1. Introduction
Infectious diseases have always been a major threat to human health. Throughout history, epidemics have posed a serious threat to human survival and nations’ economies. Currently, there are four main research methods for infectious diseases: descriptive research, analytical research, experimental research, and theoretical research [
1]. Regarding theoretical research, it is useful to establish mathematical models to describe infectious diseases. The earliest mathematical models designed to describe infectious diseases were established by Kermack and McKendrick [
2], and their compartment model, the susceptible–infected–removed (SIR) model, has been widely used and developed. However, for diseases with latent periods of infection and asymptomatic transmission (such as SARS, COVID-19, and foot-and-mouth disease), the susceptible–exposed–infectious–asymptomatic–removed (SEIAR) model is more in line with reality; see [
3,
4,
5,
6,
7,
8] and the references therein for details.
With the rapid development of the internet, people’s access to information is becoming broader and faster. It is well known that media coverage reduces the prevalence of infectious diseases and shortens the duration of infection [
9]. The influence of media coverage on diseases is mainly reflected during the initial stages of outbreaks, in which people learn about information related to the disease (transmission principles, routes, and prevention or treatment measures) through media coverage and strengthen their own protection and treatment. Additionally, through official media reports and health education, people’s safety awareness is raised, which may, to some extent, reduce the contact rate between people, as seen with the SARS outbreak of 2002. Regarding research from recent years, Kar and Nandi et al. [
10] introduced the media effect through the parameter M, and then Xing and Gao et al. [
11], based on the characteristics of media coverage with information implementation rate and reporting lossless rate, expressed the effective contact rate of the disease affected by media coverage as
, where M is a constant.
For diseases related to age (such as measles, pertussis, mumps, and COVID-19), considering age factors is more realistic. Age structure is divided into physiological age structure and quasi-age structure, where physiological age is the age that changes over time, and quasi-age refers to age calculated from the day of illness or vaccination. Since the 1970s, many scholars have noted the significant impact of physiological age structure on disease transmission and have established many infectious disease models with age structure to investigate the dynamic behavior for certain infectious models; see [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23] and the references therein.
Based on the above analysis, we will establish a physiological age SEIAR infectious disease model that considers the media effect and uses the exponential function method to demonstrate the impact of media effects on disease transmission. It is assumed that individuals who successfully receive vaccinations will not fall ill again due to immunity and that the natural death rate, vaccination rate, infection rate, and recovery rate are all related to age. Firstly, the existence of both disease-free and endemic equilibrium states is discussed; then, the stability of both disease-free and endemic equilibrium states is studied. Finally, we present numerical simulations to validate our theoretical results.
2. Model Description
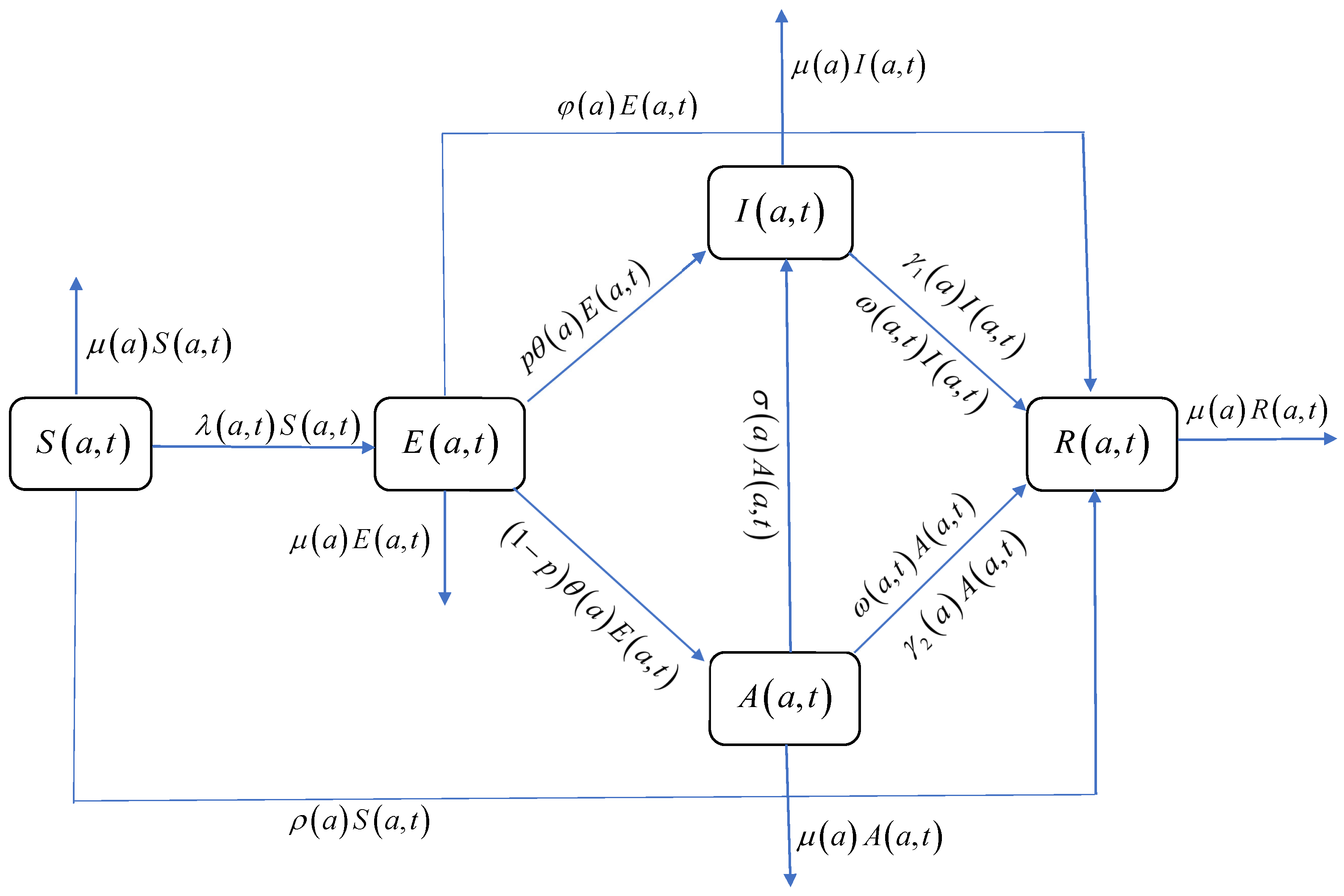
