Hybrid Euler–Lagrange Approach for Fractional-Order Modeling of Glucose–Insulin Dynamics
Abstract
1. Introduction
2. Preliminaries
- Linearity: The Caputo fractional derivative is linear, meaning that for any constants and , and functions and ,
- Initial Conditions: The Caputo derivative allows for standard initial conditions of integer-order differential equations. This is particularly useful for applications in biomedical modeling, where initial conditions are typically well defined based on empirical data.
- Derivative of a Constant Function: The fractional derivative of a constant function is zero:
- Power Function Property: For a power function , the Caputo fractional derivative is given by the following:This property is useful in understanding how the fractional derivative interacts with power-law behaviors, which are often encountered in physical and biological systems.
- The fractional integral of order of a function is defined as
- The Euler method is a simple numerical technique for approximating the solutions of differential equations. It is often used as a basis for more advanced methods, such as the hybrid Euler–Lagrange method introduced in this paper.
- Lagrange interpolation is a method of constructing polynomials that pass through a given set of data points. In the hybrid approach, this technique is used to approximate the fractional derivatives and simulate the memory effects in the glucose–insulin model.
- The existence and uniqueness of solutions to the proposed fractional-order model are proven using fixed-point theory, which guarantees that a unique solution exists under certain conditions, ensuring the validity of the numerical simulations.
- These foundational concepts will be used throughout the paper to develop and analyze the proposed fractional-order model and its numerical implementation.
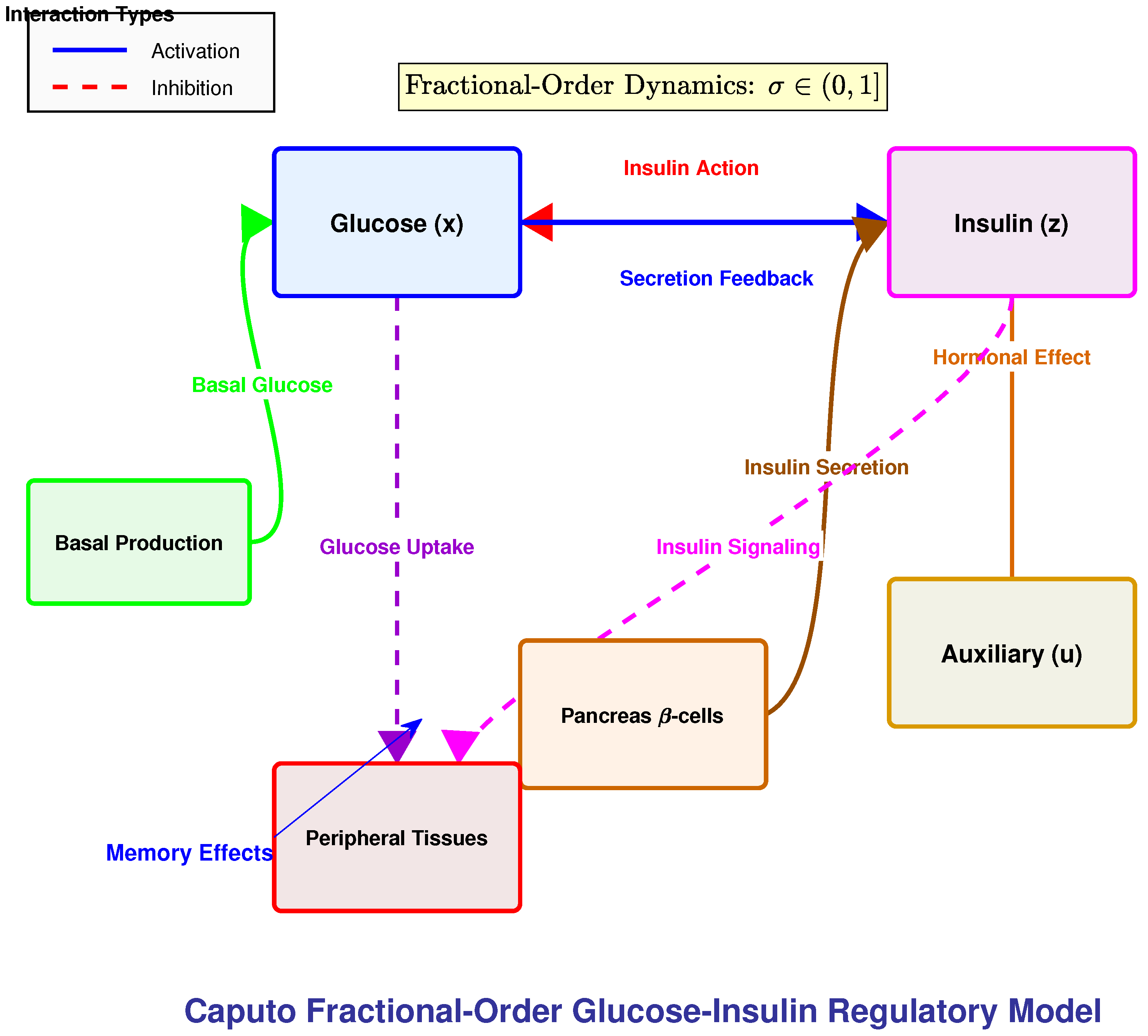
3. Model Description and Fractional Extensions
3.1. Physiological Motivation and Model Rationale
- Glucose utilization through both insulin-dependent and insulin-independent pathways.
