Muscle Selection Using ICA Clustering and Phase Variable Method for Transfemoral Amputees Estimation of Lower Limb Joint Angles
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Data
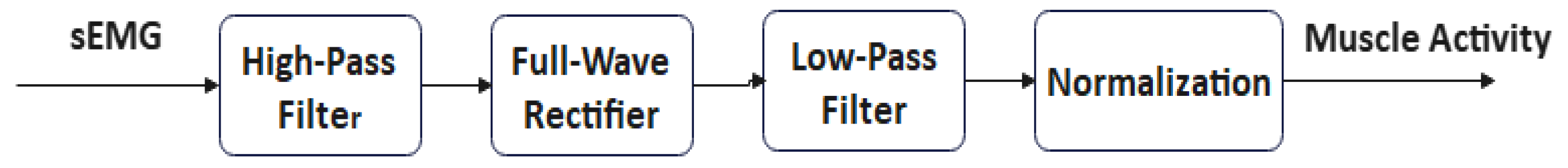
2.2. Signal Preprocessing
2.3. ICA Clustering Method
2.3.1. Independent Component Analysis of sEMG
2.3.2. Interlayer Clustering
2.4. Feature Extraction and Dimensionality Reduction
2.5. Mapping of Neural Networks and Phase Variable Methods
2.6. Evaluation of the Angle Estimation
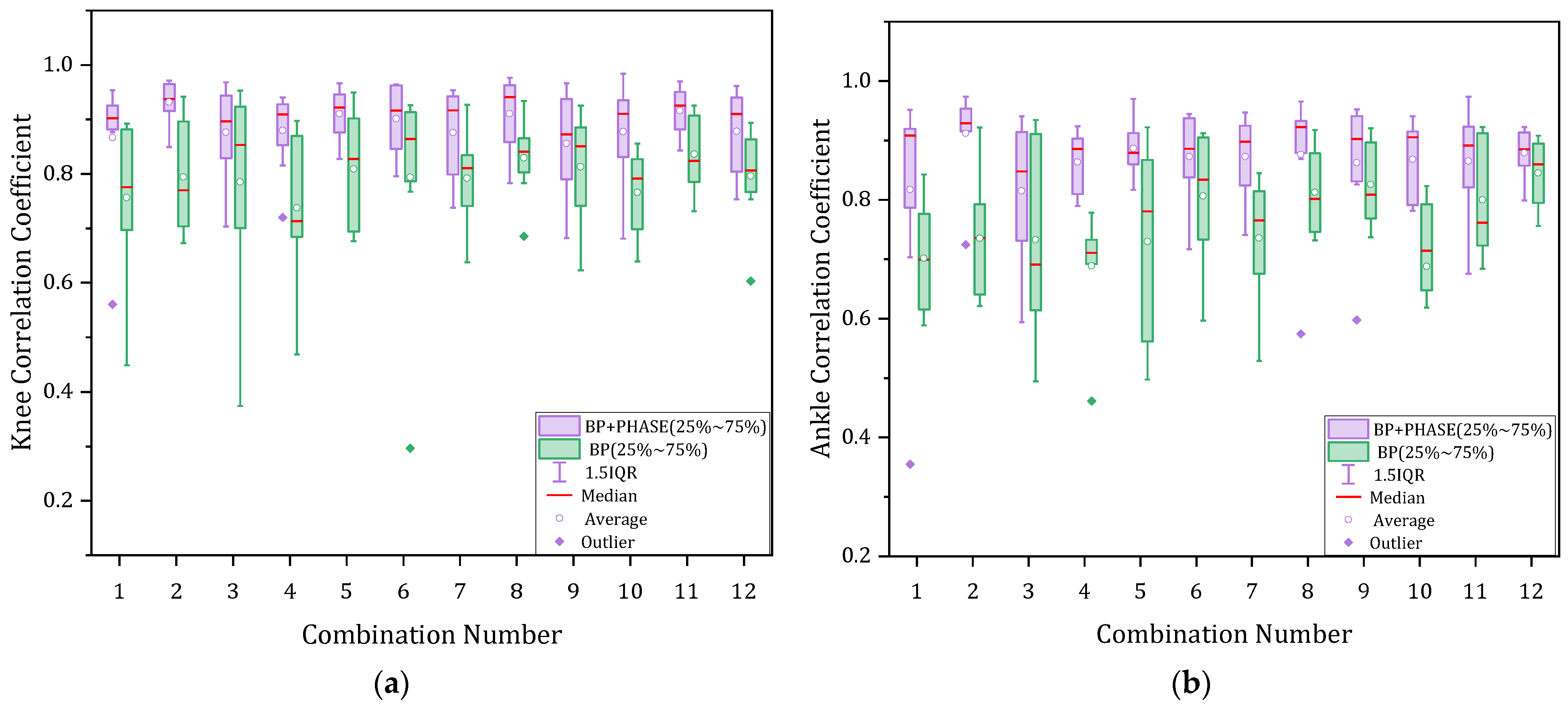
3. Results
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, Y.U.; Zhang, Y.; Xia, C. Stydy of Gait Pattern Recognition Based on Fusion of Mechanomyography and Attitude Angle Signal. J. Mech. Med. Biol. 2020, 20, 1069–1072. [Google Scholar]
- Torrealba, R.R.; Fonseca-Rojas, E.D. Toward the Development of Knee Prostheses: Review of Current Active Devices. Appl. Mech. Rev. 2019, 73, 030801. [Google Scholar] [CrossRef]
- Hussain, T.; Iqbal, N.; Maqbool, H.F.; Khan, M.; Tahir, M. Amputee walking mode recognition based on mel frequency cepstral coefficients using surface electromyography sensor. Int. J. Sens. Netw. 2020, 32, 139. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Miran, S.M.; Xi, X.; Xue, A. Surface Electromyography Based Estimation of Knee Joint Angle by Using Correlation Dimension of Wavelet Coefficient. IEEE Access 2019, 7, 60522–60531. [Google Scholar] [CrossRef]
