Phosphate Waste Rock Piles as a Secondary Resource: Insights into Composition and Strategic Element Potential
Abstract
1. Introduction
2. Materials and Methods
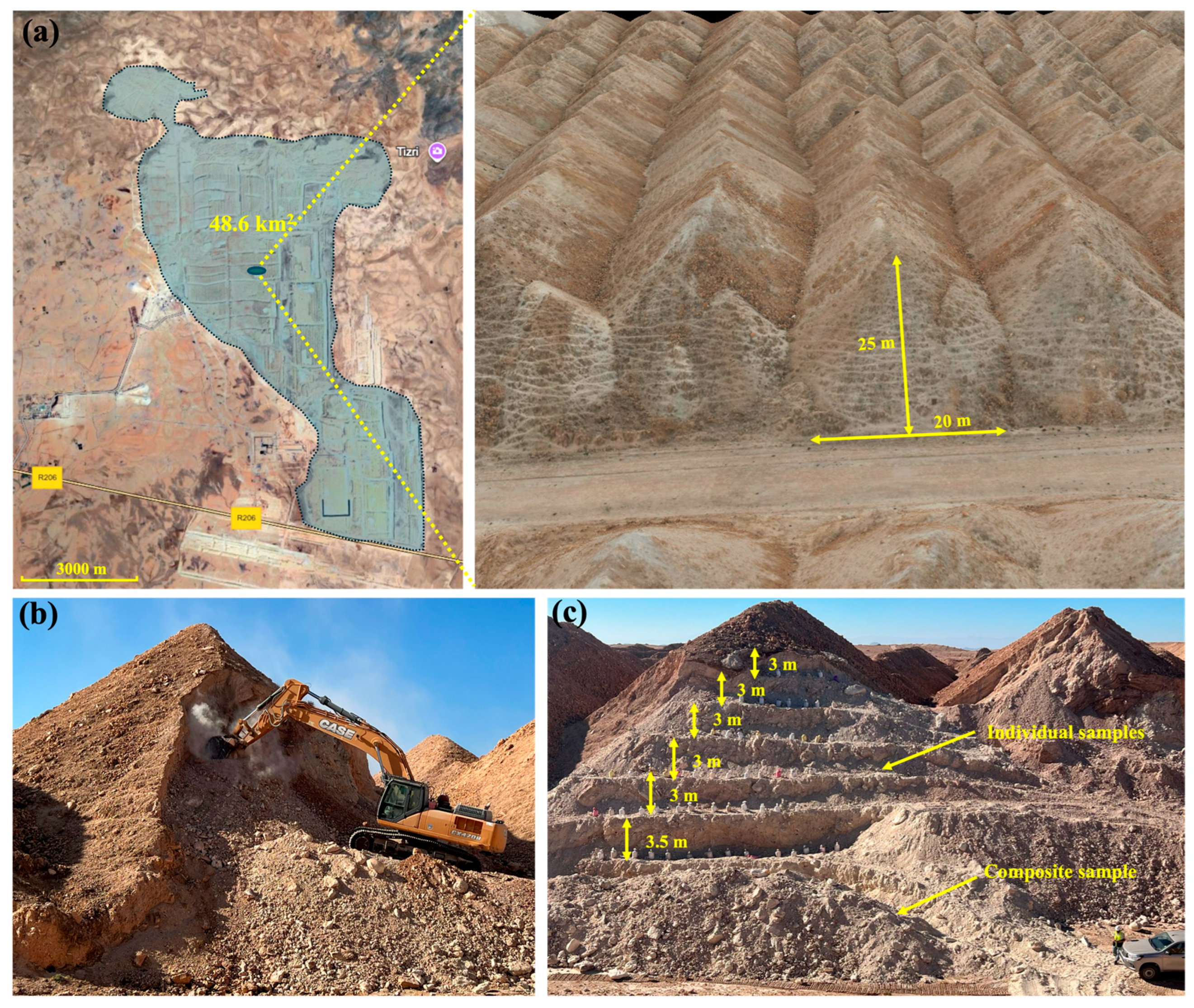
2.1. Sampling and Material Preparation
2.2. Physical and Chemical Characterization of Samples
2.3. Mineralogical Characterization
2.4. Calculation of Enrichment Factors
3. Results and Discussion