- A threshold-triggered secretion mechanism, reflecting the switch-like response of pancreatic -cells.
- Delayed regulatory effects, represented by an auxiliary hormonal process.
- Explicit representation of basal equilibrium states for glucose and insulin.
3.2. Model Formulation
3.3. Physiological Interpretation
- : blood glucose concentration (mg/dL).
- : insulin action on glucose disposal (1/min), representing insulin-mediated uptake efficiency.
- : plasma insulin concentration (μU/mL).
- : auxiliary variable modeling delayed hormonal processes (e.g., hepatic glucose suppression).
- Glucose suppression: combines basal utilization and insulin-driven clearance.
- Basal glucose production: represents hepatic glucose release at rest.
- Insulin activation: quantifies insulin-stimulated uptake above basal levels.
- Threshold-regulated secretion: mimics the switch-like activation of -cells.
- Hormonal clearance: captures insulin degradation by liver and kidneys.
- Delayed feedback: The auxiliary dynamics reproduce slow hormonal effects.
3.4. Parameters
3.5. Rationale for Fractional Order
3.6. Model Assumptions
- The plasma compartment is homogeneous and well mixed.
- Counter-regulatory hormones (e.g., glucagon) are indirectly accounted for in basal terms.
- The auxiliary variable suffices to approximate delayed secretion without explicit time delays.
- Parameters are constant for a given subject during the observation period.
4. Non-Negativity and Boundedness of Solutions
5. Existence and Uniqueness of Solutions
5.1. Mathematical Framework
5.2. Practical Implications for Model Applicability
5.3. Implications for Complex Biological Systems Modeling
6. Hyers–Ulam Stability Analysis
6.1. Definition and Main Result
6.2. Biological and Numerical Implications
6.3. Comparative Analysis of Mathematical Properties
6.4. Methodological Framework
6.5. Interplay Between Stability, Non-Negativity, and Boundedness
- Boundedness ⇒ Finite Lipschitz constants ⇒ Controllable error propagation
- Non-negativity ⇒ Well-defined threshold operations ⇒ Predictable error dynamics
- Both properties ⇒ Physically meaningful solutions ⇒ Biologically relevant stability bounds
- Non-negativity ensures physiological realism: The stability of non-negative solutions means that the model maintains biological plausibility even under perturbations. For example, measurement errors in glucose monitoring will not produce negative glucose predictions.
- Boundedness enables clinical applicability: The stability of bounded solutions ensures that predictions remain within physiologically possible ranges. This is crucial for clinical applications where extreme predictions could lead to inappropriate treatment decisions.
- Combined robustness: The interplay between these properties means that the model can handle the types of uncertainties typically encountered in clinical practice—measurement errors, individual variations, and modeling approximations—while still producing realistic, bounded, non-negative predictions.
- Numerical schemes should preserve non-negativity to maintain the error structure used in the stability proof.
- The boundedness property suggests that adaptive step-size control could focus on regions where variables approach their physiological bounds.
- The stability constants provide quantitative targets for numerical error control.