- Duan, F.; Dai, L.; Chang, W.; Chen, Z.; Zhu, C.; Li, W. sEMG-Based Identification of Hand Motion Commands Using Wavelet Neural Network Combined With Discrete Wavelet Transform. IEEE Trans. Ind. Electron. 2016, 63, 1923–1934. [Google Scholar] [CrossRef]
- Scheme, E.; Englehart, K. Electromyogram pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. J. Rehabil. Res. Dev. 2011, 48, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Keleş, A.D.; Yucesoy, C.A. Development of a neural network based control algorithm for powered ankle prosthesis. J. Biomech. 2020, 113, 110087. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, I.S.; Agarwal, R.; Ryait, H.S. Principal component analysis-based muscle identification for myoelectric-controlled exoskeleton knee. J. Appl. Stats 2017, 44, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Smirnov, K.; Lucio, M.; Gougeon, R.D.; Alexandre, H.; Schmitt-Kopplin, P. MetICA: Independent component analysis for high-resolution mass-spectrometry based non-targeted metabolomics. BMC Bioinform. 2016, 17, 114. [Google Scholar] [CrossRef]
- Han, J.; Ding, Q.; Xiong, A.; Zhao, X. A State-Space EMG Model for the Estimation of Continuous Joint Movements. IEEE Trans. Ind. Electron. 2015, 62, 4267–4275. [Google Scholar] [CrossRef]
- Cheron, G.; Leurs, F.A.; Bengoetxea, A.; Draye, J.P.; Dan, B. A dynamic recurrent neural network for multiple muscles electromyographic mapping to elevation angles of the lower limb in human locomotion. J. Neurosci. Methods 2003, 129, 95–104. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Cheng, Y.; Xi, N. Surface EMG based continuous estimation of human lower limb joint angles by using deep belief networks. Biomed. Signal Process. Control. 2018, 40, 335–342. [Google Scholar] [CrossRef]