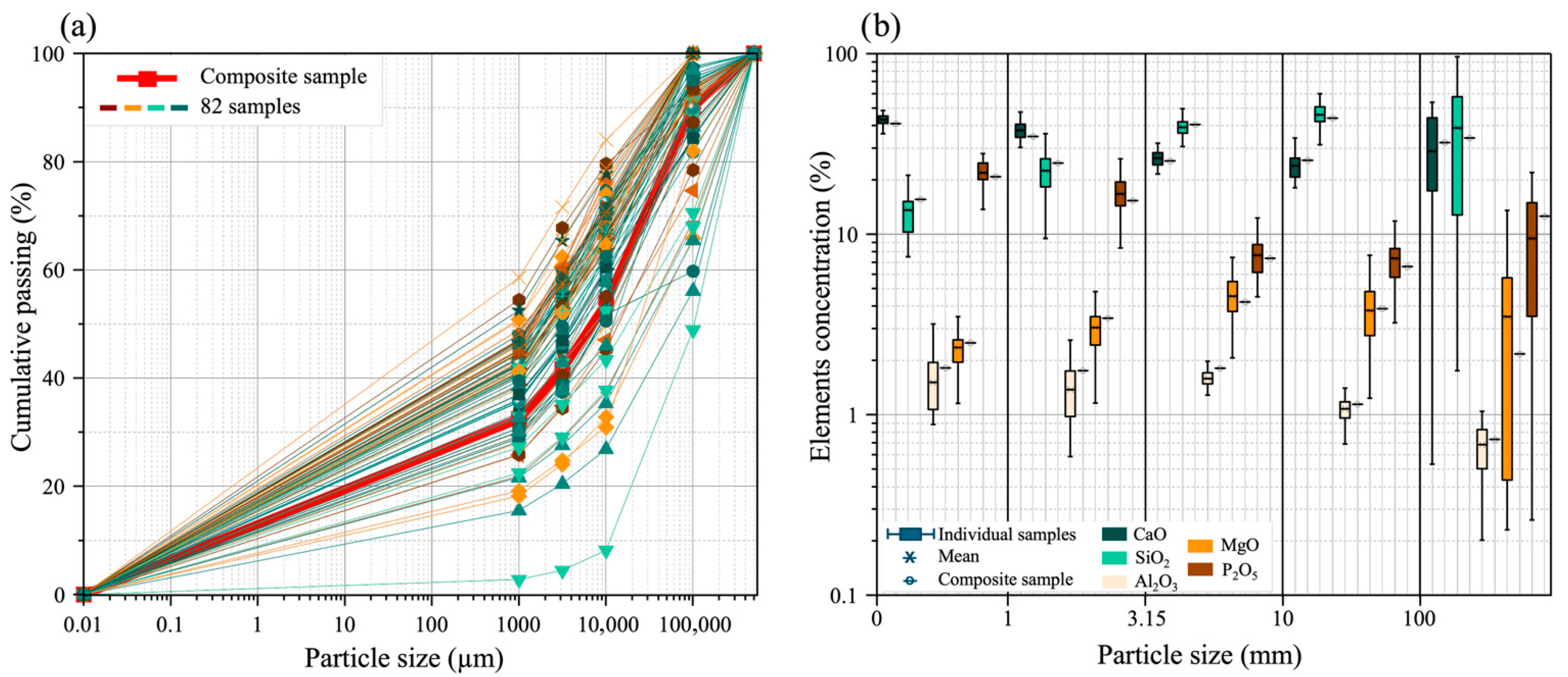
3.1. Evaluation of Sampling Strategy
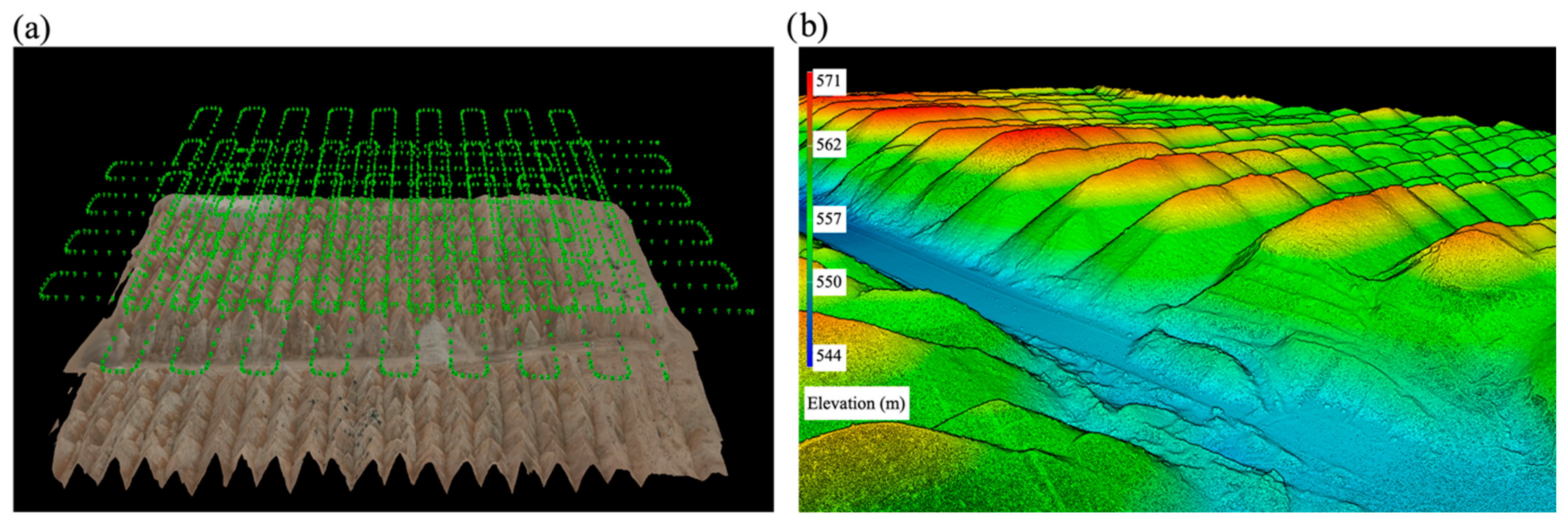
3.2. Three-Dimensional Topographic Model and Spatial Distribution of CFA and Particle Size
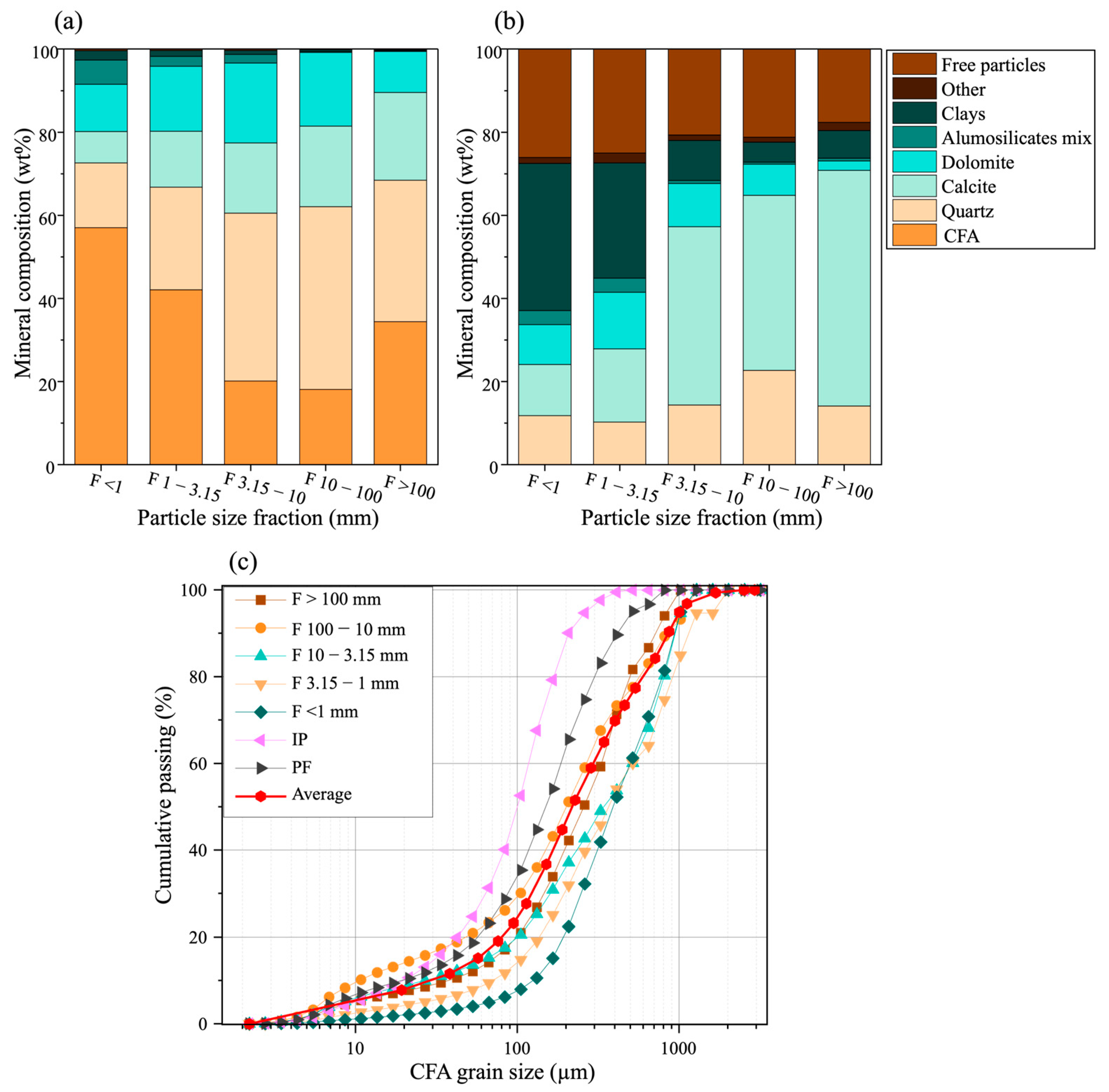
3.3. Mineralogical Characterization
3.4. Chemical Characterization
3.5. Correlation
4. Phosphate Mine Waste Rock Valorization
5. Conclusions
- The developed sampling strategy ensured the collection of representative samples, as validated through comparisons of the physical and chemical properties of the composite and individual samples. However, the observed granulometric heterogeneity within the waste rock emphasizes the necessity of a second sampling phase, covering multiple zones, to achieve more comprehensive and accurate results.