- In healthy metabolism, glucose and insulin levels are both bounded and non-negative, and the regulatory system is robust to perturbations (stable).
- In metabolic disorders, this robustness can be compromised—for example, in diabetes, the system may exhibit poorer stability properties with larger oscillations and reduced ability to maintain boundedness under perturbations.
- The fractional order appears in both the boundedness bounds and the stability constants, suggesting a mathematical basis for understanding how metabolic memory affects both regulatory capacity and robustness.
7. Caputo–Lagrange Discretization Method (CLDM)
7.1. Method Formulation
7.2. Solution via Newton–Raphson Method
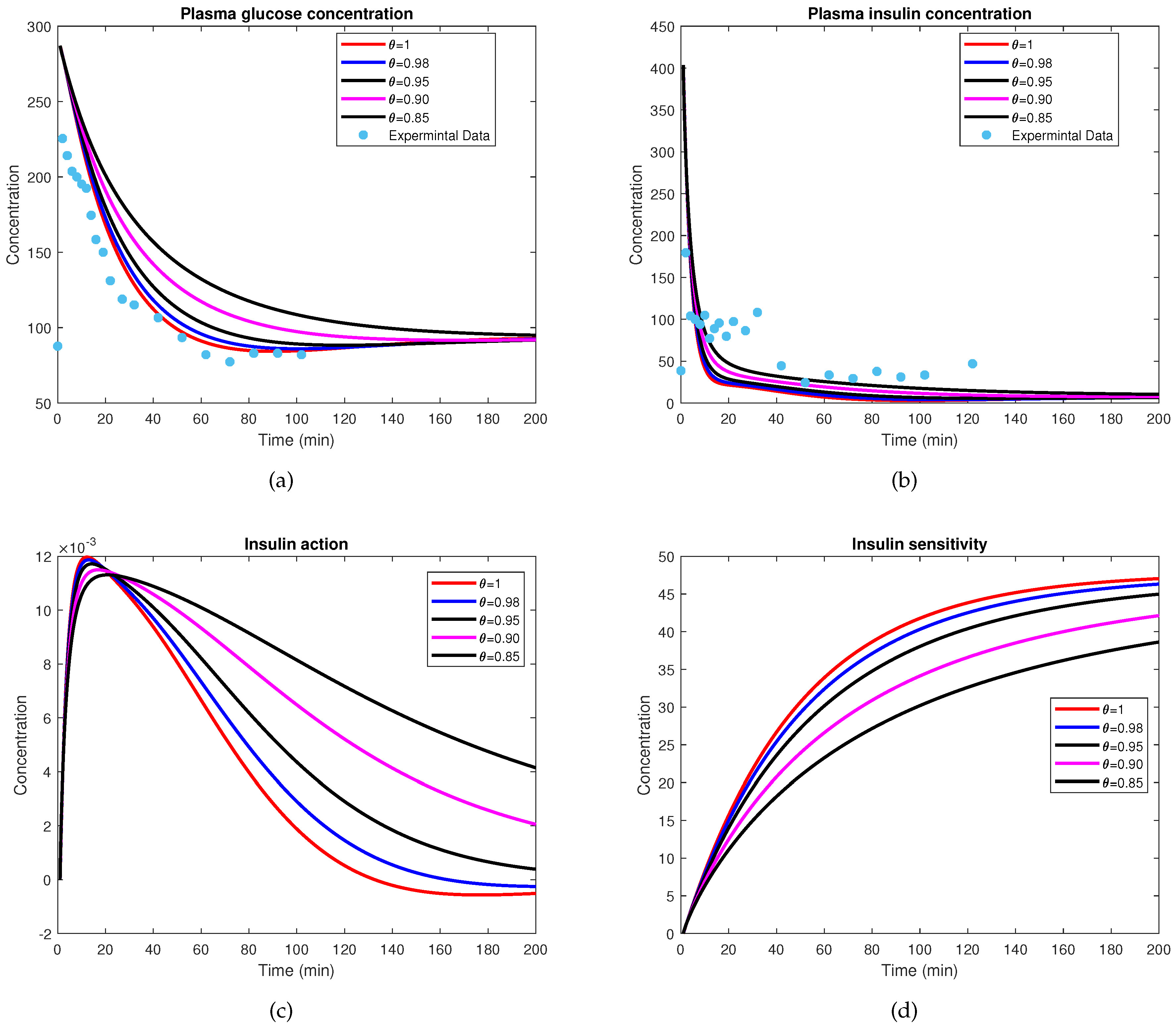
7.3. Numerical Results and Physiological Validation
7.4. Error Metrics and Convergence
7.5. Comparison with Alternative Methods
7.6. Broader Applications
8. Fractional Euler Method with Caputo Derivatives
9. Numerical Simulation
Clarification on the Experimental Data Representation
10. Discussion
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Arena, P.; Caponetto, R.; Fortuna, L.; Porto, M. Nonlinear Noninteger Order Systems: Theory and Applications; World Scientific Publishing: Singapore, 2001. [Google Scholar]
- Ahmad, W.M.; El-Khazali, R. Fractional-order dynamical models of Love. Chaos Solitons Fractals 2007, 33, 1367–1375. [Google Scholar] [CrossRef]
- Nisar, K.S.; Farman, M.; Abdel-Aty, M.; Ravichandran, C. A review of fractional order epidemic models for life sciences problems: Past, present and future. Alex. Eng. J. 2024, 95, 283–305. [Google Scholar] [CrossRef]
- Lin, J.; Xu, C.; Xu, Y.; Zhao, Y.; Pang, Y.; Liu, Z.; Shen, J. Bifurcation and controller design in a 3D delayed predator-prey model. AIMS Math. 2024, 9, 33891–33929. [Google Scholar] [CrossRef]
- Khan, M.A.; Atangana, A.; Khan, A. Modeling and analysis of tuberculosis dynamics with the Caputo–Fabrizio derivative. Alex. Eng. J. 2021, 60, 3677–3690. [Google Scholar] [CrossRef]
- Atangana, A.; Araz, S.I. Modeling the spread of COVID-19 with new fractal–fractional operators. Chaos Solitons Fractals 2020, 139, 110256. [Google Scholar] [CrossRef]
- Althubyani, M.; Saber, S. Hyers–Ulam stability of fractal–fractional computer virus models with the Atangana–Baleanu operator. Fractal Fract. 2025, 9, 158. [Google Scholar] [CrossRef]
- Baleanu, D.; Jajarmi, A.; Sajjadi, S.S.; Mozyrska, D. A new fractional model and optimal control of a tumor-immune surveillance with non-singular derivative operator. Chaos 2019, 29, 083127. [Google Scholar] [CrossRef]
- Baleanu, D.; Shekari, P.; Torkzadeh, L.; Ranjbar, H.; Jajarmi, A.; Nouri, K. Stability analysis and system properties of Nipah virus transmission: A fractional calculus case study. Chaos Solitons Fractals 2023, 166, 112990. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Cao, J.; Abdel-Aty, M. Dynamics and backward bifurcations of SEI tuberculosis models in homogeneous and heterogeneous populations. J. Math. Anal. Appl. 2025, 543, 128924. [Google Scholar] [CrossRef]
