Spatiotemporal Dynamics of a Diffusive Immunosuppressive Infection Model with Nonlocal Competition and Crowley–Martin Functional Response
Abstract
1. Introduction
2. Stability and Bifurcation
2.1. Stability and Bifurcation of the System with Nonlocal Competition
- (i)
- (ii)
- (iii)
- (i)
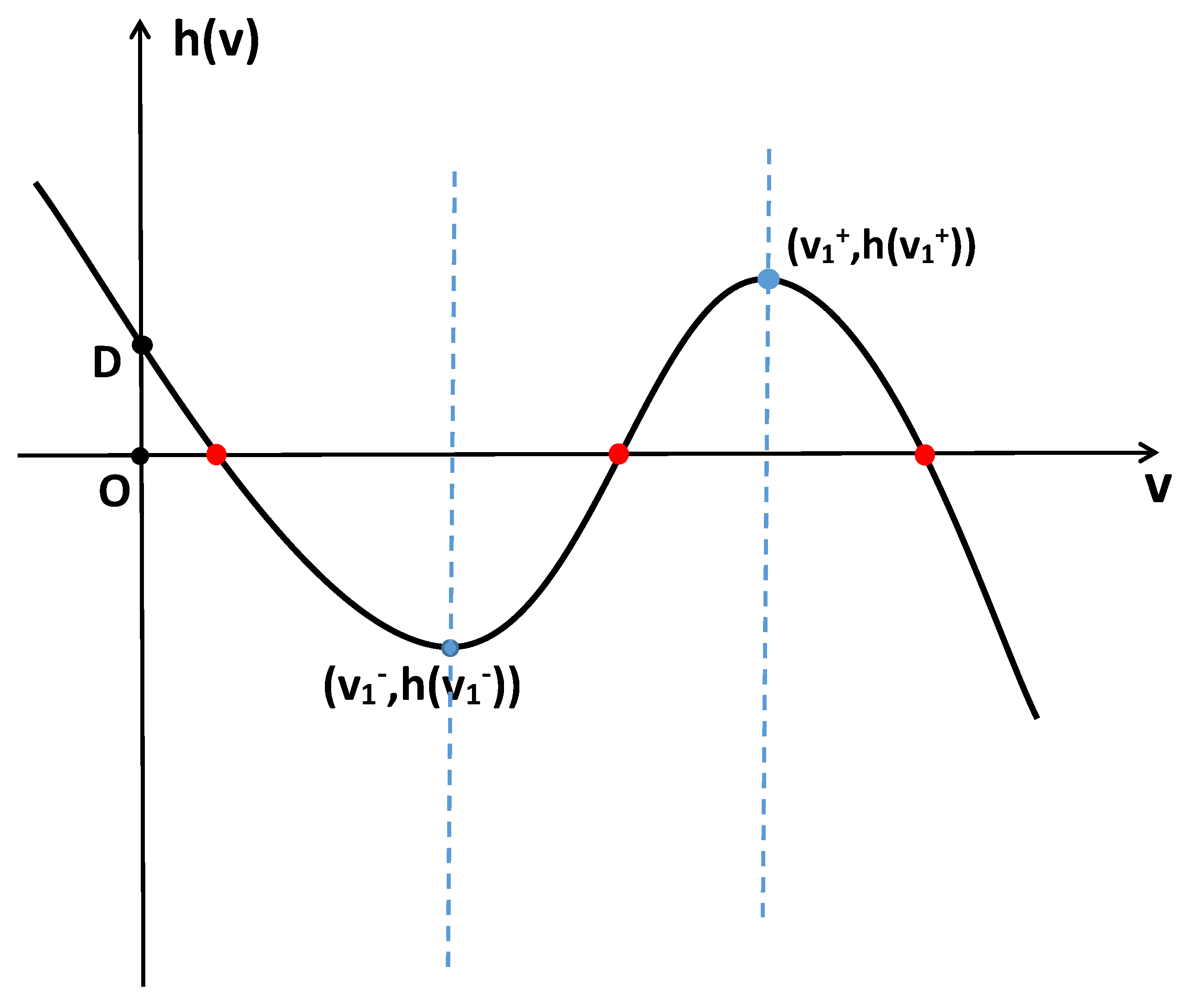
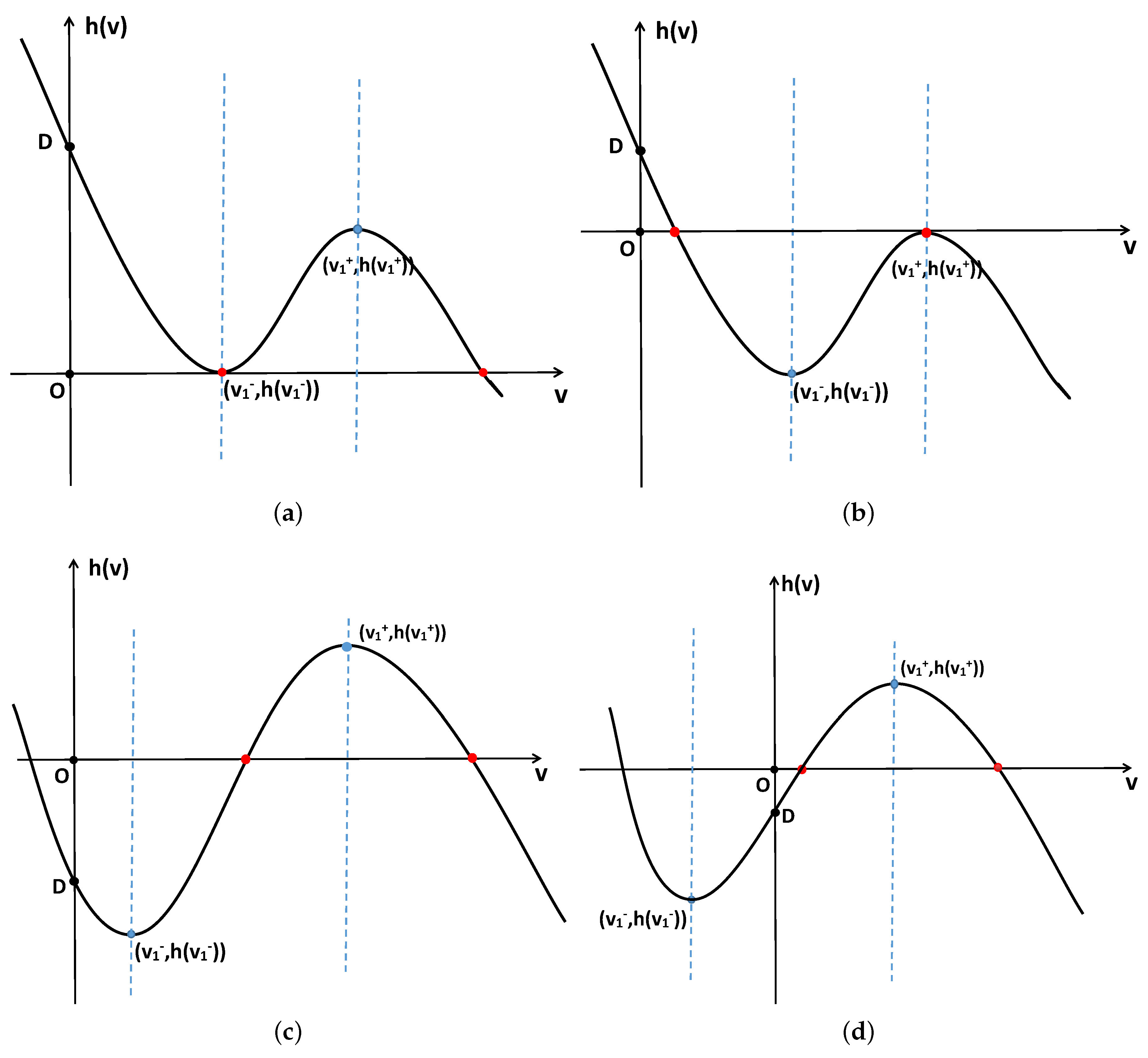
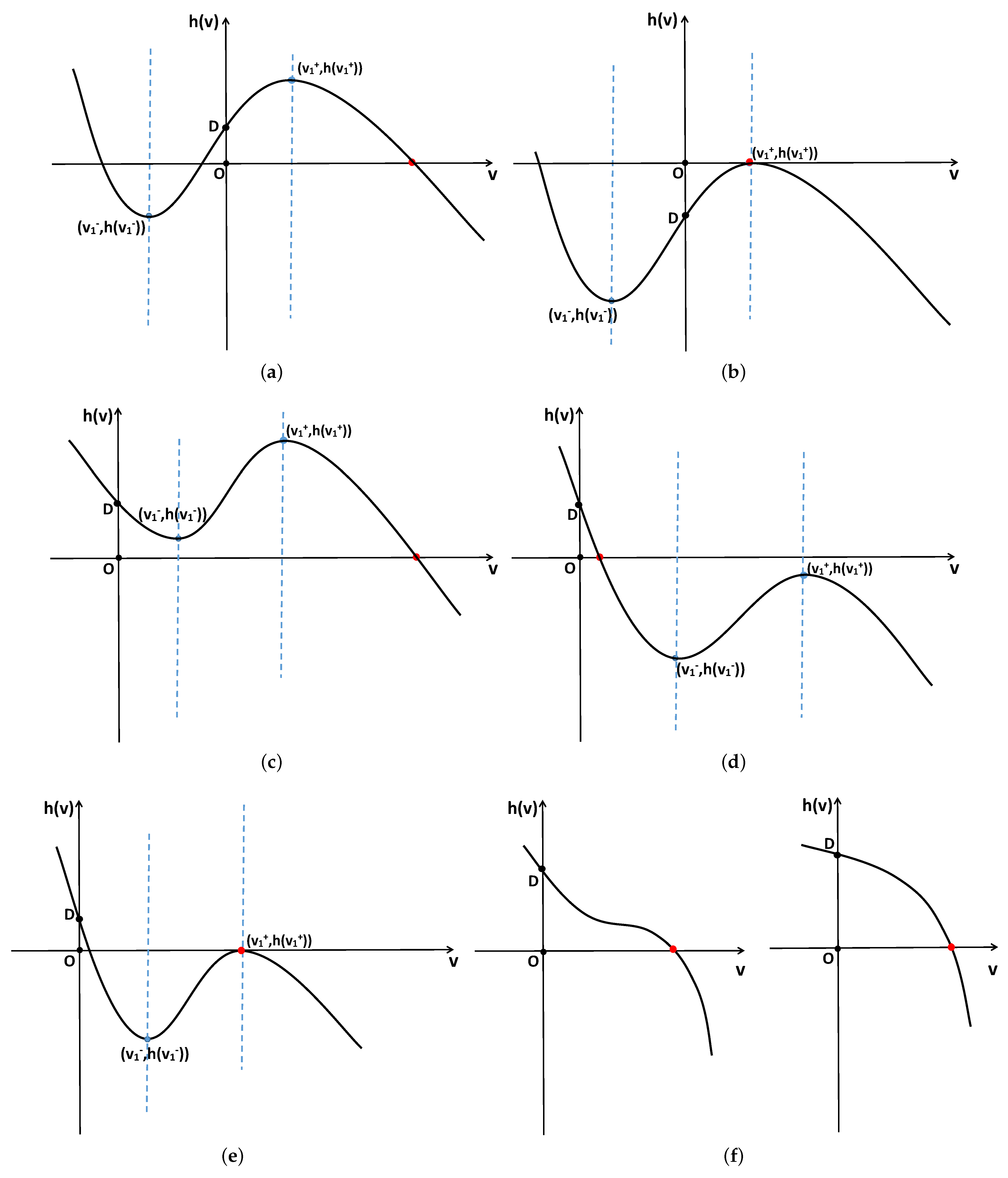
- When and , the positive constant steady state is locally asymptotically stable for and unstable for . Additionally, system (2) undergoes -mode Hopf bifurcation at when ;
- (ii)
- When , the positive constant steady state is unstable for ;
- (iii)
- When , then system (2) undergoes -mode Turing–Hopf bifurcation at ,
2.2. Stability and Bifurcation of the System without Nonlocal Competition
3. Stability and Direction of Hopf Bifurcation
3.1. Stability and Direction of Hopf Bifurcation of the System with Nonlocal Competition
- (i)
- If , the bifurcating periodic solutions reduced on the center manifold are unstable, and when ( ), the direction of bifurcation is forward (backward);
- (ii)
- If , the bifurcating periodic solutions reduced on the center manifold are stable, and when ( ), the direction of bifurcation is forward (backward).