We assume that the population can be classified into five different groups, that is, susceptible, exposed, infectious, asymptomatic, and recovered, respectively. , , , , and represent the densities of susceptible, exposed, infectious, asymptomatic, and recovered individuals at time t with age a, respectively. The total population is denoted by and the maximum age of an individual by .
It is noted that exposed individuals can become symptomatic infectives with a probability of
p and asymptomatic infectives with a probability of
through contact with symptomatic or asymptomatic infectives. Clinical manifestations indicate that asymptomatic individuals cannot become symptomatic infectives through their own toxins, while asymptomatically infected individuals can become symptomatic infectives through contact with infectious individuals with an infectivity rate of
[
14].
In the initial stages of outbreaks, media coverage can reduce the spread of the disease [
21]. Therefore, this paper uses an exponential function method to demonstrate the role of media effects in epidemic prevention and control. The exposure rate
is defined as
, where
represents the impact of media effects on the average number of people exposed, the parameter
h represents the intensity of media coverage,
j quantifies the effect of city-level control measures on the infection rate following media dissemination,
denotes the age-dependent probability of an individual being exposed to infectious disease information through media channels, and
is the reduction in infection risk due to enhanced self-protective behaviors adopted after receiving such media reports.
Furthermore, self-examination enables the earlier detection of infections, significantly reducing diagnosis time and facilitating prompt treatment. This proactive approach not only enhances recovery rates but also exhibits a positive correlation with the contemporaneous proportion of infected individuals in the population. Therefore, the recovery rates
and
become
and
, respectively. Here,
,
represents the increased recovery rate due to prompt treatment after learning about the disease from the media, and
represents the total proportion of infections at time
t. The interactions between these state variables are shown in the flowchart of the model (
Figure 1).
From an epidemiological modeling perspective, we establish the following system of partial differential equations:
with initial value conditions (IVCs)
and boundary value conditions (BVCs)
,
.
Detailed biological considerations of the parameters of the model (1) can be found in
Table 1.
The primary objective of this study was to investigate the existence and stability conditions of both disease-free and endemic equilibrium states in system (1). For this purpose, we formulate the following well-founded assumptions considering human activity patterns and age demographics. Analogous assumptions can be found in references [
20,
21].
Assumption 1. We provide the following assumptions throughout the paper:
(A1) Population sizes without immigration and emigration are set at a certain level.
(A2) Deaths due to disease are not considered.
(A3) Both asymptomatic and symptomatic individuals are infectious with an infection rate of β, and the infection rate of asymptomatic individuals is k times that of symptomatic individuals.
(A4) , which ensures no possibility of survival at the maximum age.
Using the same argument as that in [
21] with obvious changes, we may prove the following:
Lemma 1. The total population is bounded if and are bounded.