- Alshehri, M.H.; Duraihem, F.Z.; Alalyani, A.; Saber, S. A Caputo (discretization) fractional-order model of glucose–insulin interaction: Numerical solution and comparisons with experimental data. J. Taibah Univ. Sci. 2021, 15, 26–36. [Google Scholar] [CrossRef]
- Ahmed, K.I.; Adam, H.D.; Youssif, M.Y.; Saber, S. Different strategies for diabetes by mathematical modeling: Applications of fractal–fractional derivatives in the sense of Atangana–Baleanu. Results Phys. 2023, 52, 106892. [Google Scholar] [CrossRef]
- Ahmed, K.I.; Adam, H.D.; Youssif, M.Y.; Saber, S. Different strategies for diabetes by mathematical modeling: Modified minimal model. Alex. Eng. J. 2023, 80, 74–87. [Google Scholar] [CrossRef]
- Ahmed, K.I.; Mirgani, S.M.; Seadawy, A.; Saber, S. A comprehensive investigation of fractional glucose–insulin dynamics: Existence, stability, and numerical comparisons using residual power series and generalized Runge–Kutta methods. J. Taibah Univ. Sci. 2025, 19, 2460280. [Google Scholar] [CrossRef]
- Alhazmi, M.; Aljohani, A.F.; Taha, N.E.; Abdel-Khalek, S.; Bayram, M.; Saber, S. Application of a fractal fractional operator to nonlinear glucose–insulin systems: Adomian decomposition solutions. Comput. Biol. Med. 2025, 196, 110453. [Google Scholar] [CrossRef]
- Saber, S.; Mirgani, S.M. Numerical analysis and stability of a system (2) using the Laplace residual power series method incorporating the Atangana–Baleanu derivative. Int. J. Model. Simul. Sci. Comput. 2025, 16, 2550030. [Google Scholar] [CrossRef]
- Saber, S.; Mirgani, S.M. Numerical solutions, stability, and chaos control of Atangana–Baleanu variable-order derivatives in glucose–insulin dynamics. J. Appl. Math. Comput. Mech. 2025, 24, 44–55. [Google Scholar] [CrossRef]
- Saber, S.; Mirgani, S. Analyzing fractional glucose–insulin dynamics using Laplace residual power series methods via the Caputo operator: Stability and chaotic behavior. Beni-Suef Univ. J. Basic Appl. Sci. 2025, 14, 28. [Google Scholar] [CrossRef]
- Alhazmi, M.; Saber, S. Application of a fractal fractional derivative with a power-law kernel to the glucose–insulin interaction system based on Newton’s interpolation polynomials. Fractals 2025, 25402017. [Google Scholar] [CrossRef]
- Saber, S.; Dridi, B.; Alahmari, A.; Messaoudi, M. Application of Jumarie–Stancu Collocation Series Method and Multi-Step Generalized Differential Transform Method to fractional glucose–insulin. Int. J. Optim. Control Theor. Appl. 2025, 2025, 025120054. [Google Scholar] [CrossRef]
- Saber, S.; Dridi, B.; Alahmari, A.; Messaoudi, M. Hyers–Ulam stability and control of fractional glucose–insulin systems. Eur. J. Pure Appl. Math. 2025, 18, 6152. [Google Scholar] [CrossRef]