3.2. Stability and Bifurcation of the System without Nonlocal Competition
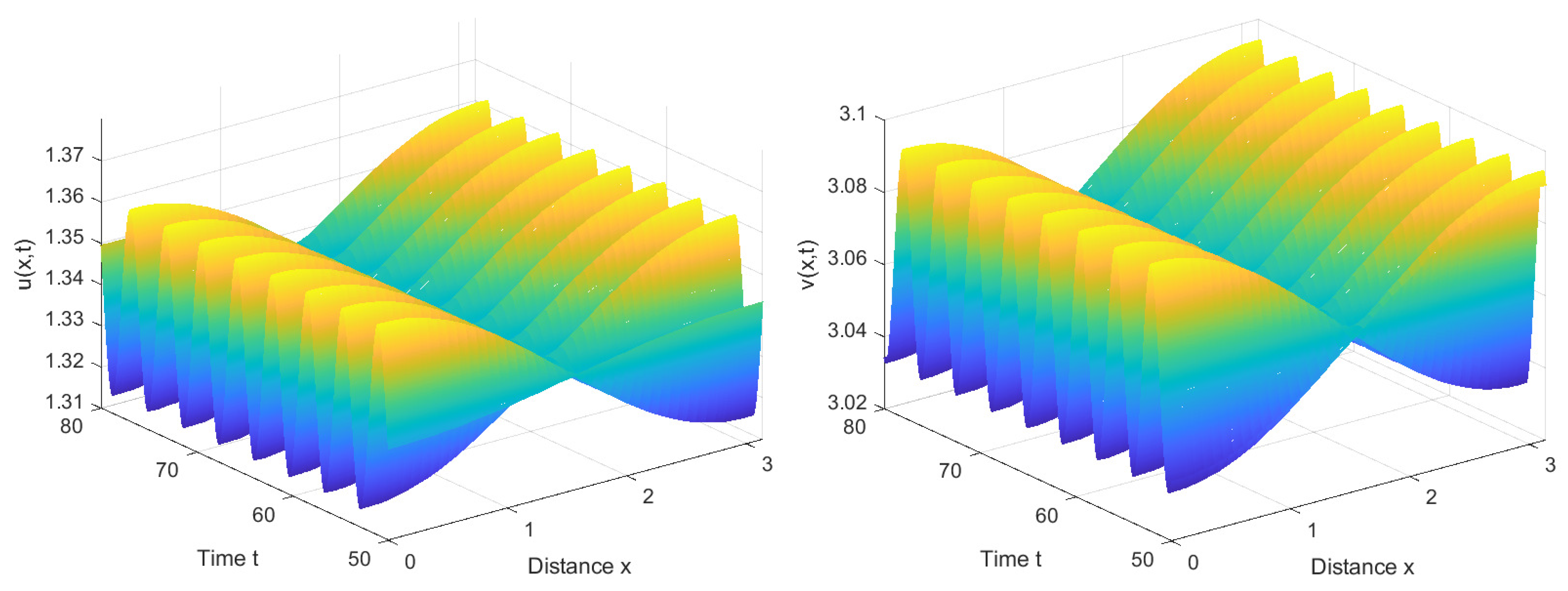
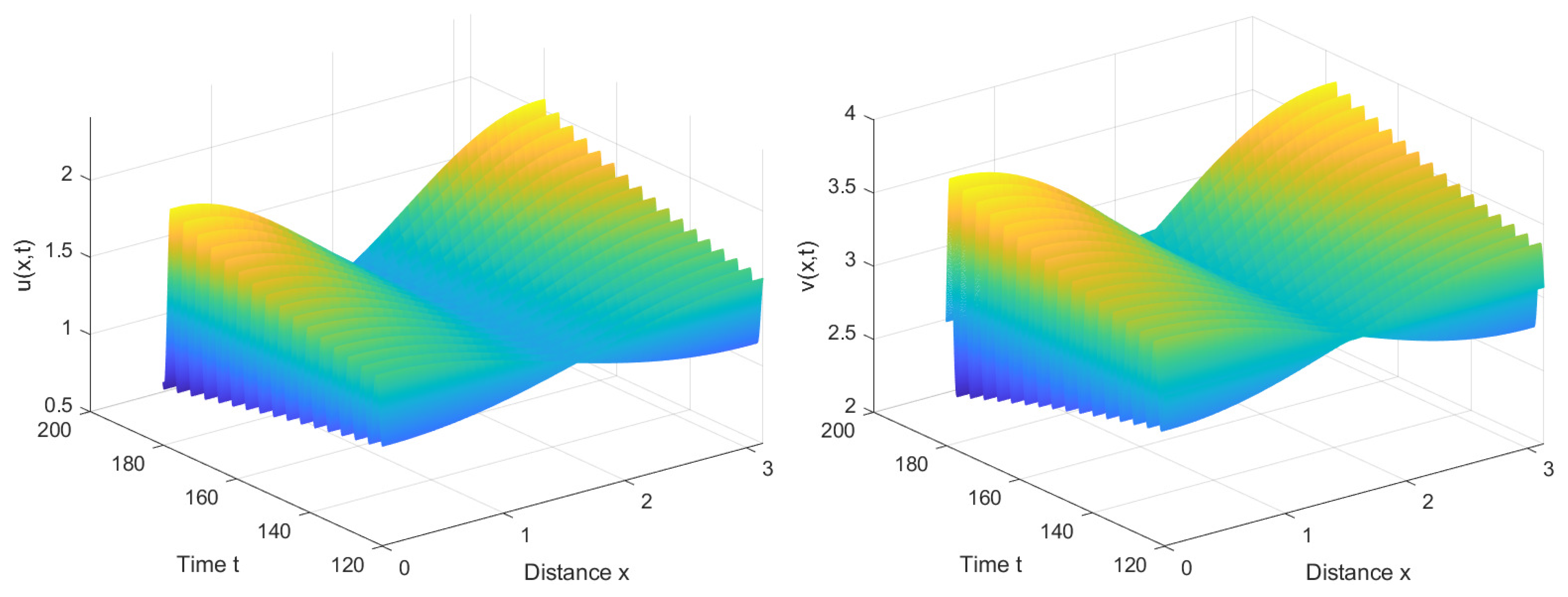
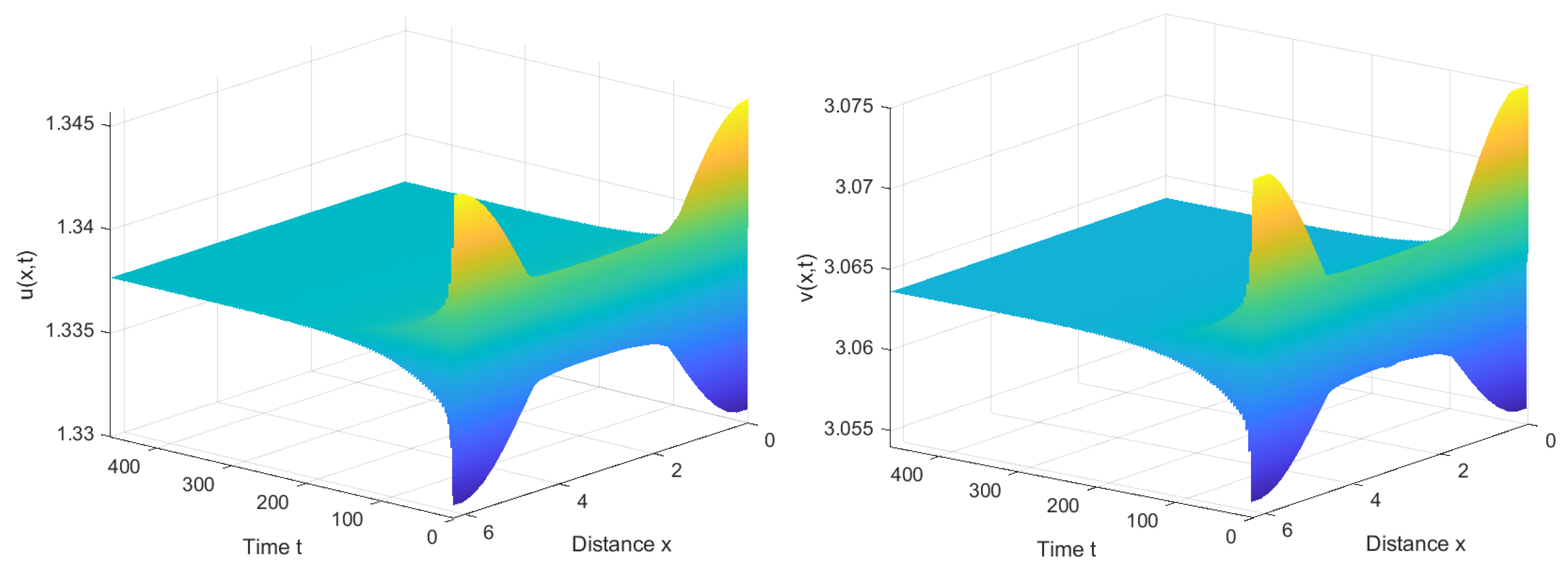
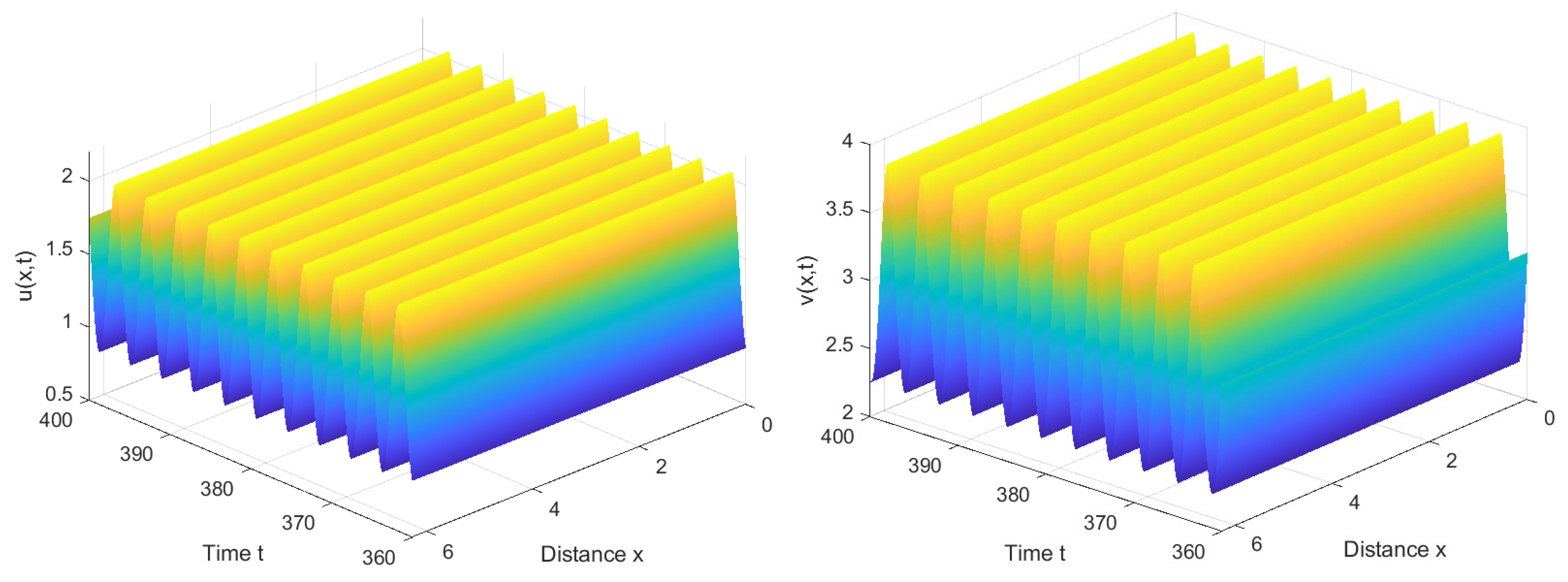
4. Numerical Simulations
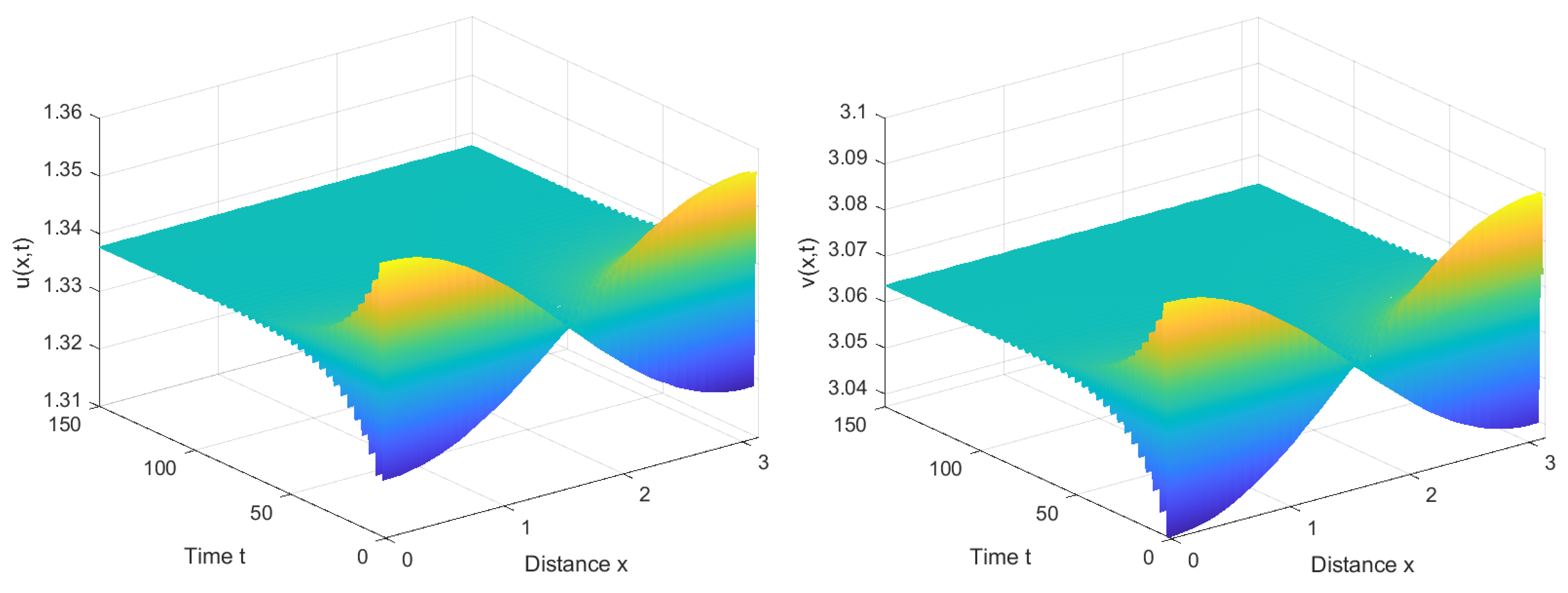
4.1. Numerical Simulations of the System with Nonlocal Competition
4.2. Numerical Simulations of the System without Nonlocal Competition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Komarova, N.L.; Barnes, E.; Klenerman, P.; Wodarz, D. Boosting immunity by antiviral drug therapy: A simple relationship among timing, efficacy, and success. Proc. Natl. Acad. Sci. USA 2003, 100, 1855–1860. [Google Scholar] [CrossRef]
- Sheppard, H.W.; Celum, C.; Michael, N.L.; O’Brien, S.; Dean, M.; Carrington, M.; Dondero, D.; Buchbinder, S.P. HIV-1 infection in individuals with the CCR5-Δ32/32/Δ32 genotype: Acquisition of syncytium-inducing virus at seroconversion. J. Acquir. Immune Defic. Syndr. 2002, 29, 307–313. [Google Scholar] [CrossRef]
- Walker, B.D. Elite control of HIV Infection: Implications for vaccines and treatment. Top. HIV Med. 2007, 15, 134–136. [Google Scholar]
- Hersperger, A.R.; Martin, J.N.; Shin, L.Y.; Sheth, P.M.; Kovacs, C.M.; Cosma, G.L.; Betts, M.R. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 2011, 117, 3799–3808. [Google Scholar] [CrossRef]
- Saag, M.; Deeks, S.G. How do HIV elite controllers do what they do? Clin. Infect. Dis. 2010, 51, 239–241. [Google Scholar] [CrossRef][Green Version]
- Deeks, S.G.; Walker, B.D. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007, 27, 406–416. [Google Scholar] [CrossRef]
