2020, 11, 96. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhong, F.; Xu, H. Numerical Study on a Diesel/Dissociated Methanol Gas Compression Ignition Engine with Exhaust Gas Recirculation. Appl. Sci 2023, 13, 9612. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol production and applications: An overview. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. [Google Scholar] [CrossRef]
- Karvounis, P.; Theotokatos, G.; Vlaskos, I.; Hatziapostolou, A. Methanol Combustion Characteristics in Compression Ignition Engines: A Critical Review. Energies 2023, 16, 8069. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Borisut, P.; Nuchitprasittichai, A. Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation-Based Optimization. Front. Energy Res. 2019, 7, 00081. [Google Scholar] [CrossRef]
- Drakon, A.; Eremin, A. The Influence of Biofuels Addition on Shock-Induced Ignition and Combustion of Methane–Hydrogen Mixtures. Fire 2023, 6, 460. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, M.; Cui, Y.; Ming, Z.; Wang, T.; Zhang, C.; Ampah, J.D.; Jin, C.; Huang, H.; Liu, H. Effects of Methanol Application on Carbon Emissions and Pollutant Emissions Using a Passenger Vehicle. Processes 2022, 10, 525. [Google Scholar] [CrossRef]
- Zhennan, H.; Sulong, G.; Xi, Z.; Shipei, X.; Ping, A.; Jiguang, C.; Jun, Y.; Feng, L.; Suyi, Z.; Miao, L.; et al. Reaction decoupling in thermochemical fuel conversion and technical progress based on decoupling using fluidized bed. Carbon Resour. Convers. 2018, 1, 109–125. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Tong, L.; Zheng, Z.; Yao, M. Investigation on the potential of high efficiency for internal combustion engines. Energies 2018, 11, 513. [Google Scholar] [CrossRef]
- Ma, B.; Yao, A.; Yao, C.; Chen, C.; Qu, G.; Wang, W.; Ai, Y. Multiple combustion modes existing in the engine operating in diesel methanol dual fuel. Energy 2021, 234, 121285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).