- Chai, R.; Tran, Y.; Ling, S.H.; Craig, A.; Nguyen, H.T. Combining ICA Clustering and Power Spectral Density for Feature Extraction of Mental Fatigue of Spinal Cord Injury Patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Ding, Q.C.; Xiong, A.B.; Zhao, X.G.; Han, J.D. A Review on Researches and Applications of sEMG-based Motion Intent Recognition Methods. Acta Autom. Sin. 2016, 42, 13–25. [Google Scholar]
- Himberg, J.; Hyvrinen, A.; Esposito, F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage 2004, 22, 1214–1222. [Google Scholar] [CrossRef]
- Wei, P.; Bao, R.; Fan, Y. Comparing the reliability of different ICA algorithms for fMRI analysis. PloS ONE 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Camargo, J.; Ramanathan, A.; Flanagan, W.; Young, A. A comprehensive, open-source dataset of lower limb biomechanics in multiple conditions of stairs, ramps, and level-ground ambulation and transitions. J. Biomech. 2021, 119, 110320. [Google Scholar] [CrossRef]
- Naik, G.R.; Al-Timemy, A.H.; Nguyen, H.T. Transradial Amputee Gesture Classification Using an Optimal Number of sEMG Sensors: An Approach Using ICA Clustering. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 837–846. [Google Scholar] [CrossRef]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the dimensionality of data with neural networks. Dep. Comput. Sci. Univ. Tor. 2006, 313, 504–507. [Google Scholar] [CrossRef]