- Chemical characterization identified substantial concentrations of critical and strategic elements, particularly phosphate, magnesium, rare earth elements (REEs), strontium (Sr), vanadium (V), and uranium (U), with significant potential for recovery and valorization. REEs, Sr, V, and U were notably found to be highly enriched in the finer fractions and showed a positive correlation with phosphate, except for magnesium.
- This positive correlation suggests that their preconcentration can be effectively achieved through phosphate beneficiation processes, including desliming, grinding, and flotation. Such processes not only upgrade the waste to achieve marketable phosphate quality but also concentrate these valuable elements.
- The volume estimation of the waste rock, conducted in August 2024, revealed a total volume of approximately 419,612,367 m3, underscoring the enormous resource potential embedded within these materials.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chernoburova, O.; Chagnes, A. Mining and Processing Residues: Future’s Source of Critical Raw Materials; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Hofmann, M.; Hofmann, H.; Hagelüken, C.; Hool, A. Critical Raw Materials: A Perspective from the Materials Science Community. Sustain. Mater. Technol. 2018, 17, e00074. [Google Scholar] [CrossRef]
- Patricia, A.D.; Silvia, B.; Samuel, C.; Beatrice, P. The Role of Rare Earth Elements in Wind Energy and Electric Mobility: An Analysis of Future Supply/Demand Balances; Publications Office of the European Union: Luxembourg, 2020; ISBN 9789276270164. [Google Scholar]
- Buijs, B.; Sievers, H.; Espinoza, L.A.T. Limits to the Critical Raw Materials Approach. Proc. Inst. Civil. Eng. Waste Resour. Manag. 2012, 165, 201–208. [Google Scholar] [CrossRef]
- Ferro, P.; Bonollo, F. Materials Selection in a Critical Raw Materials Perspective. Mater. Des. 2019, 177, 107848. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Survey Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; ISBN 9781411345447.
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023; European Commission: Brussels, Belgium, 2023; ISBN 9789279680519. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 13 December 2025).
- Blengini, G.; Mathieux, F.; Mancini, L.; Nyberg, M.; Cavaco Viegas, H. Recovery of Critical and Other Raw Materials from Mining Waste and Landfills State of Play on Existing Practices; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Kumari, R.; Samadder, S.R. A Critical Review of the Pre-Processing and Metals Recovery Methods from e-Wastes. J. Environ. Manag. 2022, 320, 115887. [Google Scholar] [CrossRef] [PubMed]
- Dold, B. Sourcing of Critical Elements and Industrial Minerals from Mine Waste—The Final Evolutionary Step Back to Sustainability of Humankind? J. Geochem. Explor. 2020, 219, 106638. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Gilmour, S.; Fox, N.; Olin, P. Geometallurgical Characterization of Non-Ferrous Historical Slag in Western Tasmania: Identifying Reprocessing Options. Minerals 2019, 9, 415. [Google Scholar] [CrossRef]
- Rosario-Beltré, A.J.; Sánchez-España, J.; Rodríguez-Gómez, V.; Fernández-Naranjo, F.J.; Bellido-Martín, E.; Adánez-Sanjuán, P.; Arranz-González, J.C. Critical Raw Materials Recovery Potential from Spanish Mine Wastes: A National-Scale Preliminary Assessment. J. Clean. Prod. 2023, 407, 137163. [Google Scholar] [CrossRef]
- Gomez, D.V.; Salgado, E.S.; Mejías, O.; Pat-Espadas, A.M.; Torres, L.A.P.; Jackson, L.; Parbhakar-Fox, A. Data Integration of Critical Elements from Mine Waste in Mexico, Chile and Australia. Minerals 2022, 12, 122. [Google Scholar] [CrossRef]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; de la Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of Mining Tailings in México for the Possible Recovery of Strategic Elements. J. S. Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for World Population and Global Food Availability for Global Health; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131480. [Google Scholar]
- U.S. Geological Survey. Survey Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023; ISBN 9781411345041.
