Bio-Inspired Artificial Receptor with Integrated Tactile Sensing and Pain Warning Perceptual Abilities
Abstract
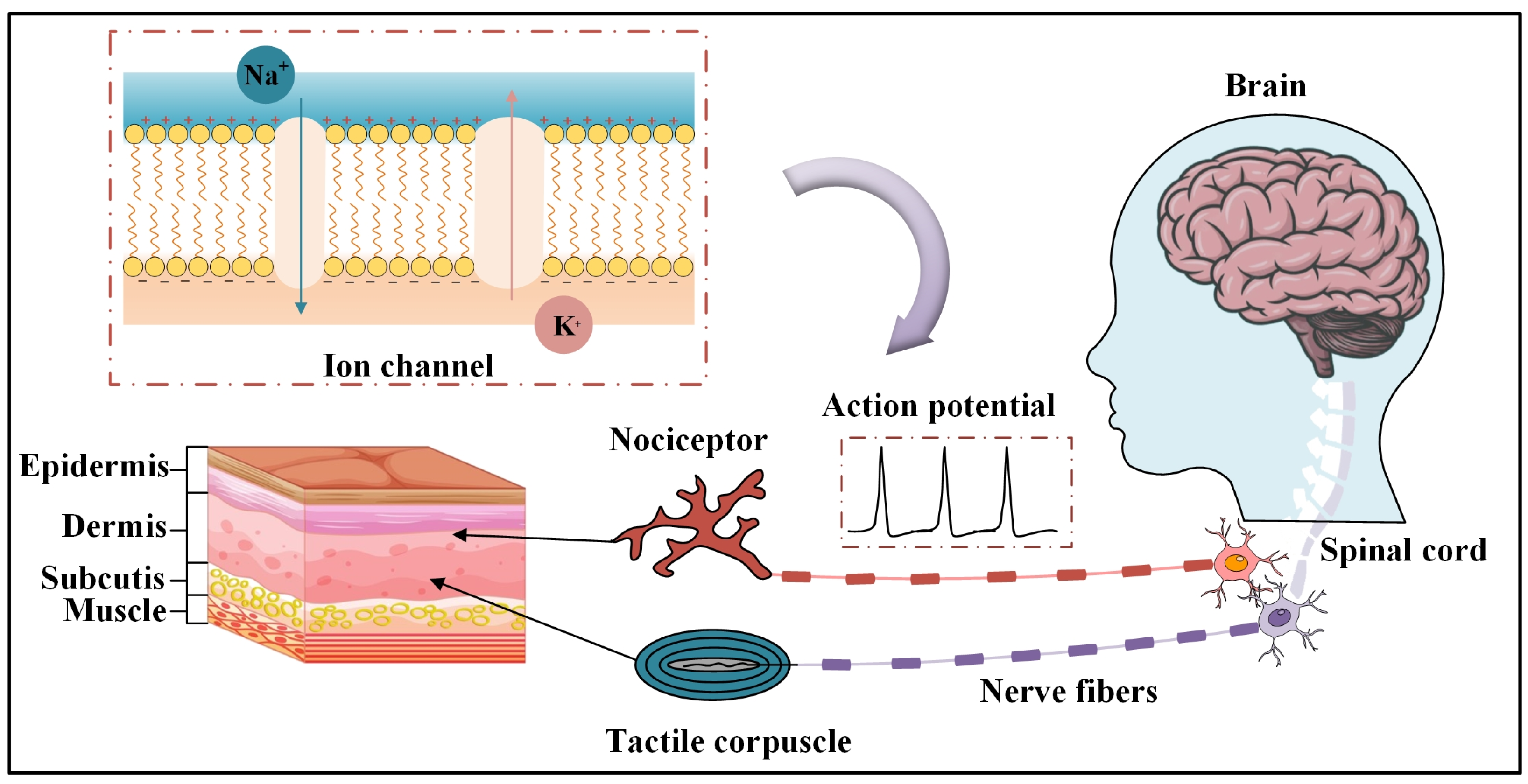
1. Introduction
2. Materials and Methods
2.1. Materials
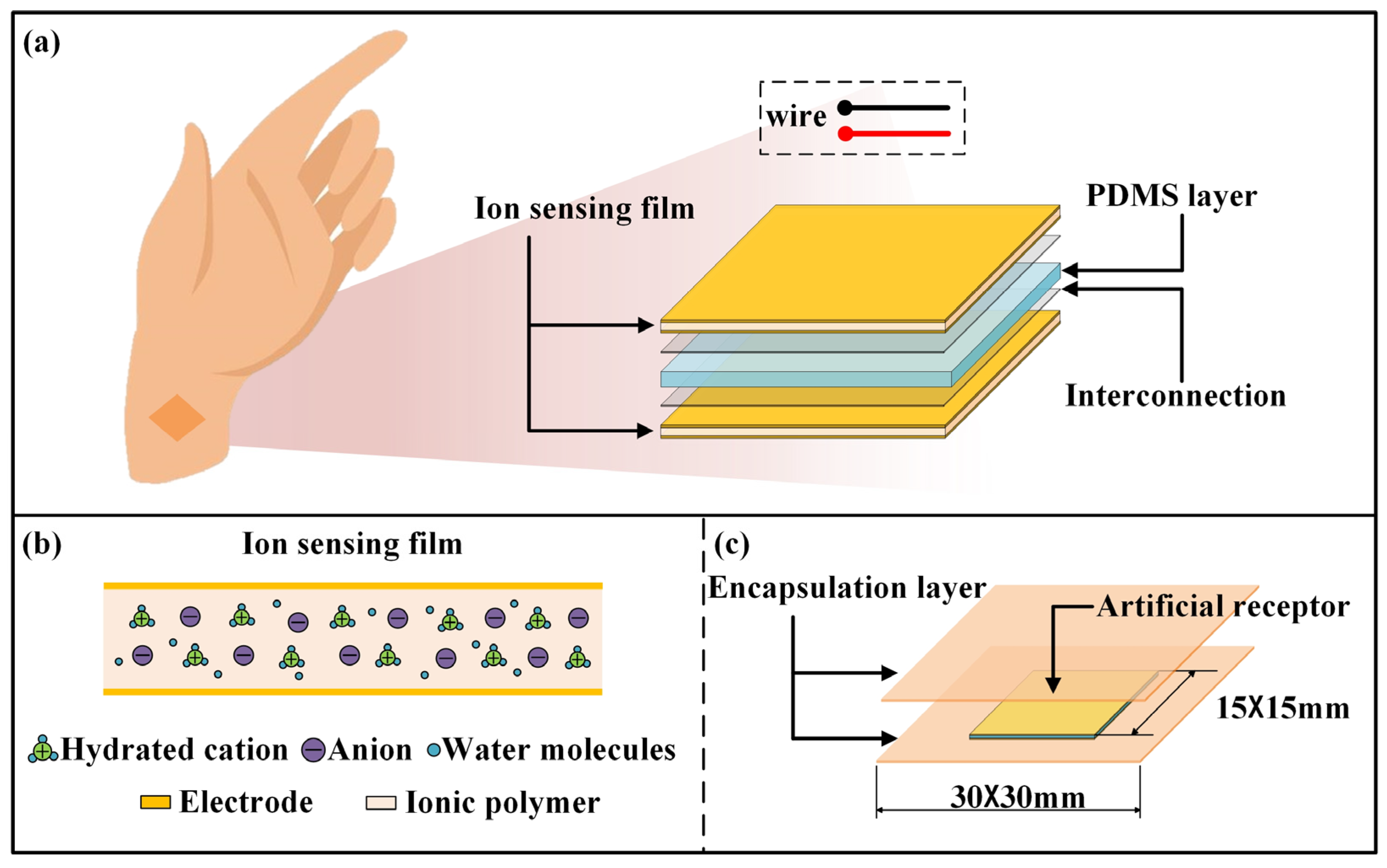
2.2. Preparation of Receptor
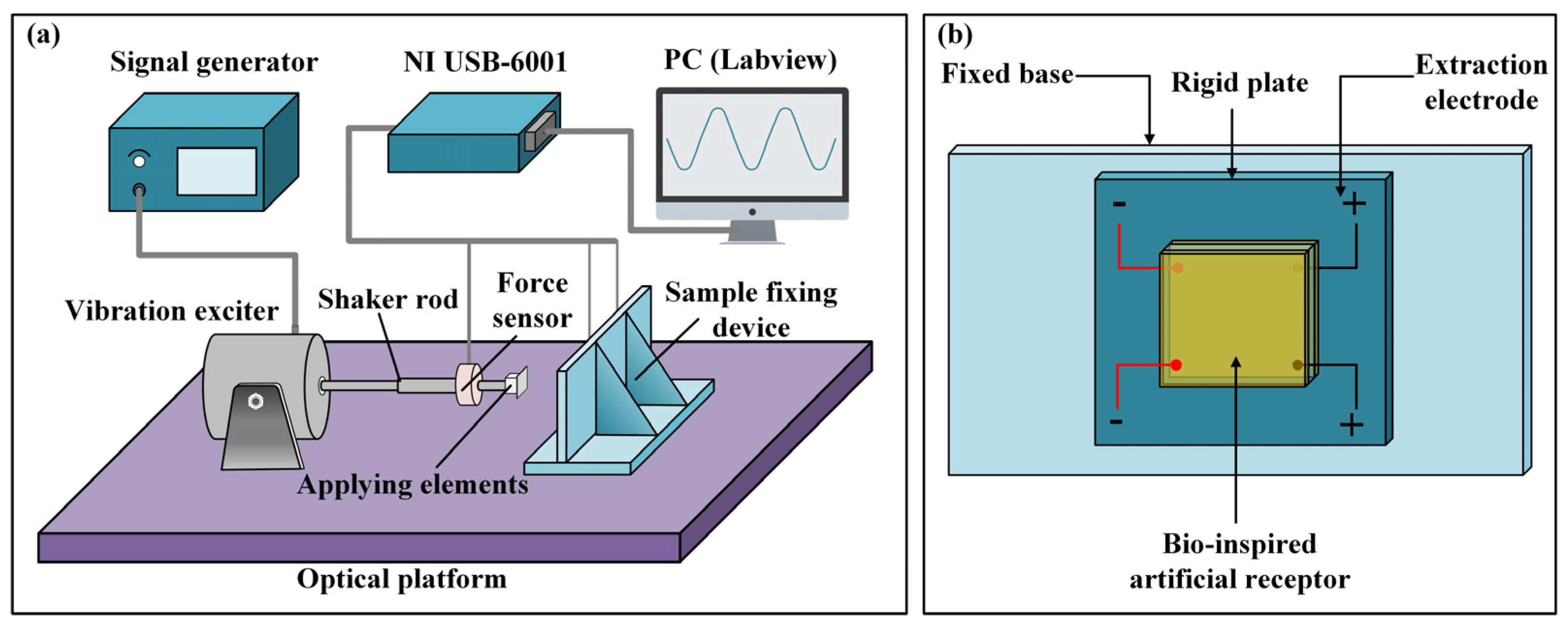
2.3. Experimental Set-Up
3. Results
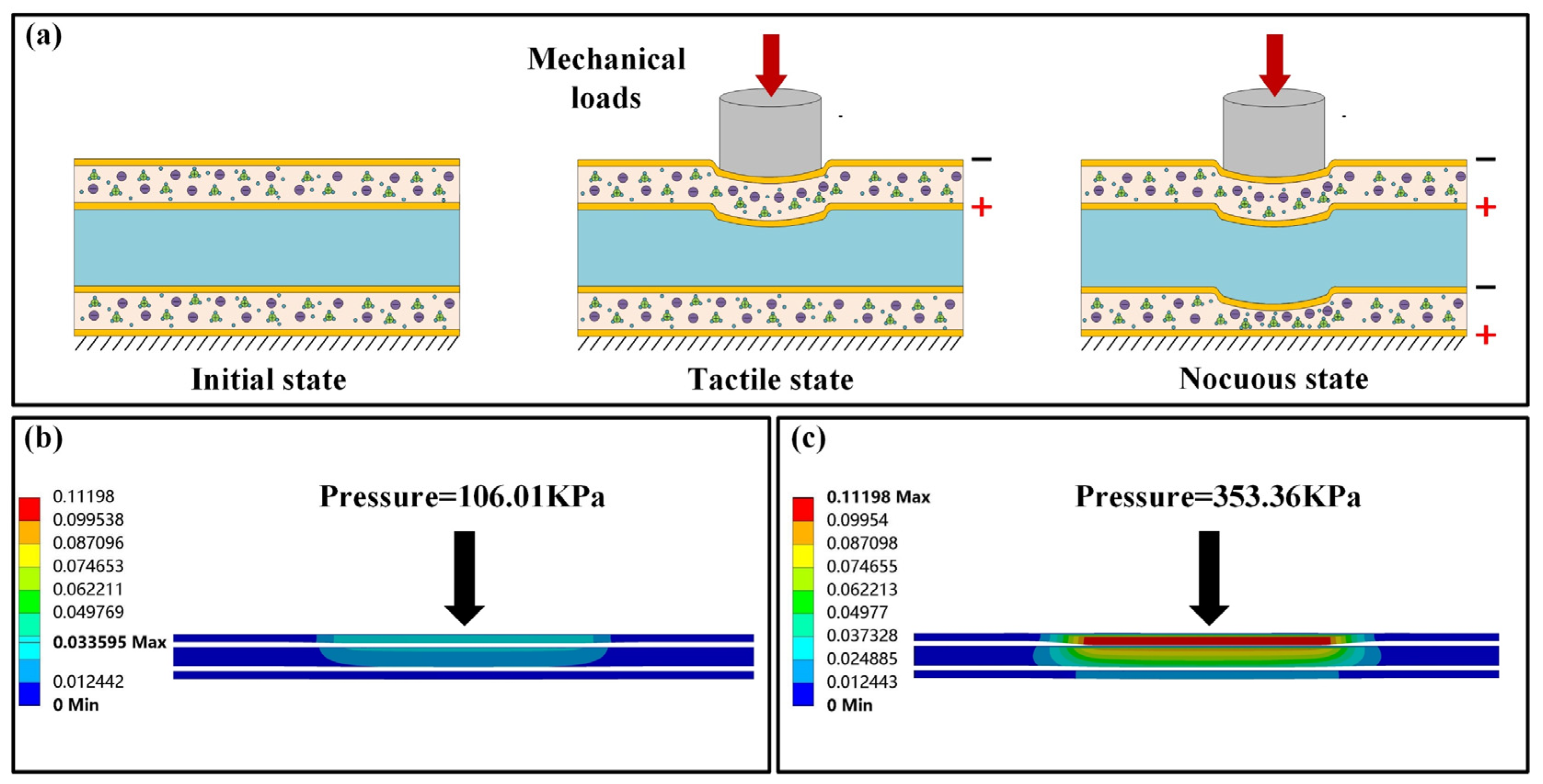
3.1. Sensing Analysis of the Receptor
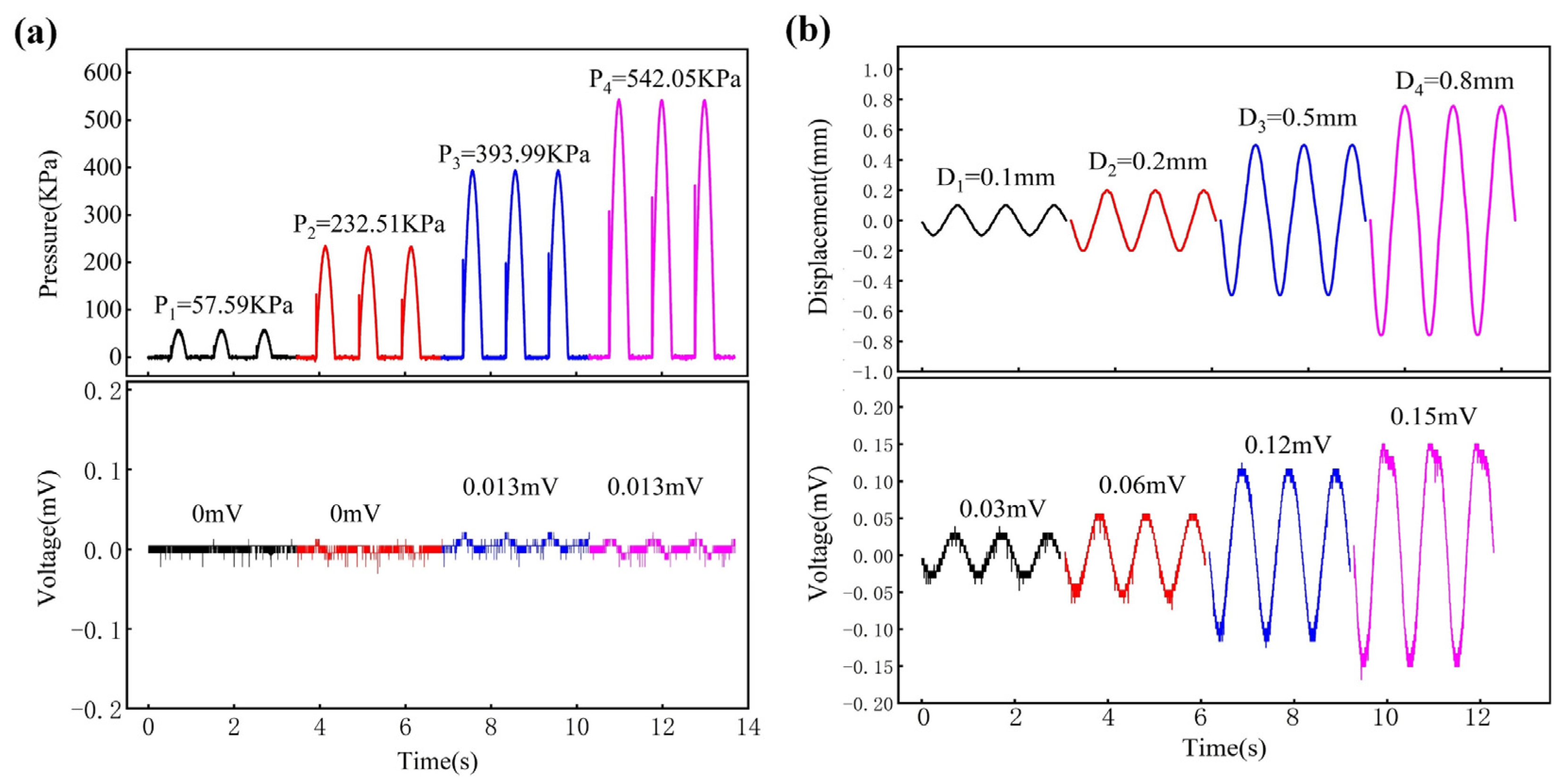
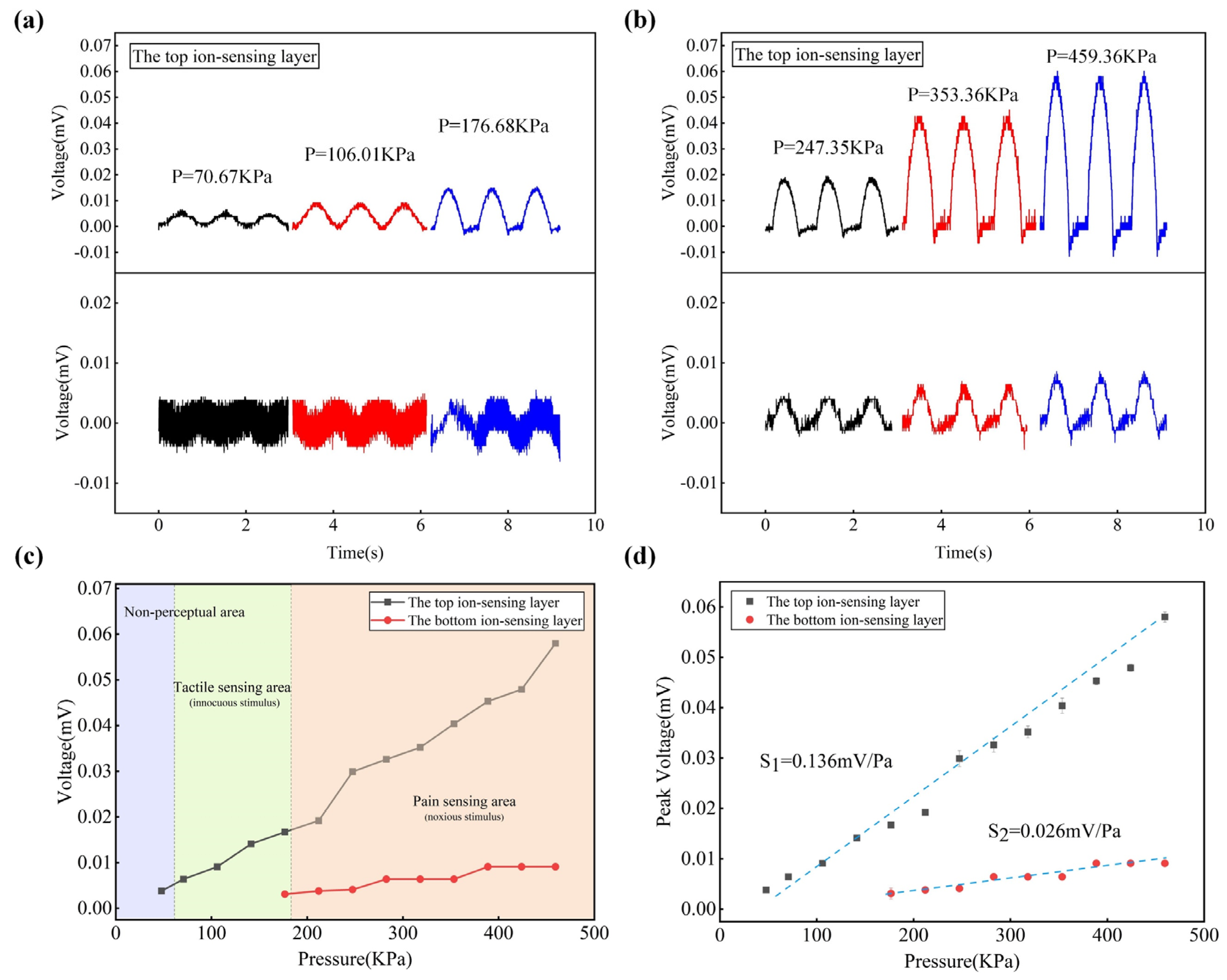
3.2. Characterization of Touch and Pain
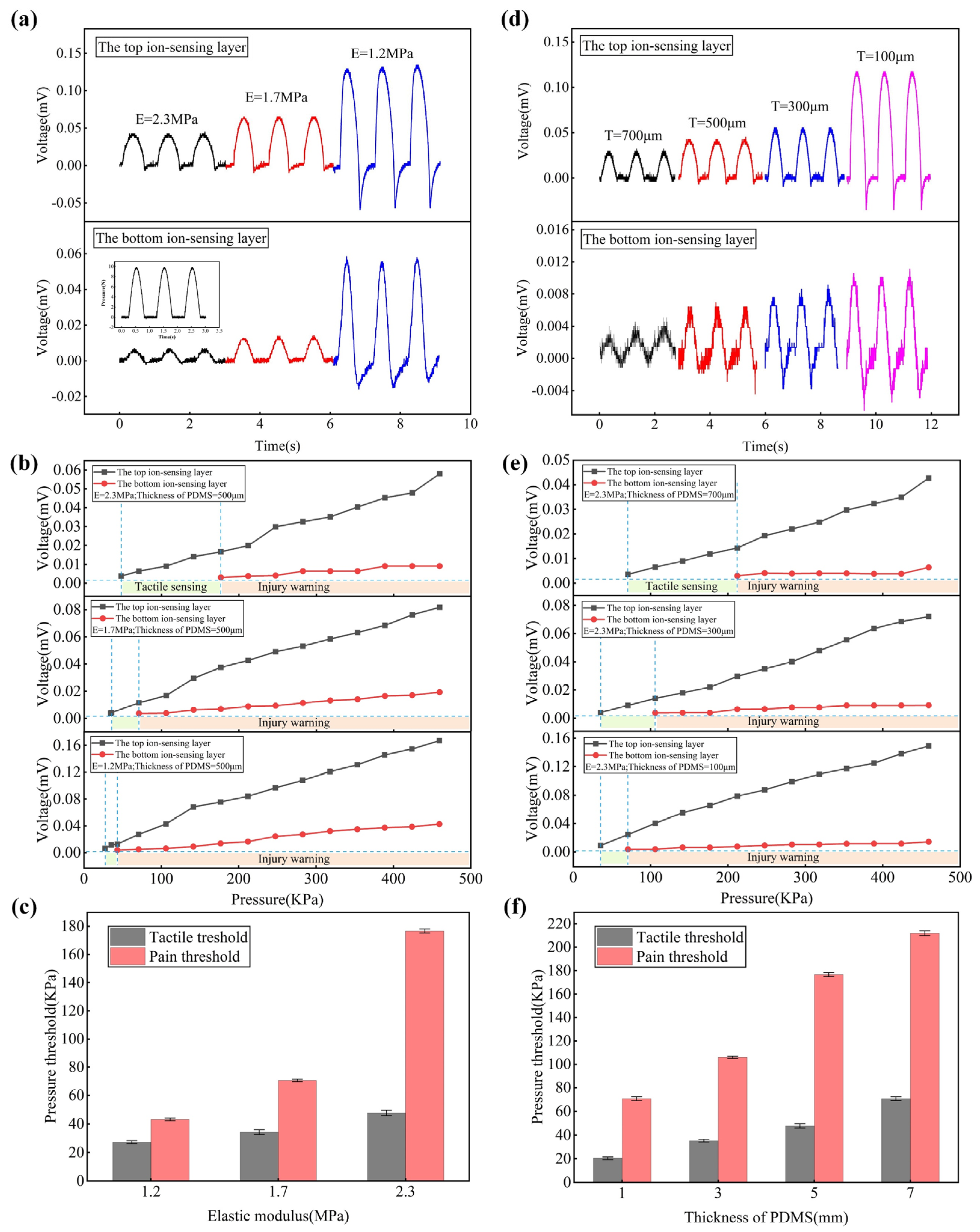
3.3. PDMS Layer Effects
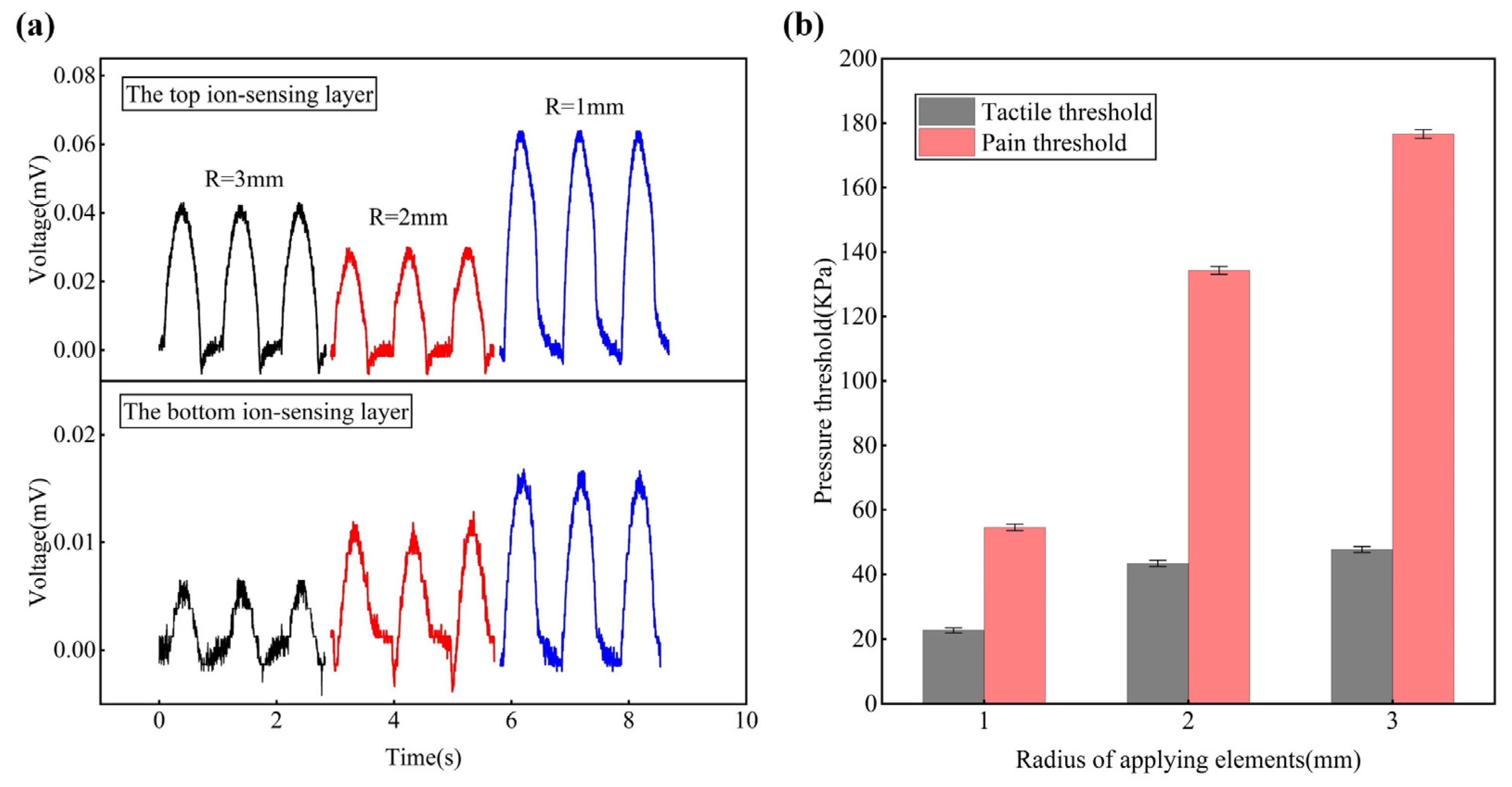
3.4. Contract Area Effects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McGlone, F.; Wessberg, J.; Olausson, H. Discriminative and Affective