- Villarreal, D.J.; Poonawala, H.A.; Gregg, R.D. A Robust Parameterization of Human Gait Patterns Across Phase-Shifting Perturbations. IEEE Trans. Neural. Syst. Rehabil. Eng. 2017, 25, 265–278. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Zhou, M.; Xu, W. An adaptive framework of real-time continuous gait phase variable estimation for lower-limb wearable robots. Robot. Auton. Syst. 2021, 143, 103842. [Google Scholar] [CrossRef]
- Embry, K.R.; Gregg, R.D. Analysis of Continuously Varying Kinematics for Prosthetic Leg Control Applications. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 29, 262–272. [Google Scholar] [CrossRef] [PubMed]
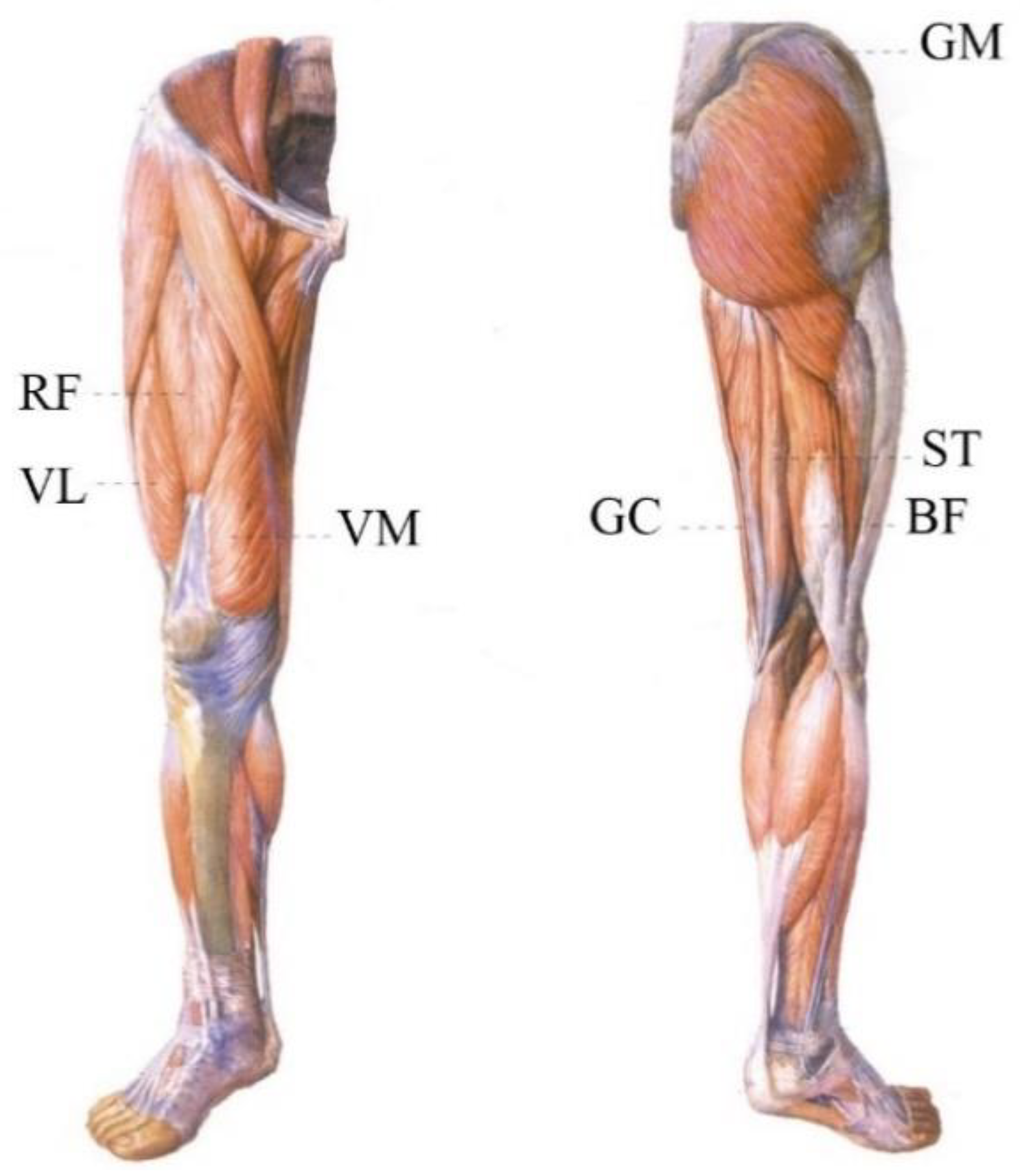


| Subject ID | Age | Gender | Height (m) | Mass (kg) | Subject ID | Age | Gender | Height (m) | Mass (kg) |
|---|---|---|---|---|---|---|---|---|---|
| AB09 | 21 | F | 1.63 | 63.5 | AB18 | 19 | F | 1.82 | 60.1 |
| AB10 | 22 | M | 1.75 | 83.9 | AB23 | 20 | M | 1.80 | 76.8 |
| AB11 | 21 | M | 1.75 | 77.1 | AB24 | 21 | F | 1.73 | 72.6 |
| AB12 | 24 | M | 1.74 | 86.2 | AB25 | 20 | F | 1.63 | 52.2 |
| AB14 | 22 | F | 1.52 | 58.4 | AB28 | 33 | F | 1.69 | 62.1 |
| AB15 | 21 | M | 1.78 | 96.2 | AB30 | 31 | M | 1.77 | 77.0 |
| Subject ID | Class | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|---|---|---|---|---|
| AB09 | IC | 6, 7, 5 | 4, 1 | 3, 2 | |
| Sensors | RF, VL, VM | BF, ST | GC, GM | ||
| AB10 | IC | 6, 7, 5, 4 | 1 | 2, 3 | |