- Al-Ajeel, A.W.A.; Toama, H.Z. Mining and beneficiation of phosphate rocks: Prospects of unexploited phosphate deposits in the western desert, IRAQ. In Iraqi Bulletin Of Geology And Mining; Iraq Geological Survey (GEOSURV, Iraq); Ministry of Industry and Minerals: Baghdad, Iraq, 2017; pp. 59–66. [Google Scholar]
- Amar, H.; Benzaazoua, M.; Elghali, A.; Hakkou, R.; Taha, Y. Waste Rock Reprocessing to Enhance the Sustainability of Phosphate Reserves: A Critical Review. J. Clean. Prod. 2022, 381, 135151. [Google Scholar] [CrossRef]
- Taha, Y.; Elghali, A.; Hakkou, R.; Benzaazoua, M. Towards Zero Solid Waste in the Sedimentary Phosphate Industry: Challenges and Opportunities. Minerals 2021, 11, 1250. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T.E. The Potential for Mining Trace Elements from Phosphate Rock. J. Clean. Prod. 2015, 91, 337–346. [Google Scholar] [CrossRef]
- Loutou, M.; Hajjaji, M.; Mansori, M.; Favotto, C.; Hakkou, R. Phosphate Sludge: Thermal Transformation and Use as Lightweight Aggregate Material. J. Environ. Manag. 2013, 130, 354–360. [Google Scholar] [CrossRef]
- Yang, B.; He, J. New Insights into Selective Depression Mechanism of Tamarindus Indica Kernel Gum in Flotation Separation of Fluorapatite and Calcite. Sep. Purif. Technol. 2025, 354, 128787. [Google Scholar] [CrossRef]
- Yang, B.; Li, J.; He, J.; Zhu, L.; Guo, S.; Zhang, S. Differentiated Regulation of Mineral Interface Properties Using an Eco-Friendly Polysaccharide Depressant for Enhanced Apatite–Dolomite Sustainable Flotation Separation. Green. Chem. 2025, 27, 15211–15224. [Google Scholar] [CrossRef]
- El-bahi, A.; Ait-Khouia, Y.; Benzaazoua, M.; Hakkou, R.; Taha, Y. Biobased Collectors for Sustainable Phosphate Ore Flotation: Enhanced Performance and Selectivity. Adv. Colloid Interface Sci. 2025, 341, 103506. [Google Scholar] [CrossRef]
- Ji, B.; Li, Q.; Huang, Q.; Zhang, W. Enhanced Leaching Recovery of Rare Earth Elements from a Phosphatic Waste Clay through Calcination Pretreatment. J. Clean Prod. 2021, 319, 128654. [Google Scholar] [CrossRef]
- Chlahbi, S.; Belem, T.; Elghali, A.; Rochdane, S.; Zerouali, E.; Inabi, O.; Benzaazoua, M. Geological and Geomechanical Characterization of Phosphate Mine Waste Rock in View of Their Potential Civil Applications: A Case Study of the Benguerir Mine Site, Morocco. Minerals 2023, 13, 1291. [Google Scholar] [CrossRef]
- Amar, H.; Benzaazoua, M.; Elghali, A.; Taha, Y.; El Ghorfi, M.; Krause, A.; Hakkou, R. Mine Waste Rock Reprocessing Using Sensor-Based Sorting (SBS): Novel Approach toward Circular Economy in Phosphate Mining. Miner. Eng. 2023, 204, 108415. [Google Scholar] [CrossRef]
- El Ghorfi, M.; Inabi, O.; Amar, H.; Taha, Y.; Elghali, A.; Hakkou, R.; Benzaazoua, M. Design and Implementation of Sampling Wells in Phosphate Mine Waste Rock Piles: Towards an Enhanced Composition Understanding and Sustainable Reclamation. Minerals 2024, 14, 286. [Google Scholar] [CrossRef]
- Sädbom, S.; Bäckström, M. Sampling of Mining Waste-Historical Background, Experiences and Suggested Methods Carbonate Hosted Magnetite; Bergskraft Bergslagen AB, Report BKBAB: Kumla, Sweden, 2018; pp. 18–109. [Google Scholar]
- Maknoon, M.; Aubertin, M. On the Use of Bench Construction to Improve the Stability of Unsaturated Waste Rock Piles. Geotech. Geol. Eng. 2021, 39, 1425–1449. [Google Scholar] [CrossRef]
- Gy, P. Sampling of Particulate Materials Theory and Practice, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Akoto, R.; Anning, A.K. Heavy Metal Enrichment and Potential Ecological Risks from Different Solid Mine Wastes at a Mine Site in Ghana. Environ. Adv. 2021, 3, 100028. [Google Scholar] [CrossRef]