- Ulam, S.M. A Collection of Mathematical Problems; Interscience Tracts in Pure and Applied Mathematics; Interscience Publishers: New York, NY, USA, 1960; Volume 8. [Google Scholar]
- Hyers, D.H.; Isac, G.; Rassias, T.M. Stability of Functional Equations in Several Variables; Birkhäuser: Basel, Switzerland, 1998. [Google Scholar]
- Diethelm, K.; Ford, N.J.; Freed, A.D. A predictor-corrector approach for the numerical solution of fractional differential equations. Nonlinear Dyn. 2002, 29, 3–22. [Google Scholar] [CrossRef]
- Li, C.; Zeng, F. Numerical Methods for Fractional Calculus; Chapman & Hall/CRC: Boca Raton, FL, USA, 2015. [Google Scholar]
- Diethelm, K. The Analysis of Fractional Differential Equations: An Application-Oriented Exposition Using Differential Operators of Caputo Type; Springer: Berlin, Germany, 2010. [Google Scholar]
- Kilbas, A.A.; Srivastava, H.M.; Trujillo, J.J. Theory and Applications of Fractional Differential Equations; Elsevier: Amsterdam, The Netherland, 2006. [Google Scholar]
- Bagley, R.L.; Calico, R.A. Fractional order state equations. J. Guid. Control Dyn. 1991, 14, 304–311. [Google Scholar] [CrossRef]
- Kusnezov, D.; Bulgac, A.; Dang, G.D. Quantum Lévy processes. Phys. Rev. Lett. 1999, 82, 1136–1139. [Google Scholar] [CrossRef]
- Hammad, H.A.; Qasymeh, M.; Abdel-Aty, M. Langevin systems with Caputo–Hadamard operators. Int. J. Geom. Methods Mod. Phys. 2024, 21, 2450218. [Google Scholar] [CrossRef]
- Shukla, V.K.; Joshi, M.C.; Mishra, P.K.; Xu, C. Adaptive fixed-time difference synchronization for different classes of chaotic dynamical systems. Phys. Scr. 2024, 99, 095264. [Google Scholar] [CrossRef]
- Alsulami, A.; Alharb, R.A.; Albogami, T.M.; Eljaneid, N.H.; Adam, H.D.; Saber, S.F. Controlled chaos of a fractal–fractional Newton-Leipnik system. Therm. Sci. 2024, 28, 5153–5160. [Google Scholar] [CrossRef]
- Yan, T.; Alhazmi, M.; Youssif, M.Y.; Elhag, A.E.; Aljohani, A.F.; Saber, S. Analysis of a Lorenz model using Adomian decomposition and fractal–fractional operators. Therm. Sci. 2024, 28, 5001–5009. [Google Scholar] [CrossRef]
- Alhazmi, M.; Dawalbait, F.M.; Aljohani, A.; Taha, K.O.; Adam, H.D.; Saber, S. Numerical approximation method and chaos for a chaotic system in the sense of Caputo–Fabrizio operator. Therm. Sci. 2024, 28, 5161–5168. [Google Scholar] [CrossRef]
- Evirgen, F.; Bayram, M.; Yüzbaşı, Ş. Numerical analysis of an influenza model with Caputo–Fabrizio fractional derivative. Math. Model. Numer. Simul. Appl. 2023, 3, 58–73. [Google Scholar]
- Ozdemir, N.; Kara, S.; Kiris, E. Stability and sensitivity analysis of fractional SVIR epidemic models. Facta Univ. Ser. Math. Inform. 2022, 37, 605–619. [Google Scholar]