| Sensors | VM, VL, RF, GM | GC | BF, ST | ||
| AB11 | IC | 6, 7, 3, 1 | 5, 4 | 2 | |
| Sensors | VM, VL, RF, GC | BF, ST | GM | ||
| AB12 | IC | 4, 3 | 2 | 1 | 6, 7, 5 |
| Sensors | BF, ST | GC | GM | VM, VL, RF | |
| AB14 | IC | 6, 7, 3, 1 | 4, 5 | 2 | |
| Sensors | VM, VL, RF, GM | BF, ST | GC | ||
| AB15 | IC | 6, 7, 5, 3 | 2, 4 | 1 | |
| Sensors | VM, VL, RF, GM | BF, ST | GC | ||
| AB18 | IC | 6, 7, 4, 2 | 1 | 3, 5 | |
| Sensors | VM, VL, RF, GM | GC | BF, ST | ||
| AB23 | IC | 5, 6, 7 | 1, 3 | 2, 4 | |
| Sensors | VM, VL, RF | GC, GM | BF, ST | ||
| AB24 | IC | 6, 7, 4, 5 | 3 | 1, 2 | |
| Sensors | VM, VL, RF, GM | GC | ST, BF | ||
| AB25 | IC | 6, 5, 4 | 1 | 2, 3 | 7 |
| Sensors | RF, VM, VL | GM | ST, BF | GC | |
| AB28 | IC | 6, 7, 4 | 3, 5 | 2 | 1 |
| Sensors | VM, VL, RF | ST, BF | GM | GC | |
| AB30 | IC | 2, 3, 7 | 1 | 5, 6 | 4 |
| Sensors | VM, VL, RF | GM | BF, ST | GC |
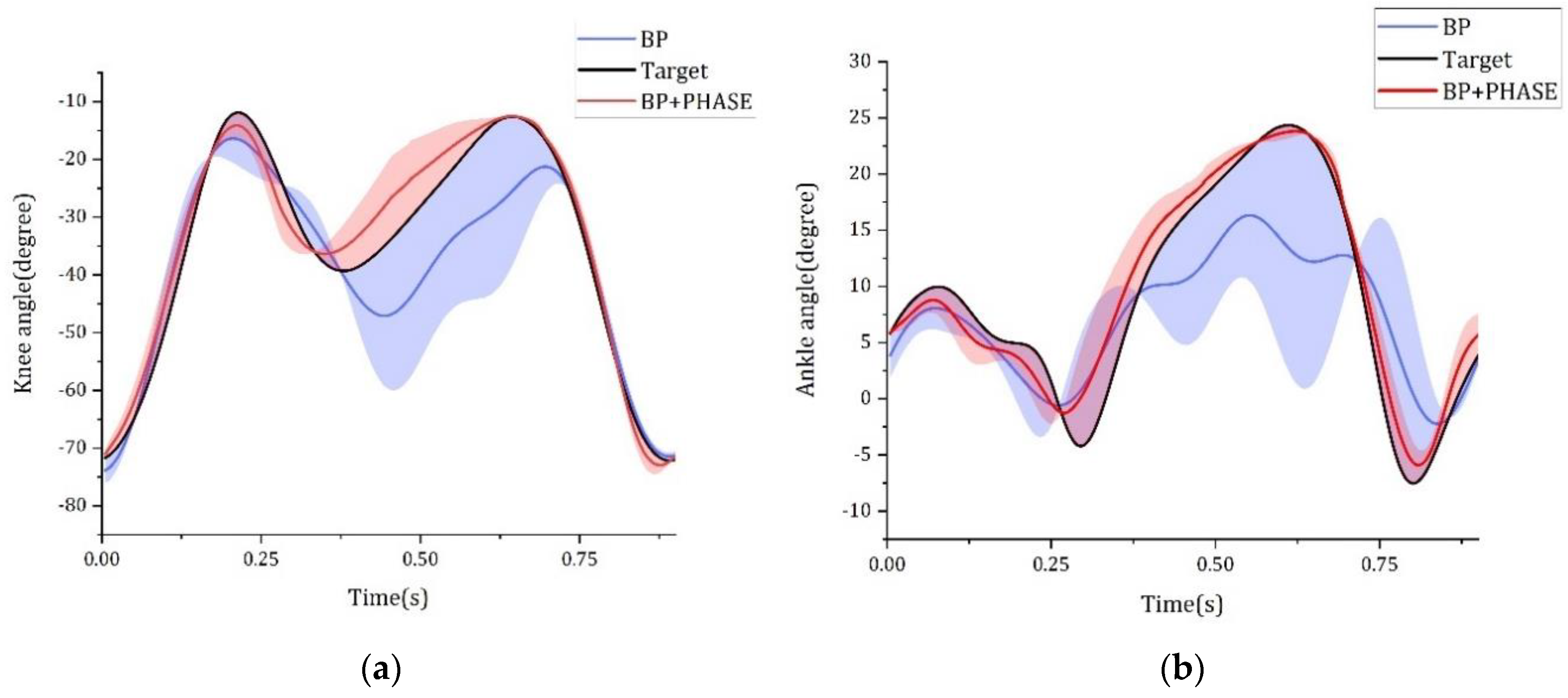
| Variation | BP | BP + PHASE | ||||||
|---|---|---|---|---|---|---|---|---|
| Knee | Ankle | Knee | Ankle | |||||
| RMSE [Deg] | γ (r) | RMSE [Deg] | γ (r) | RMSE [Deg] | γ (r) | RMSE [Deg] | γ (r) | |
| VM + BF + GC (1) | 7.071 ± 0.105 | 0.933 | 4.605 ± 0.124 | 0.875 | 4.817 ± 0.129 | 0.971 | 2.896 ± 0.066 | 0.958 |
| VL + BF + GC (2) | 9.926 ± 0.513 | 0.860 | 5.624 ± 0.226 | 0.810 | 2.813 ± 0.066 | 0.989 | 1.794 ± 0.058 | 0.985 |
| RF + BF + GC (3) | 9.173 ± 0.675 | 0.888 | 4.995 ± 0.137 | 0.847 | 6.129 ± 0.248 | 0.948 | 3.304 ± 0.078 | 0.942 |
