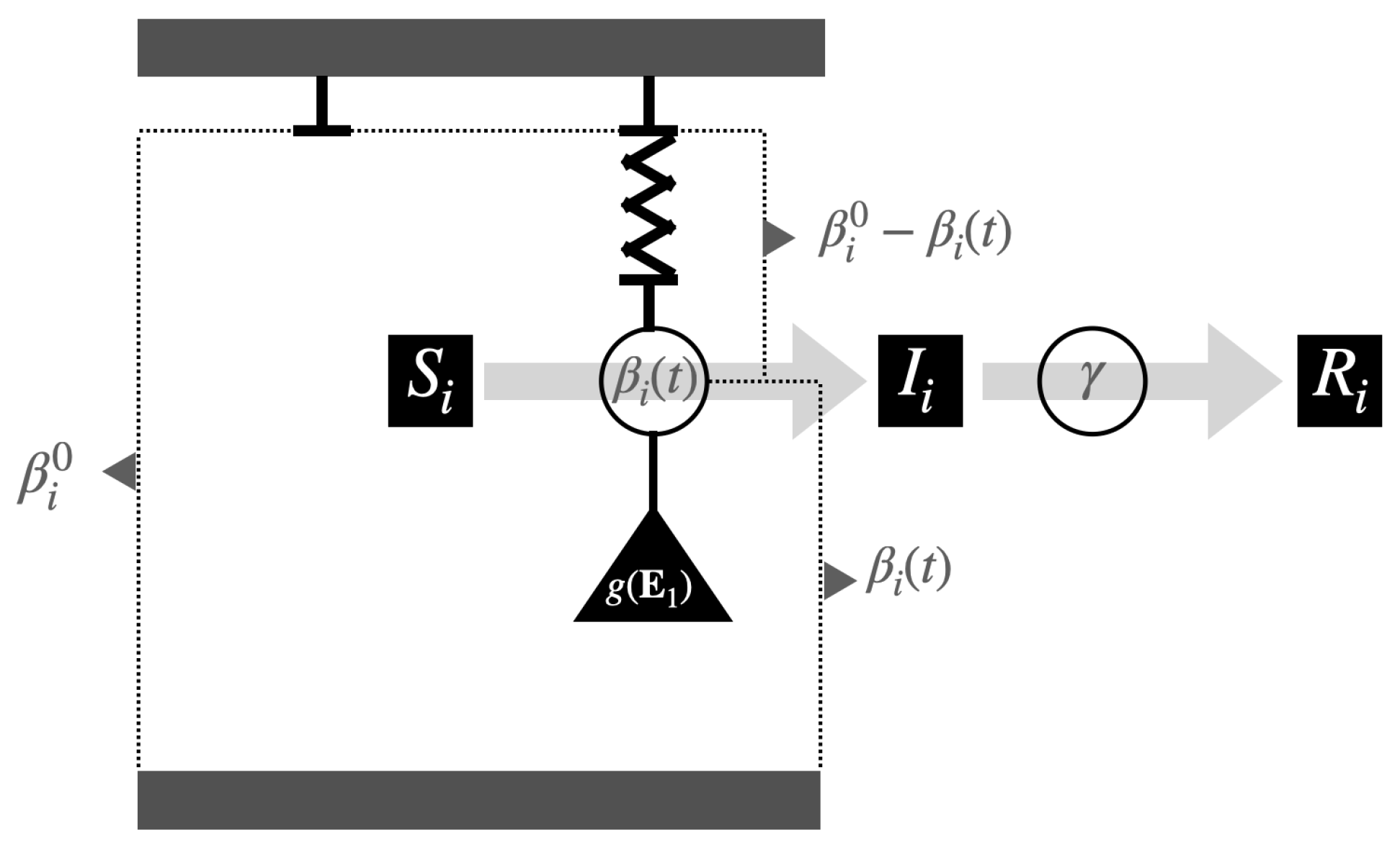
1. Introduction
From an ecological perspective, two populations of the same species, even if they are indistinguishable in terms of genetics, local adaptation, or growth and reproduction strategies, can still be differentiated primarily by geographical factors, climatic conditions, resource availability, intrapopulation mobility, and ecological interactions. These differences may, to a greater or lesser extent, determine the epidemiological risk associated with a specific pathogen. In the case of human populations, cultural and economic factors further contribute to these variations in risk [
1,
2,
3,
4,
5].