- Li, X.-P.; Wang, Y.; Zhang, T. A novel fractional-order model for coronavirus dynamics via the fractal–fractional approach. Results Phys. 2022, 34, 105179. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.A.; Khan, M.A.; Farooq, M.; Ullah, S.; Alzahrani, E.O. A fractional order model for Hepatitis B virus with treatment via Atangana–Baleanu derivative. Physica A 2020, 538, 122636. [Google Scholar] [CrossRef]
- Althubyani, M.; Taha, N.; Taha, K.; Alharb, R.; Saber, S. Epidemiological modeling of pneumococcal pneumonia: Insights from ABC fractal–fractional derivatives. CMES-Comp. Model. Eng. Sci. 2025, 143, 3491–3521. [Google Scholar] [CrossRef]
- Althubyani, M.; Adam, H.D.; Alalyani, A.; Taha, N.E.; Taha, K.O.; Alharbi, R.A.; Saber, S. Understanding zoonotic disease spread with a fractional order epidemic model. Sci. Rep. 2025, 15, 13921. [Google Scholar] [CrossRef]
- Adam, H.D.; Althubyani, M.; Mirgani, S.M.; Saber, S. An application of Newton’s interpolation polynomials to the zoonotic disease transmission between humans and baboons system based on a time-fractal fractional derivative with a power-Law kernel. AIP Adv. 2025, 15, 045217. [Google Scholar] [CrossRef]
- Saber, S.; Solouma, E. The generalized Euler method for analyzing zoonotic disease dynamics in baboon–human populations. Symmetry 2025, 17, 541. [Google Scholar] [CrossRef]
- Saber, S.; Solouma, E.; Althubyani, M.; Messaoudi, M. Statistical insights into zoonotic disease dynamics: Simulation and control strategy evaluation. Symmetry 2025, 17, 733. [Google Scholar] [CrossRef]
- Saber, S.; Alahmari, A. Mathematical insights into zoonotic disease spread: Application of the Milstein method. Eur. J. Pure Appl. Math. 2025, 18, 58–81. [Google Scholar] [CrossRef]
- Bergman, R.N.; Phillips, L.S.; Cobelli, C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J. Clin. Investig. 1981, 68, 1456–1467. [Google Scholar] [CrossRef]
- Pacini, G.; Bergman, R.N. MINMOD: A computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput. Methods Programs Biomed. 1987, 23, 113–122. [Google Scholar] [CrossRef]
- Cobelli, C.; Carson, E.R.; Finkelstein, L.A. Introduction to Modeling in Physiology and Medicine; Academic Press: London, UK, 2009. [Google Scholar]



| Parameter | Physiological Meaning | Units |
|---|---|---|
| Initial glucose concentration (fasting measurement). | mg/dL | |
| Insulin-independent glucose disposal rate and baseline hepatic production. | ||
| Decay rate of insulin action (lifetime of receptor binding and downstream signaling). | ||
| Insulin sensitivity coefficient; reduction indicates insulin resistance. | mL/μU/min | |
| Maximal pancreatic insulin secretion gain above threshold. | μU/mL/min | |
| Threshold glucose level for insulin secretion activation. | mg/dL | |
| Clearance rate of plasma insulin (hepatic/renal degradation). | ||
| Initial deviation of insulin from basal level. | μU/mL | |