| VM + ST + GC (4) | 7.171 ± 0.107 | 0.935 | 4.501 ± 0.092 | 0.887 | 4.421 ± 0.052 | 0.975 | 2.722 ± 0.063 | 0.963 |
| VL + ST + GC (5) | 8.257 ± 0.121 | 0.914 | 5.641 ± 0.010 | 0.811 | 3.738 ± 0.085 | 0.980 | 2.278 ± 0.046 | 0.973 |
| RF + ST + GC (6) | 9.231 ± 1.076 | 0.898 | 5.054 ± 0.324 | 0.857 | 6.981 ± 0.790 | 0.931 | 3.645 ± 0.299 | 0.926 |
| VM + BF + GM (7) | 8.056 ± 0.256 | 0.911 | 4.711 ± 0.221 | 0.878 | 6.300 ± 0.960 | 0.942 | 2.483 ± 0.286 | 0.963 |
| VL + BF + GM (8) | 12.864 ± 0.429 | 0.747 | 6.464 ± 0.171 | 0.726 | 4.553 ± 0.593 | 0.972 | 1.736 ± 0.333 | 0.983 |
| RF + BF + GM (9) | 9.156 ± 0.467 | 0.886 | 4.975 ± 0.229 | 0.854 | 6.541 ± 1.103 | 0.937 | 3.531 ± 0.507 | 0.928 |
| VM + ST + GM (10) | 7.440 ± 0.179 | 0.924 | 4.252 ± 0.113 | 0.896 | 9.115 ± 0.575 | 0.882 | 5.051 ± 0.253 | 0.857 |
| VL + ST + GM (11) | 8.730 ± 0.385 | 0.900 | 5.468 ± 0.242 | 0.827 | 9.819 ± 0.736 | 0.863 | 5.277 ± 0.330 | 0.838 |
| RF + ST + GM (12) | 9.238 ± 0.467 | 0.891 | 4.747 ± 0.084 | 0.873 | 12.033 ± 1.276 | 0.794 | 6.679 ± 0.657 | 0.737 |
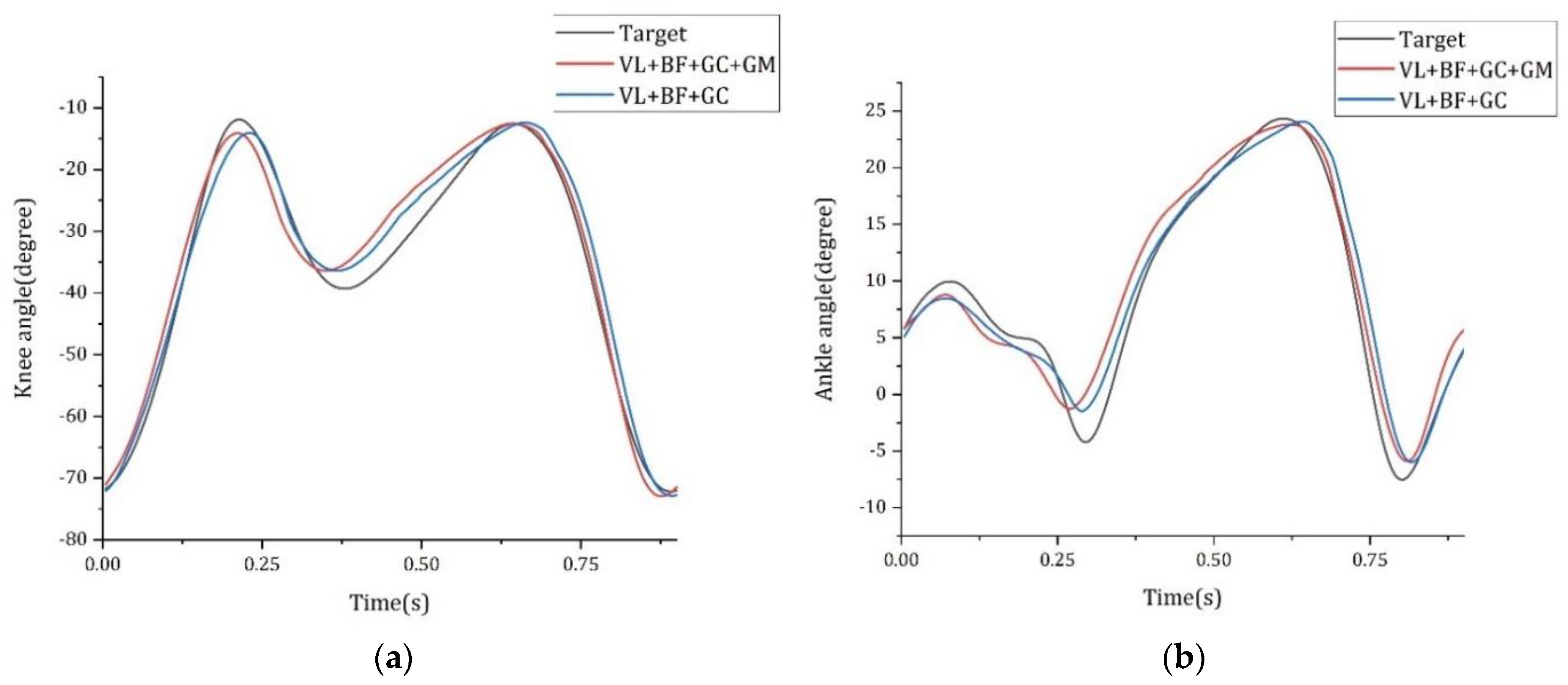
| Variation | BP | BP + PHASE | ||||||
|---|---|---|---|---|---|---|---|---|
| Knee | Ankle | Knee | Ankle | |||||
| RMSE [Deg] | γ (r) | RMSE [Deg] | γ (r) | RMSE [Deg] | γ (r) | RMSE [Deg] | γ (r) | |
| VM + BF + GC + GM | 6.339 ± 0.986 | 0.944 | 4.223 ± 0.069 | 0.905 | 5.503 ± 0.243 | 0.960 | 3.072 ± 0.050 | 0.950 |
| VL + BF + GC + GM | 8.553 ± 0.286 | 0.898 | 5.259 ± 0.193 | 0.844 | 3.443 ± 0.073 | 0.984 | 2.152 ± 0.062 | 0.976 |