Indeed, if we consider not only the geographical elements that naturally differentiate two populations, even within the same nationality, but also demographic aspects (e.g., size, structure, and geographical distribution), socioeconomic factors (e.g., education, income, types of employment, housing conditions, or access to goods and services), and cultural factors (e.g., customs, traditions, values, beliefs, practices, or lifestyles), it is likely that the risk associated with the transmission of an infectious disease will vary significantly between populations. All of these variables can play a crucial role, influencing epidemiological dynamics in different ways—either positively or negatively—and can affect the speed and magnitude of contagion, as was observed during the COVID-19 pandemic [
6,
7,
8,
9].
The basic reproductive number (
), particularly its transmission rate component, commonly referred to as beta, is a crucial epidemiological indicator for assessing the potential spread of infectious diseases within a population. This indicator incorporates various geographic, demographic, and sociocultural factors that influence its magnitude, enabling differentiation between human populations that do not share the same territory. From a geographic standpoint,
reflects how environmental characteristics, such as population density and mobility, impact disease transmission. Demographically, this number accounts for population structure, including age distribution, which can affect the average transmissibility of the infectious agent. Socioculturally,
is influenced by health practices and behaviors that vary among communities, such as interpersonal distancing and personal healthcare customs. Consequently, basic reproductive numbers or transmission rates can vary qualitatively and quantitatively in their calculation, which can differ from one location to another, thus determining different risk values as an indicator [
10,
11,
12,
13].
Without considering individual biological factors, one notable example is the epidemiological differences between urban and rural areas. In urban settings, higher population density may account for variations in transmissibility due to increased interactions and physical proximity. It is well established that economic conditions significantly influence the spread of diseases; thus, in an economically segregated city, the risks of contagion can vary by sector. Cultural practices and levels of education, such as literacy, play a crucial role, as they shape hygiene and preventive beliefs and behaviors that can either facilitate or hinder disease propagation. The geographical environment also serves as a discriminator in transmission dynamics, particularly in relation to climate and the characteristics of the landscape. Furthermore, health systems themselves may differ in terms of resources, intensity, and the quality of preventive or mitigation efforts [
14,
15,
16].
Once a contagious disease is introduced into a territory, health efforts to slow its spread, in the absence of alternatives, typically involve the implementation of non-pharmaceutical mitigation measures, such as restrictions on movement, isolation, mask-wearing, and frequent handwashing. These measures are informed by diagnostic assessments and projections of the epidemiological status of the affected population. However, when the territory is not homogeneous, mitigation measures are not always based on indicators that reflect the situation of the entire area. Instead, decisions may be made for specific subsectors that are more accessible or relevant, even if not in health terms but rather in political, economic, or geographic contexts. For example, in societies with strong centralism, large cities define the health authority’s implementation of ad hoc health management strategies, which may not always be appropriate for the entire territory. This is exemplified by quarantine measures aimed at preventing urban overcrowding, which may not be relevant in rural areas where such conditions do not exist.
In the context of a country, territories can exhibit various forms of fragmentation. Beyond the urban–rural dichotomy mentioned earlier, these territories can also be classified based on geographic factors (objective conditions) or cultural aspects (subjective conditions), including social, economic, ethnic, or religious origins. A mitigation measure perceived as beneficial in one territory may prove detrimental in another. For instance, home quarantines implemented in the same city may seem reasonable overall; however, they can exacerbate overcrowding in social sectors that occupy small residential spaces, such as tenement buildings or cramped houses, which often lack adequate ventilation and increase the risk of contagion. Moreover, territorial fragmentation within a country can extend beyond political–administrative divisions and significantly impact health outcomes. A clear example is found in multinational states, where diversity among national groups can influence pathogen transmission dynamics, leading to variations in the probability of close contact and transmissibility among these groups.
In a fragmented population, where it is distributed across two conceptual or physical patches of a territory, the public health decisions or measures applied to one zone may not reflect its own specific epidemiological reality. Instead, they may be based on assessments from the complementary patch, often the dominant one in this context. When health protocols are imposed from outside, variations may arise in both the intensity of implementation and in the resistance to these measures. Additionally, while the arrival of the disease to the different patches may be diachronic, for simplicity, we will not address that case here. Our focus is on the epidemiological consequences for a temporarily demographically independent subpopulation (e.g., due to the imposition of a preventive lockdown) when a mitigation policy is applied based on epidemiological information from the other patch. For the purposes of this analysis, we will assume that, from the moment the contagion process begins, the patches remain isolated, with no flow of individuals between them.
Methodologically, we will assume two independent subpopulations, each homogeneously distributed within its respective territory, and compartmentalized into the classic susceptible, infected, and recovered groups of the standard SIR model. However, the model will incorporate variable transmission rates for both populations, specifically governed by coupled reaction–restoration dynamic laws of the beta-SIR model, as introduced in [
17,
18] and complemented in [
19,
20,
21].