| Basal glucose level (fasting equilibrium). | mg/dL | |
| Basal insulin level at equilibrium. | μU/mL | |
| Decay rate of the auxiliary delayed effect . |
| Property | Mathematical Role | Biological Significance |
|---|---|---|
| Non-negativity | Ensures solutions remain within a physically meaningful domain | Maintains physiological realism by preventing negative concentrations |
| Boundedness | Provides uniform bounds on all system variables | Reflects homeostatic regulation and prevents unphysical growth |
| Existence and Uniqueness | Guarantees well-posedness of the model | Ensures deterministic predictions for given initial conditions and parameters |
| Hyers–Ulam Stability | Controls error propagation from approximations | Provides robustness against biological variability and measurement errors |
| n | (Glucose) | (Insulin Action) | (Insulin) | (Auxiliary) |
|---|---|---|---|---|
| 0 | 287.000000 | 0.000000 | 403.400000 | 0.000000 |
| 1 | 286.164909 | 0.000144 | 403.354672 | 0.130897 |
| 2 | 285.439650 | 0.000268 | 404.303207 | 0.243904 |
| 3 | 284.749925 | 0.000386 | 405.966856 | 0.350857 |
| 4 | 284.081848 | 0.000503 | 408.268607 | 0.453976 |
| 5 | 283.429070 | 0.000619 | 411.159885 | 0.554280 |
| 6 | 282.787921 | 0.000737 | 414.605416 | 0.652354 |
| 7 | 282.155996 | 0.000856 | 418.577477 | 0.748578 |
| 8 | 281.531575 | 0.000978 | 423.053182 | 0.843219 |
| 9 | 280.913360 | 0.001104 | 428.012997 | 0.936473 |
| 10 | 280.300315 | 0.001234 | 433.439846 | 1.028493 |
| Characteristic | CLDM | RPSM | FRK4 |
|---|---|---|---|
| Accuracy () | |||
| Computational cost | |||
| Memory requirements | Low | Medium | High |
| Adaptivity | Yes | Limited | Yes |
| Implementation | Simple | Complex | Moderate |
| h | Error | Rate | Error | Rate |
|---|---|---|---|---|
| 0.1000 | 0.01063 | – | 0.03541 | – |
| 0.0500 | 0.01017 | 0.063 | 0.03061 | 0.210 |
| 0.0250 | 0.00991 | 0.037 | 0.03747 | −0.292 |
| 0.0125 | 0.01020 | −0.042 | 0.03979 | −0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhazmi, M.; Mirgani, S.M.; Saber, S. Hybrid Euler–Lagrange Approach for Fractional-Order Modeling of Glucose–Insulin Dynamics. Axioms 2025, 14, 800. https://doi.org/10.3390/axioms14110800
Alhazmi M, Mirgani SM, Saber S. Hybrid Euler–Lagrange Approach for Fractional-Order Modeling of Glucose–Insulin Dynamics. Axioms. 2025; 14(11):800. https://doi.org/10.3390/axioms14110800
Chicago/Turabian StyleAlhazmi, Muflih, Safa M. Mirgani, and Sayed Saber. 2025. "Hybrid Euler–Lagrange Approach for Fractional-Order Modeling of Glucose–Insulin Dynamics" Axioms 14, no. 11: 800. https://doi.org/10.3390/axioms14110800
APA StyleAlhazmi, M., Mirgani, S. M., & Saber, S. (2025). Hybrid Euler–Lagrange Approach for Fractional-Order Modeling of Glucose–Insulin Dynamics. Axioms, 14(11), 800. https://doi.org/10.3390/axioms14110800