- Khalil, A.; Hanich, L.; Bannari, A.; Zouhri, L.; Pourret, O.; Hakkou, R. Assessment of Soil Contamination around an Abandoned Mine in a Semi-Arid Environment Using Geochemistry and Geostatistics: Pre-Work of Geochemical Process Modeling with Numerical Models. J. Geochem. Explor. 2013, 125, 117–129. [Google Scholar] [CrossRef]
- Chester, R.; Stoner, J.H. Pb in Particulates from the Lower Atmosphere of the Eastern. Nature 1973, 245, 27–28. [Google Scholar] [CrossRef]
- Schiff, K.C.; Weisberg, S.B. Iron as a Reference Element for Determining Trace Metal Enrichment in Southern California Coastal Shelf Sediments. Mar. Environ. Res. 1999, 48, 161–176. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2003; Volume 3–9, pp. 1–64. ISBN 9780080548074. [Google Scholar]
- Qiu, P.; Pabst, T. Effect of Construction Method and Bench Height on Particle Size Segregation during Waste Rock Disposal. Int. J. Min. Reclam. Environ. 2024, 38, 677–700. [Google Scholar] [CrossRef]
- Brough, C.P.; Warrender, R.; Bowell, R.J.; Barnes, A.; Parbhakar-Fox, A. The Process Mineralogy of Mine Wastes. Miner. Eng. 2013, 52, 125–135. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical Characterization of Mine Waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Aleksandrova, T.; Elbendari, A.; Nikolaeva, N. Beneficiation of a Low-Grade Phosphate Ore Using a Reverse Flotation Technique. Miner. Process. Extr. Metall. Rev. 2022, 43, 22–27. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Yassin, K.E.; Boulos, T.R. Processing of an East Mediterranean Phosphate Ore Sample by an Integrated Attrition Scrubbing/Classification Scheme (Part One). Sep. Sci. Technol. 2020, 55, 967–979. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M. Physical and Thermal Treatment of Phosphate Ores—An Overview. Int. J. Miner. Process 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Shariati, S.; Ramadi, A.; Salsani, A. Beneficiation of Low-Grade Phosphate Deposits by a Combination of Calcination and Shaking Tables: Southwest Iran. Minerals 2015, 5, 367–379. [Google Scholar] [CrossRef]
- Leißner, T.; Mütze, T.; Bachmann, K.; Rode, S.; Gutzmer, J.; Peuker, U.A. Evaluation of Mineral Processing by Assessment of Liberation and Upgrading. Miner. Eng. 2013, 53, 171–173. [Google Scholar] [CrossRef]
- Sousa, R.; Simons, B.; Bru, K.; de Sousa, A.B.; Rollinson, G.; Andersen, J.; Martin, M.; Machado Leite, M. Use of Mineral Liberation Quantitative Data to Assess Separation Efficiency in Mineral Processing—Some Case Studies. Miner. Eng. 2018, 127, 134–142. [Google Scholar] [CrossRef]
- Ryszko, U.; Rusek, P.; Kołodyńska, D. Quality of Phosphate Rocks from Various Deposits Used in Wet Phosphoric Acid and P-Fertilizer Production. Materials 2023, 16, 793. [Google Scholar] [CrossRef]
- Boujlel, H.; Daldoul, G.; Tlil, H.; Souissi, R.; Chebbi, N.; Fattah, N.; Souissi, F. The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia). Minerals 2019, 9, 2. [Google Scholar] [CrossRef]
- Sarker, S.K.; Haque, N.; Bhuiyan, M.; Bruckard, W.; Pramanik, B.K. Recovery of Strategically Important Critical Minerals from Mine Tailings. J. Environ. Chem. Eng. 2022, 10, 107622. [Google Scholar] [CrossRef]
- Balagh, Z.; Ait-khouia, Y.; Benzaazoua, M.; Taha, Y. Magnesium and Calcium Extraction from Phosphate Mine Waste Rock Using Phosphoric Acid: Thermodynamics, Parameter Optimization, Kinetics, and Reaction Mechanism. J. Ind. Eng. Chem. 2025, 146, 812–825. [Google Scholar] [CrossRef]