In
Section 2, from a SIR-type baseline model perspective, we present our model of the evolution of transmission rates for two populations: one of these populations subordinated to the other for the effects of a mitigation intervention based on the epidemiology information of only one of them. The specification of the questions, mainly regarding the impact of the distinction among the behavioral parameters of reaction–restitution, are also found in this section.
Section 3 is reserved to show some analytical and numerical answers. This paper closes with a discussion and conclusion, respectively, in
Section 4 and
Section 5.
3. Results
If, from the beginning, the population is connected with the mitigation policy, we see that when in contact with an external infected person with the population:
- (a)
In
, i.e., initial condition
, the system reacts according to
So, the transmission rate, in both populations, would go down.
- (b)
In
, i.e., initial condition
, the system reacts only by the
subsystem, i.e.,
Then, without disease in the population, there is no reaction in it, and the population continues with its SIR development following its intrinsic transmission rate.
3.1. Some Analytical Results
We observe that, clearing the term involving
from the first two equations of (1), the identity linking the transmission rates of the subpopulations is:
from their integral equations, alternatively
Note that when
, from (
2) and (
3), we have:
where
and
.
Theorem 1. Given system (1) and an equilibrium point , then and , with , . So, in the long run infection rates return to the intrinsic rate. That is, the effect that will matter to us, assuming mitigation of another population, is mainly in the early stages of epidemiological development. In what follows, we will denote by , , the fraction that represents the transmission rate of its intrinsic value for the respective population, which will be called relative transmission rate.
Theorem 2. If in system (1) it has , then , with In addition, y .
Note that to know under what conditions one relative transmission rate is greater or less than another, it is necessary to establish when is greater or less than one. At the moment, we know that and that if the initial conditions for the transmission rates are their intrinsic values, then . Furthermore, since , we have . Regarding the comparison of these relative values in a neighborhood at the beginning of the contagion process, we have the following result.
Notice that if the system (
1) it has
, i.e.,
, then
and
as can also be deduced directly from (
1).
Theorem 3. If in system (1) it has , then: - (i)
in a neighborhood to the right of , if , i.e., .
- (ii)
in a neighborhood to the right of , if , i.e., .
Let us observe the products , ; they represent some measure of the subpopulation resistance to respect the mitigation in consideration of the intrinsic value of the transmission rate. In this idea, Theorem 3 tells us that a lower (or higher) resistance implies a lower (or higher) relative expression of the rate, as expected a priori.
We can also observe that in terms of
,
, the first two equations of (
1) are such that
This implies that since as , these relative transmission rates evolve toward the value one, similarly to a logistic equation.
An indicator associated with the relative transmission rate is the relative variation in the transmission rate, defined by , .
Theorem 4. If for system (1) there exists tal que , then , con . Thus, - (i)
If , then . In addition, implies .
- (ii)
If , then . In addition, implies .
3.1.1. Comparing According to Intrinsic Rates (, and )
Denoting by and the common values of the restitution and reaction factors, we have the consequences of Theorems 3 and 4. That is, (resp. >) in a neighborhood to the right of , if (resp. >). In addition, considering that , if (resp. ≤), then (resp. ≥).
3.1.2. Comparing According Reaction Factors (, and )
Denoting by
and
the common values of the factor of restitution and intrinsic rate, respectively, Theorem 4 states that if for system (
1) there exists
such that
, then
. Thus,
- (i)
If , then . In addition, implies .
- (ii)
If , then . In addition, implies .
3.1.3. Comparing According to Restitution Factors (, and )
Denoting by and the common values of the factor of reaction and intrinsic rate, respectively, Theorem 3 tells us that in a neighborhood of , we have:
- (i)
If , then .
- (ii)
If , then .
However, Theorem 4 states that if for system (
1) there exists
tal que
, then
. Thus,
- (i)
If , then . In addition, implies .
- (ii)
If , then . In addition, implies .