| RF + BF + GC + GM | 7.386 ± 0.162 | 0.927 | 4.594 ± 0.418 | 0.878 | 4.952 ± 0.082 | 0.966 | 2.840 ± 0.040 | 0.957 |
| VM + ST + GC + GM | 5.999 ± 0.212 | 0.951 | 4.067 ± 0.055 | 0.907 | 3.884 ± 0.189 | 0.979 | 2.566 ± 0.074 | 0.967 |
| VL + ST + GC + GM | 7.194 ± 0.130 | 0.931 | 5.156 ± 0.164 | 0.842 | 3.645 ± 0.146 | 0.981 | 2.337 ± 0.030 | 0.972 |
| RF + ST + GC + GM | 8.167 ± 0.498 | 0.910 | 4.671 ± 0.049 | 0.874 | 5.766 ± 0.306 | 0.954 | 3.235 ± 0.131 | 0.944 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wei, Q.; Ma, H.; An, H.; Liu, Y. Muscle Selection Using ICA Clustering and Phase Variable Method for Transfemoral Amputees Estimation of Lower Limb Joint Angles. Machines 2022, 10, 944. https://doi.org/10.3390/machines10100944
Liu X, Wei Q, Ma H, An H, Liu Y. Muscle Selection Using ICA Clustering and Phase Variable Method for Transfemoral Amputees Estimation of Lower Limb Joint Angles. Machines. 2022; 10(10):944. https://doi.org/10.3390/machines10100944
Chicago/Turabian StyleLiu, Xingyu, Qing Wei, Hongxu Ma, Honglei An, and Yi Liu. 2022. "Muscle Selection Using ICA Clustering and Phase Variable Method for Transfemoral Amputees Estimation of Lower Limb Joint Angles" Machines 10, no. 10: 944. https://doi.org/10.3390/machines10100944
APA StyleLiu, X., Wei, Q., Ma, H., An, H., & Liu, Y. (2022). Muscle Selection Using ICA Clustering and Phase Variable Method for Transfemoral Amputees Estimation of Lower Limb Joint Angles. Machines, 10(10), 944. https://doi.org/10.3390/machines10100944