Touch: Sensing and Feeling. Neuron 2014, 82, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.S.; Marshall, A.G.; Makdani, A.; Jarocka, E.; Liljencrantz, J.; Ridderström, M.; Shaikh, S.; Saade, D.; Donkervoort, S.; Olausson, H.; et al. An ultrafast system for signaling mechanical pain in human skin. Sci. Adv. 2019, 5, eaaw1297. [Google Scholar] [CrossRef]
- Li, X.; Toyoda, H. Role of leak potassium channels in pain signaling. Brain Res. Bull. 2015, 119, 73–79. [Google Scholar] [CrossRef]
- Wood, J.N.; Abrahamsen, B.; Baker, M.D.; Boorman, J.D.; Donier, E.; Drew, L.J.; Nassar, M.A.; Okuse, K.; Seereeram, A.; Zhao, J.; et al. Ion channel activities implicated in pathological pain. Novartis Found. Symp. 2004, 261, 32–40. [Google Scholar]
- Deutch, A.Y. Neuroscience: Exploring the brain. J. Clin. Psychiatry 1999, 60, 59. [Google Scholar] [CrossRef]
- De Maria, G.; Natale, C.; Pirozzi, S. Directions Toward Effective Utilization of Tactile Skin: A Review. IEEE Sens. J. 2014, 14, 4109. [Google Scholar] [CrossRef]
- Lin, W.; Wang, B.; Peng, G.; Shan, Y.; Hu, H.; Yang, Z. Skin-Inspired Piezoelectric Tactile Sensor Array with Crosstalk-Free Row plus Column Electrodes for Spatiotemporally Distinguishing Diverse Stimuli. Adv. Sci. 2021, 8, 2002817. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tao, L.-Q.; Yu, J.; Wang, Z.; Zhu, C.; Chen, X. Integrated Sensing and Warning Multifunctional Devices Based on the Combined Mechanical and Thermal Effect of Porous Graphene. ACS Appl. Mater. Interfaces 2020, 14, 53049–53057. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.; Liu, H.; Zhang, Y.; Lin, Q.; Chen, T.; Sun, L. Multifunctional Self-Powered E-Skin with Tactile Sensing and Visual Warning for Detecting Robot Safety. Adv. Mater. Interfaces 2020, 7, 2000536. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Li, J.; Zhou, Q.; Xiao, Y.; Zhang, K.; Luo, B.; Zhou, J.; Hu, B. Dual-Mode Electronic Skin with Integrated Tactile Sensing and Visualized Injury Warning. ACS Appl. Mater. Interfaces 2017, 9, 37493–37500. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, S.; Lu, Y.; Asghar, W.; Cao, J.; Hu, C.; Li, R. Bio-Inspired Multi-Mode Pain-Perceptual System (MMPPS) with Noxious Stimuli Warning, Damage Localization, and Enhanced Damage Protection. Adv. Sci. 2021, 8, 2004208. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Walia, S.; Naznee, S.; Taha, M.; Nirantar, S.; Rahman, F.; Bhaskaran, M.; Sriram, S. Artificial Somatosensors: Feedback Receptors for Electronic Skins. Adv. Intell. Syst. 2020, 2, 2000094. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Tang, G.; Ru, J.; Zhu, Z.; Li, B.; Guo, C.F.; Li, L.; Zhu, D. Ionic Flexible Sensors: Mechanisms, Materials, Structures, and Applications. Adv. Funct. Mater. 2022, 32, 2110417. [Google Scholar] [CrossRef]
- Hao, M.; Wang, Y.; Zhu, Z.; He, Q.; Zhu, D.; Luo, M. A Compact Review of IPMC as Soft Actuator and Sensor: Current Trends, Challenges, and Potential Solutions from Our Recent Work. Front. Robot. AI 2019, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kim, Y.M.; Moon, H.C. Porous Ion Gel: A Versatile Ionotronic Sensory Platform for High-Performance, Wearable Ionoskins with Electrical and Optical Dual Output. ACS Nano 2021, 