- Funk, G.A.; Jansen, V.A.; Bonhoeffer, S.; Killingback, T. Spatial models of virus-immune dynamics. J. Theor. Biol. 2005, 233, 221–236. [Google Scholar] [CrossRef]
- Browne, C.J.; Smith, H.L. Dynamics of virus and immune response in multi-epitope network. J. Math. Biol. 2018, 77, 1833–1870. [Google Scholar] [CrossRef]
- Wodarz, D.; Nowak, M.A. Mathematical models of HIV pathogenesis and treatment. Bioessays 2002, 24, 1178–1187. [Google Scholar] [CrossRef]
- Shu, H.; Wang, L.; Watmough, J. Sustained and transient oscillations and chaos induced by delayed antiviral immune response in an immunosuppressive infection model. J. Math. Biol. 2014, 68, 477–503. [Google Scholar] [CrossRef]
- Tian, C.; Gan, W.; Zhu, P. Stability analysis in a diffusional immunosuppressive infection model with delayed antiviral immune response. Math. Methods Appl. Sci. 2017, 40, 4001–4013. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Li, J.; Zhang, D. Dynamics of a chronic virus infection model with viral stimulation delay. Appl. Math. Lett. 2021, 122, 107547. [Google Scholar] [CrossRef]
- Fenton, A.; Lello, J.; Bonsall, M.B. Pathogen responses to host immunity: The impact of time delays and memory on the evolution of virulence. Proc. R. Soc. B 2006, 273, 2083–2090. [Google Scholar] [CrossRef]
- Johansen, P.; Storni, T.; Rettig, L.; Manolova, V.; Lang, K.S.; Qiu, Z. Antigen kinetics determines immune reactivity. Swiss Med. Wkly. 2007, 137, 23S. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Liu, Z.; Wang, Y. Complex dynamics for an immunosuppressive infection model with virus stimulation delay and nonlinear immune expansion. Qual. Theory Dyn. Syst. 2023, 22, 118. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, J.; Ding, Y. Dynamics analysis of a diffusional immunosuppressive infection model with Beddington-DeAngelis functional response. Electron. Res. Arch. 2023, 31, 6071–6088. [Google Scholar] [CrossRef]