To simplify system (1), a normalization transformation is applied. Let Then, system (1) takes the following form:with IVCsand BVCs , , where 3. Stability of the Disease-Free Steady State
This section focuses on establishing the existence and stability of the disease-free steady state in system (2). Since
, system (2) can be simplified as follows:
Due to the time independence of the disease-free steady state, system (3) reduces to the following equations:
with IVCs
where
For the disease-free equilibrium , then , it is implied that Thus,
In summary, it can be seen that the disease-free equilibrium exists and is unique; that is,
For the purpose of studying the local stability of
, a linear transformation is performed on system (3) as follows:
Then, the linear part of the corresponding system is as follows:
with BVCs
, where
Consider the exponential solution form of system (5),
System (5) takes the following form
with IVCs
where
If we let
, then
Therefore, we obtain the solution of system (6) below:
Substituting
and
into
, we have
Thus, the characteristic equation is
where
Let the basic reproduction number
; that is,
Theorem 1. The disease-free steady state is locally asymptotically stable if and unstable if .
Proof. We can obtain some properties of the function
:
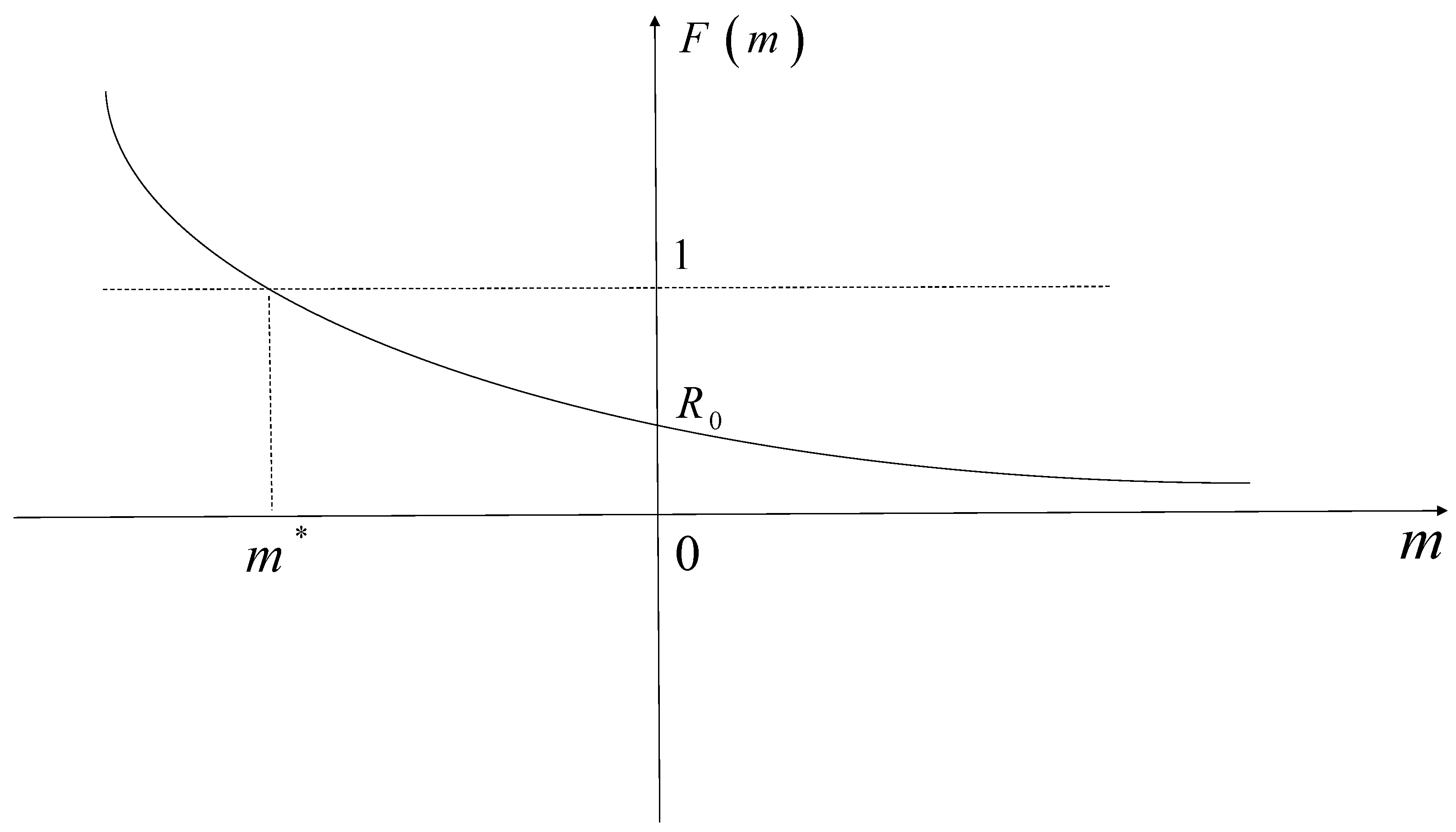
Then, the equation has a unique real root .