3.2. Simulations and Numerical Results
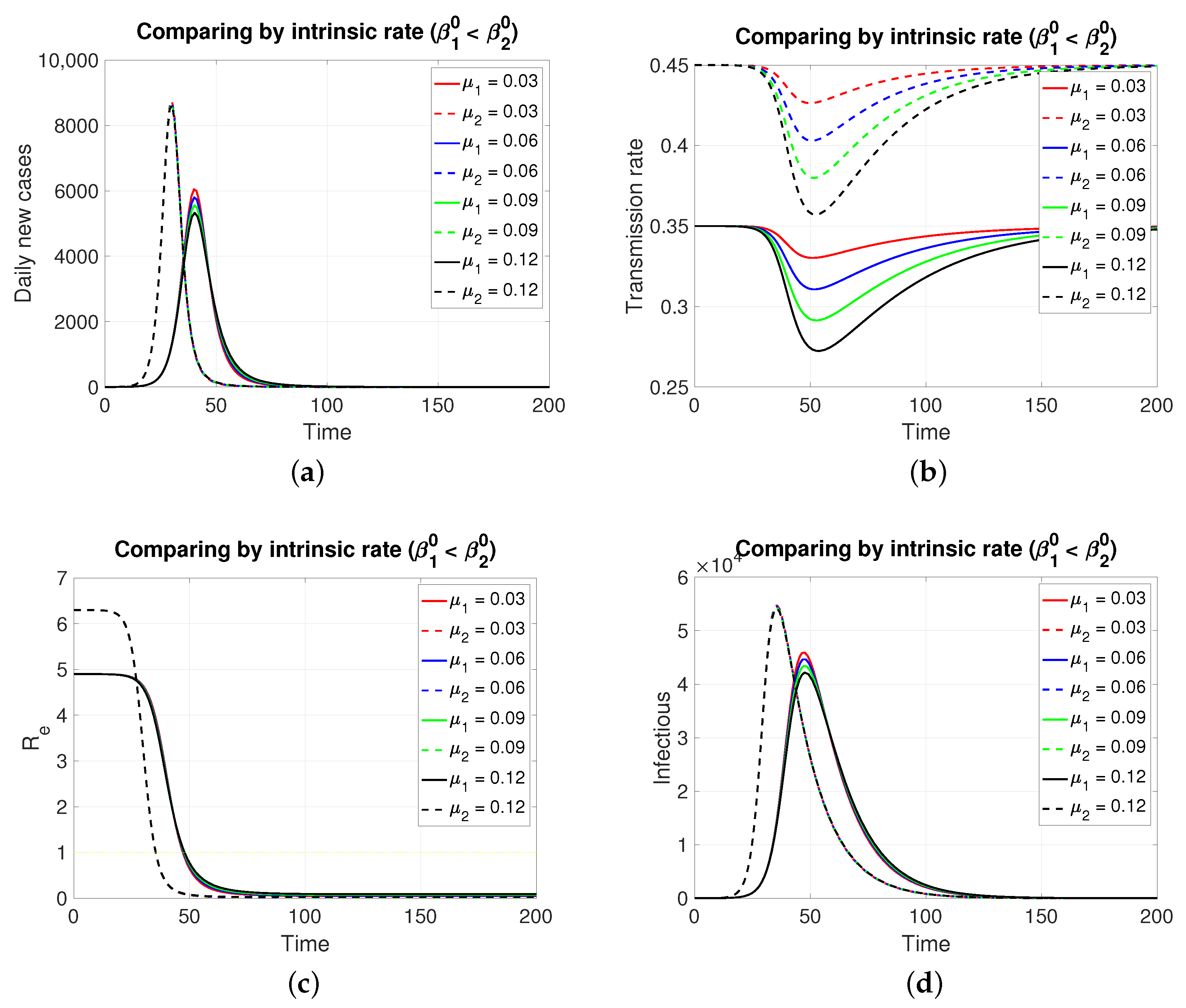
3.2.1. Comparing by Intrinsic Rate (, and )
Since both populations share the same restitution factors (
) and the same reaction factors (
), an increasing series of values for the latter implies a decreasing series of transmission rates for both populations, which is expressed transiently. As expected (
Figure 2), the population in
, having a lower intrinsic rate than population 1, presents its pulse (segmented lines) of lesser height and later, which is typical of an SIR model. Inversely, if the case is (
Figure 3), the population in
, having a lower intrinsic rate than population 2, presents its pulse (continuous line) of lesser height and later.
Although, in both cases, the graphs of daily new cases and also the infectious one do not show significant differences for the four values of in , it is clear that in a population of tens of thousands at the peaks of these epidemic curves, the difference in the maximum number of infectious individuals can be counted in units of thousands. Regarding the effective reproductive number, the reduction to zero of its value is faster in the population that presents a higher intrinsic value, i.e., a greater basic reproductive number.
A differentiating aspect appears when comparing
Figure 2b with
Figure 3b. It is that the minimum for the transmission rates, when
, occurs at an earlier time concerning the corresponding instant for the case
.