- Crowley, P.H.; Martin, E.K. Functional responses and interference within and between year classes of a dragonfly population. J. N. Am. Benthol. Soc. 1989, 8, 211–221. [Google Scholar] [CrossRef]
- Hossain, S.; Haque, M.M.; Kabir, M.H.; Gani, M.O.; Sarwardi, S. Complex spatiotemporal dynamics of a harvested prey-predator model with Crowley-Martin response function. Results Control Optim. 2021, 5, 100059. [Google Scholar] [CrossRef]
- Chen, S.; Wei, J.; Yu, J. Stationary patterns of a diffusive predator-prey model with Crowley-Martin functional response. Nonlinear Anal. Real World Appl. 2018, 39, 33–57. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Raw, S.N.; Rai, V. Dynamical complexities in a tri-trophic hybrid food chain model with Holling type II and Crowley-Martin functional responses. Nonlinear Anal. Model. Control. 2010, 15, 361–375. [Google Scholar] [CrossRef]
- Skalski, G.T.; Gilliam, J.F. Functional responses with predator interference: Viable alternatives to the holling type II model. Ecology 2001, 82, 3083–3092. [Google Scholar] [CrossRef]
- Bocharov, G.A. Modelling the dynamics of LCMV infection in mice: Conventional and exhaustive CTL responses. J. Theor. Biol. 1998, 192, 283–308. [Google Scholar] [CrossRef]
- Keşmir, C.; De Boer, R.J. Clonal exhaustion as a result of immune deviation. Bull. Math. Biol. 2003, 65, 359–374. [Google Scholar] [CrossRef]
- Naik, P.A.; Zu, J.; Ghoreishi, M. Stability analysis and approximate solution of SIR epidemic model with Crowley-Martin type functional response and Holling type-II treatment rate by using homotopy analysis method. J. Appl. Anal. Comput. 2020, 10, 1482–1515. [Google Scholar] [CrossRef]