Case 1: The equation
has exactly one root, so the root must be
. Therefore, the root
is negative provided
, as shown in
Figure 2.
Case 2: The equation
has complex roots
. If
, then
and if
F monotonically decreases, it follows that
.
Based on the above discussion, the roots of the equation always have negative real parts provided , which yields , which is locally asymptotically stable if .
Similarly, we can prove that is unstable if . □
Theorem 2. The disease-free steady state is globally asymptotically stable if .
Proof. According to the definition of global stability, we will show
Therefore, it suffices to discuss the solutions when
. The first equation of system (3) is integrated along the characteristic line
if
, meaning that
Let
. Then,
and
Moreover,
and
yield
Combining (7) and (9), we obtain
When
, we have
due to the non-negativity of
It follows from
that
It is easy to see from the expression
or
that
Thus, we end the proof. □
4. Stability of the Endemic Steady State
When system (3) attains a stable age-distribution, it depends solely on
a. That is, the steady-state solution of system (3) satisfies the following form:
with IVCs
where
It is not difficult to find that when , the endemic steady state becomes the disease-free steady state .
For
, substituting the expressions of
into
allows us to obtain the following:
Let the right-hand side of the above equation be denoted as
; that is,
Then, there exists a unique endemic steady state if and only if there is a unique such that and . Consequently, we shall prove the following result:
Theorem 3. If , system (3) admits a unique endemic steady state .
Proof. The properties of
are proved as follows:
which implies that the equation
has a unique positive real root
if
. Then, system (3) has a unique positive steady state
, given by the expressions of
when
. □
To analyze the local stability of the endemic steady state when
, we further need to study the linearized system of (3) at
. If we let
then the linear part of the corresponding system is as follows:
with BVCs
where
Consider the exponential solution form for the above system (11):
Then,
with IVCs
where
Given that
define
Consequently, system (12) can be rewritten as follows:
with IVCs
. Moreover,
The solution of system (13) is
Denoting
, we want to show that all the roots of the equation
P(
n) = 1 always have negative real parts provided
R0 > 1. Substituting the expressions of
and
into
P(
n), we obtain
where
According to the expression
and the fact that
, we have
Furthermore, due to
, one has
Because is a decreasing function with respect to n, it follows that, for all , . This implies that the equation holds only in the region . Thus, if , all roots of have negative real parts.
Therefore, we have the following theorem.
Theorem 4. The endemic steady state is locally asymptotically stable if .
Proof. The process is the same as in Theorem 1, so it has been omitted. □
5. Numerical Simulation
In this section, we validate the aforementioned conclusions via several numerical simulations. Because the spread of the disease can be influenced by the media, we discuss the impact of media broadcasting intensity on the disease. Some parameters are derived from reference [
11], which are not only based on real data estimates but also possess biological significance. We assume that
,
,
,
,
,
,
,
,
,
,
,
,
, and
. From the following
Figure 3, it can be seen that, under certain initial value conditions, as the intensity of media broadcasting increases, the number of exposed, infectious, and asymptomatic individuals decreases.
This indicates that increasing the intensity of media broadcasting is also an effective way to control the spread of the disease.
To obtain a numerical solution for system (2), the following initial age distribution is assumed:
From
Figure 4, we can see that young people and the elderly are more affected by the disease, which is related to their wide range of activities, more contacts, and physical conditions.
6. Conclusions
This paper investigates the SEIAR infectious disease model in which three main factors—the media effect, vaccination, and age structure—are considered, and the stability of the model is analyzed.
Firstly, it can be seen from the expression of
that strengthening media propaganda can lead more people to transition from the exposed class to the recovered class through medication or other means, reducing the density of infectives and thus reducing the further spread of the epidemic. In particular, if
in the expression of
, that is,
it can be seen that the infectious disease can disappear more quickly in the absence of asymptomatic infections.
Secondly, vaccines stimulate the body to produce antibodies and immune cells to form immunity; the higher the success vaccination rate
, the fewer the susceptible people in the population, and the easier it is to cut off the virus transmission chain (see
Figure 1), thereby suppressing the spread of the epidemic.
Finally, age is not only an important factor for population growth patterns and the prevalence of infectious diseases but also has a certain impact on fertility rates, mortality rates, infection rates, and recovery rates. Therefore, applying an age-structured model to investigate the transmission dynamics of specific infectious diseases aligns with real-world scenarios.
However, this paper does not take into account the situation wherein vaccination becomes ineffective after a period of time or the infection process has a certain time lag. Future research will continue to discuss these issues.