3.2.2. Comparing According to Reaction Factor
Note that in
Figure 4, it is assumed, for comparative purposes, that both populations have the same number of individuals and that the contagion process begins at the same instant and with the same initial number of infective individuals. Note that if the mitigation reaction coefficient is higher (
resp. lower) in the population of
compared to that of
, then a greater (
resp. lower) number of new cases will be generated in
and transiently. However, this is reversed at least after the transmission rate reaches its minimum value.
3.2.3. Comparing According to Restitution Factor
In this case,
Figure 5 (both in
Figure 5a,d, of new cases and active cases, respectively), the dynamic behavior is symmetrical to that of
Section 3.2.2 for those about the reaction factor; that is, depending on which coefficient of restitution is greater (
resp. less), it produces in a transient way more (
resp. less) cases to then invert and subsequently equalize.
4. Discussion
One aspect that the model shows us (as soon as human behavior is introduced into the system, even in the regulated case, i.e., in a specific scheme, in our case the reaction–restoration tension) that the dynamics of the transmission rates can come to mean, for the development of the disease, a great difference between the populations, although they share the rest of the epidemiological parameters. As an example, assuming two behaviorally extreme populations so that comparatively one of them (the one that provides the intervention indicator
) is much less reactive (
) and at the same time less tolerant to said intervention and the greater tendency to restoration (
), the developments of the contagion are those shown in
Figure 6, a simulation in which it is assumed that in a pair of populations of one hundred thousand individuals, with the same intrinsic transmission rate and removal time, a first infectious case appears (time zero). It can be observed in
Figure 6b that the response to mitigation is much lower in the first population (in red) compared to the subordinate control population (in blue). The difference observed in their effective reproductive numbers is notable; in the index population (red), this indicator is nulled out by the rapid contagion of its entire susceptible population. In summary, subordination mitigation measures, as shown by the simulations, can result in health detriment or benefit for a population, depending on the comparative balance (positive/negative) of key epidemiological parameters, in particular, those we have discussed: intrinsic transmission rates, reaction factors, and restitution factors.
For other side, a natural generalization of the model should consider the epidemiological interaction between the populations. This problem, although natural, turns out to be complex if we note that among the determinants of the transmission rates there are not only geographical aspects, which in principle could be assumed to be uniform to the habitats, but also social and cultural aspects that influence them. In general, although concerning proxemics, it is also possible that there may be averaging aspects in the effective encounters between individuals of different subpopulations and the existence of dominance phenomena that may depend on cultural and socioeconomic aspects that are diverse in their expression in space and time. The subject is interesting but outside the scope of this article, so we have planned it for our future research.
It is important to note that the presented model is of a basal order, and therefore, of theoretical value but with low resolution with reality. Now, in terms of advancing in realism, as future work, it is important to mention the importance of demographically connecting the populations and also incorporating the stochastic epidemic models as they are key to avoiding explaining all the noise in the data with the noise of the observation, and, as is known, it is also essential when working with less abundant populations. In this regard, guiding articles related to our topic are [
22,
23,
24], where the latter consider compartmental models and approximate inference. Regarding the connection with the data, we must also consider the tools provided by statistical inference. We highlight the ABC approaches, iterated filtering, or approximate likelihood methods; see, respectively, [
24,
25,
26].
5. Conclusions
The propagation dynamics of an infectious disease varies significantly between populations, even when these populations are under the same non-pharmaceutical mitigation measures; this is due to differences related to sociocultural, economic, and even environmental factors, among others. Therefore, mitigation efforts evaluated as effective in temporarily controlling disease in one population according to local epidemiological indicators, when implemented in another population, will not necessarily have the same control effects.
We know that for the same disease, the intrinsic transmission rate varies according to the population, but at the same time, this rate strongly predetermines the future behavior of the disease in a population. Thus, for the mathematical modeling of epidemiological developments that consider human behavior over time, which alters said rate, either positively (e.g., non-pharmaceutical mitigation) or negatively (e.g., low compliance), it is pertinent to consider a transmission rate through a specific dynamic law. In particular, the law for the coupled relative transmission rates () proposed in this work, for each population, consequently provides us with relevant information that can be considered for decision-making of subordinate population systems from the perspective of the health authority.
The reaction and restoration factors proposed in our model show the impact that a non-pharmaceutical mitigation measure has on daily infectious cases. In particular, the simulations suggest that the spread of the disease is more sensitive to the reaction factor than to the restoration factor. This leads to the conclusion that a non-pharmaceutical mitigation measure focused on raising people’s awareness can be an effective strategy for controlling the disease. However, when applied to another population, this strategy must be evaluated according to the population’s characteristics, that is, measures following the local epidemiological status and its characteristics concerning human behavior.