- Jan, M.N.; Ali, N.; Zaman, G.; Ahmad, I.; Shah, Z.; Kumam, P. HIV-1 infection dynamics and optimal control with Crowley-Martin function response. Comput. Methods Programs Biomed. 2020, 193, 105503. [Google Scholar] [CrossRef]
- Roomi, V.; Afshari, H.; Gharahasanlou, T.K. Global Stability of an HIV Dynamical Model with Crowley-Martin Functional Response. Lett. Nonlinear Anal. Appl. 2023, 1, 39–46. [Google Scholar]
- Li, X.; Fu, S. Global stability of a virus dynamics model with intracellular delay and CTL immune response. Math. Methods Appl. Sci. 2015, 38, 420–430. [Google Scholar] [CrossRef]
- Furter, J.; Grinfeld, M. Local vs. non-local interactions in population dynamics. J. Math. Biol. 1989, 27, 65–80. [Google Scholar] [CrossRef]
- Geng, D.; Jiang, W.; Lou, Y.; Wang, H. Spatiotemporal patterns in a diffusive predator-prey system with nonlocal intraspecific prey competition. Stud. Appl. Math. 2022, 148, 396–432. [Google Scholar] [CrossRef]
- Wu, S.; Song, Y. Spatiotemporal dynamics of a diffusive predator-prey model with nonlocal effect and delay. Commun. Nonlinear Sci. Numer. Simul. 2020, 89, 105310. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Niu, B. Spatiotemporal dynamics in a diffusive predator-prey model with group defense and nonlocal competition. Appl. Math. Lett. 2020, 103, 106175. [Google Scholar] [CrossRef]
- Doebeli, M.; Killingback, T. Metapopulation dynamics with quasi-local competition. Theor. Popul. Biol. 2003, 64, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Nie, C.; Jin, D. Spatiotemporal dynamics induced by nonlocal competition in a diffusive predator-prey system with habitat complexity. Nonlinear Dyn. 2022, 110, 879–900. [Google Scholar] [CrossRef]