- Yu, Y.H.; Du, C.M.; Zhang, Y.T.; Yuan, R.Y. Phosphorus Recovery from Phosphate Tailings through a Two-Stage Leaching-Precipitation Process: Toward the Harmless and Reduction Treatment of P-Bearing Wastes. Environ. Res. 2024, 248, 118328. [Google Scholar] [CrossRef]
- Yu, Y.H.; Du, C.M. Leaching of Phosphorus from Phosphate Tailings and Extraction of Calcium Phosphates: Toward Comprehensive Utilization of Tailing Resources. J. Environ. Manag. 2023, 347, 119159. [Google Scholar] [CrossRef] [PubMed]
- Abou El Anwar, E.A.; Rashwan, M.A.; Abd El Samee, M.A.; Belal, Z.L.; Salman, S.A.; Seleem, E.M.; Abdelwahab, W.; Abd El-Shakour, Z.; Kamal, M.; Ahmed, A.S. Mining and Industrial Processing Wastes of Phosphate Rocks in Egypt: Potentiality of Rare Earth Elements. Int. J. Environ. Sci. Technol. 2025, 22, 10613–10623. [Google Scholar] [CrossRef]
- Boumaza, B.; Chekushina, T.V.; Kechiched, R.; Benabdeslam, N.; Brahmi, L.; Kucher, D.E.; Rebouh, N.Y. Environmental Geochemistry of Potentially Toxic Metals in Phosphate Rocks, Products, and Their Wastes in the Algerian Phosphate Mining Area (Tébessa, NE Algeria). Minerals 2023, 13, 853. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- McClellan, G.H. Mineralogy of Carbonate Fluorapatites. Geol. Soc. 1980, 137, 675–681. [Google Scholar] [CrossRef]
- Elvine Paternie, E.D.; Hakkou, R.; Ekengele Nga, L.; Bitom Oyono, L.D.; Ekoa Bessa, A.Z.; Oubaha, S.; Khalil, A. Geochemistry and Geostatistics for the Assessment of Trace Elements Contamination in Soil and Stream Sediments in Abandoned Artisanal Small-Scale Gold Mining (Bétaré-Oya, Cameroon). Appl. Geochem. 2023, 150, 105592. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Lei, K.; Huang, J.; Zhai, Y. Contamination Assessment of Copper, Lead, Zinc, Manganese and Nickel in Street Dust of Baoji, NW China. J. Hazard. Mater. 2009, 161, 1058–1062. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Zhang, P. Extraction of Rare Earth Elements from Upgraded Phosphate Flotation Tailings. Miner. Metall. Process. 2016, 33, 23–30. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; Depaoli, D.W. Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock. Minerals 2018, 8, 416. [Google Scholar] [CrossRef]
- Rehm, T.E. Advanced Nuclear Energy: The Safest and Most Renewable Clean Energy. Curr. Opin. Chem. Eng. 2023, 39, 100878. [Google Scholar] [CrossRef]
- NEA & AIAE. Uranium 2022: Resources, Production and Demand. Nuclear Energy Agency and International Atomic Energy Agency. 2023. Available online: http://oecd-nea.org/jcms/pl_79960/uranium-2022-resources-production-and-demand?details=true (accessed on 13 December 2025).
- Ulrich, A.E.; Schnug, E.; Prasser, H.M.; Frossard, E. Uranium Endowments in Phosphate Rock. Sci. Total Environ. 2014, 478, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Qamouche, K.; Chetaine, A.; Elyahyaoui, A.; Moussaif, A.; Touzani, R.; Benkdad, A.; Amsil, H.; Laraki, K.; Marah, H. Radiological Characterization of Phosphate Rocks, Phosphogypsum, Phosphoric Acid and Phosphate Fertilizers in Morocco: An Assessment of the Radiological Hazard Impact on the Environment. Mater. Today Proc. 2020, 27, 3234–3242. [Google Scholar] [CrossRef]
- Lea, D. Agricultural and Mineral Commodities Year Book, 1st ed.; Routledge: London, UK, 2002. [Google Scholar]
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To Extract, or Not to Extract Uranium from Phosphate Rock, That Is the Question. Environ. Sci. Technol. 2017, 51, 753–754. [Google Scholar] [CrossRef]
- OECD; NEA. Uranium 2016: Resources, Production and Demand. 2016. No. 7301. Available online: https://www.oecd-nea.org/jcms/pl_15004/uranium-2016-resources-production-and-demand?details=true (accessed on 13 December 2025).