15, 15132–15141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, W.; Ren, Y.; Liu, Z.; Zou, X.; Hu, Y.; Yan, F. Moisture-Wicking, Breathable, and Intrinsically Antibacterial Electronic Skin Based on Dual-Gradient Poly (ionic liquid) Nanofiber Membranes. Adv. Mater. 2022, 34, 2106570. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, Z.; Wang, J.; He, Q.; Luo, M. The Effects of Dimensions on the Deformation Sensing Performance of Ionic Polymer-Metal Composites. Sensors 2019, 19, 2104. [Google Scholar] [CrossRef] [PubMed]
- MohdIsa, W.; Hunt, A.; HosseinNia, S.H. Sensing and Self-Sensing Actuation Methods for Ionic Polymer–Metal Composite (IPMC): A Review. Sensors 2019, 19, 3967. [Google Scholar] [CrossRef]
- Gudarzi, M.; Smolinski, P.; Wang, Q.-M. Bending mode ionic polymer-metal composite (IPMC) pressure sensors. Measurement 2017, 103, 250–257. [Google Scholar] [CrossRef]
- Gudarzi, M.; Smolinski, P.; Wang, Q.-M. Compression and shear mode ionic polymer-metal composite (IPMC) pressure sensors. Sensors Actuators A Phys. 2017, 260, 99–111. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, G.; Zhao, C.; Mei, D.; Zhao, X.; Ji, Y.; Li, B. The effects of contact area on pressure sensing of ionic polymer metal composite sensor with a soft substrate. Smart Mater. Struct. 2022, 31, 8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Tang, G.; Zhao, C.; Mei, D.; Ji, Y.; Xiang, C.; Li, L.; Li, B.; Wang, Y. Bio-Inspired Artificial Receptor with Integrated Tactile Sensing and Pain Warning Perceptual Abilities. Machines 2022, 10, 968. https://doi.org/10.3390/machines10110968
Zhao X, Tang G, Zhao C, Mei D, Ji Y, Xiang C, Li L, Li B, Wang Y. Bio-Inspired Artificial Receptor with Integrated Tactile Sensing and Pain Warning Perceptual Abilities. Machines. 2022; 10(11):968. https://doi.org/10.3390/machines10110968
Chicago/Turabian StyleZhao, Xin, Gangqiang Tang, Chun Zhao, Dong Mei, Yujun Ji, Chaoqun Xiang, Lijie Li, Bo Li, and Yanjie Wang. 2022. "Bio-Inspired Artificial Receptor with Integrated Tactile Sensing and Pain Warning Perceptual Abilities" Machines 10, no. 11: 968. https://doi.org/10.3390/machines10110968
APA StyleZhao, X., Tang, G., Zhao, C., Mei, D., Ji, Y., Xiang, C., Li, L., Li, B., & Wang, Y. (2022). Bio-Inspired Artificial Receptor with Integrated Tactile Sensing and Pain Warning Perceptual Abilities. Machines, 10(11), 968. https://doi.org/10.3390/machines10110968