- Yang, R.; Wang, F.; Jin, D. Spatially inhomogeneous bifurcating periodic solutions induced by nonlocal competition in a predator–prey system with additional food. Math. Meth. Appl. Sci. 2022, 45, 9967–9978. [Google Scholar] [CrossRef]
- Bessonov, N.; Bocharov, G.; Meyerhans, A.; Popov, V.; Volpert, V. Nonlocal reaction-diffusion model of viral evolution: Emergence of virus strains. Math 2020, 8, 117. [Google Scholar] [CrossRef]
- Banerjee, M.; Kuznetsov, M.; Udovenko, O.; Volpert, V. Nonlocal Reaction-Diffusion Equations in Biomedical Applications. Acta Biotheor. 2022, 70, 12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Xu, J.; Ding, Y. Spatiotemporal Dynamics of a Diffusive Immunosuppressive Infection Model with Nonlocal Competition and Crowley–Martin Functional Response. Axioms 2023, 12, 1085. https://doi.org/10.3390/axioms12121085
Xue Y, Xu J, Ding Y. Spatiotemporal Dynamics of a Diffusive Immunosuppressive Infection Model with Nonlocal Competition and Crowley–Martin Functional Response. Axioms. 2023; 12(12):1085. https://doi.org/10.3390/axioms12121085
Chicago/Turabian StyleXue, Yuan, Jinli Xu, and Yuting Ding. 2023. "Spatiotemporal Dynamics of a Diffusive Immunosuppressive Infection Model with Nonlocal Competition and Crowley–Martin Functional Response" Axioms 12, no. 12: 1085. https://doi.org/10.3390/axioms12121085
APA StyleXue, Y., Xu, J., & Ding, Y. (2023). Spatiotemporal Dynamics of a Diffusive Immunosuppressive Infection Model with Nonlocal Competition and Crowley–Martin Functional Response. Axioms, 12(12), 1085. https://doi.org/10.3390/axioms12121085