- Kiegiel, K.; Gajda, D.; Zakrzewska-Kołtuniewicz, G. Recovery of Uranium and Other Valuable Metals from Substrates and Waste from Copper and Phosphate Industries. Sep. Sci. Technol. 2020, 55, 2099–2107. [Google Scholar] [CrossRef]
- Bergsma, W.; Dassios, A. A Consistent Test of Independence Based on a Sign Covariance Related to Kendall’s Tau. Bernoulli 2014, 20, 1006–1028. [Google Scholar] [CrossRef]
- Sadeghi, B. Chatterjee Correlation Coefficient: A Robust Alternative for Classic Correlation Methods in Geochemical Studies—(Including “TripleCpy” Python Package). Ore Geol. Rev. 2022, 146, 104954. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.M.; Jaber, O.; Alkuisi, M.; Sadaqah, R. Rare Earth Elements and Uranium Geochemistry in the Al-Kora Phosphorite Province, Late Cretaceous, Northwestern Jordan. Arab. J. Geosci. 2016, 9, 187. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Aseri, A.A.; Ali, K.A. Geological and Geochemical Evaluation of Phosphorite Deposits in Northwestern Saudi Arabia as a Possible Source of Trace and Rare-Earth Elements. Ore Geol. Rev. 2022, 144, 104854. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Valorization of Phosphate Waste Rocks and Sludge from the Moroccan Phosphate Mines: Challenges and Perspectives. Procedia Eng. 2016, 138, 110–118. [Google Scholar] [CrossRef]




| Fraction (µm) | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | TiO2 | P2O5 | MnO | F | LOI | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| LOD | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| >100,000 | 32.19 | 34.04 | 0.73 | 0.53 | 2.17 | 0.15 | 0.58 | 0.11 | 12.55 | <0.01 | 1.22 | 15.73 | <0.01 | |
| 10,000–100,000 | 25.64 | 43.94 | 1.15 | 0.68 | 3.87 | 0.22 | 0.27 | 0.09 | 6.60 | <0.01 | 0.00 | 17.53 | <0.01 | |
| 3150–10,000 | 25.39 | 40.37 | 1.81 | 0.90 | 4.21 | 0.32 | 0.23 | 0.11 | 7.33 | <0.01 | 1.07 | 18.25 | <0.01 | |
| 1000–3150 | 34.79 | 24.71 | 1.76 | 0.87 | 3.43 | 0.30 | 0.44 | 0.14 | 15.33 | <0.01 | 2.13 | 16.09 | <0.01 | |
| 800–1000 | 38.72 | 19.24 | 1.77 | 0.89 | 2.69 | 0.32 | 0.66 | 0.13 | 18.06 | <0.01 | 2.35 | 15.16 | <0.01 | |
| 630–800 | 39.79 | 17.88 | 1.81 | 0.84 | 2.56 | 0.28 | 0.72 | <0.01 | 19.21 | <0.01 | 2.08 | 14.81 | <0.02 | |
| 400–630 | 41.51 | 15.29 | 1.72 | 0.78 | 2.10 | 0.27 | 0.70 | 0.18 | 20.95 | <0.01 | 2.43 | 14.07 | <0.01 | |
| 315–400 | 45.11 | 11.08 | 1.23 | 0.58 | 1.25 | 0.20 | 0.67 | 0.01 | 24.95 | <0.01 | 2.67 | 12.24 | <0.01 | |
| 160–315 | 46.35 | 9.37 | 1.20 | 0.59 | 1.10 | 0.20 | 0.92 | 0.12 | 26.28 | <0.01 | 2.74 | 11.11 | <0.01 | |
| 125–160 | 45.63 | 13.12 | 1.35 | 0.62 | 1.06 | 0.23 | 0.86 | 0.11 | 23.43 | <0.01 | 1.48 | 12.10 | <0.01 | |
| 80–125 | 34.01 | 25.22 | 2.65 | 1.11 | 3.14 | 0.52 | 0.59 | 0.27 | 15.43 | <0.01 | 1.81 | 15.25 | <0.01 | |
| 63–80 | 36.76 | 23.66 | 2.18 | 0.96 | 2.00 | 0.42 | 0.72 | 0.17 | 17.57 | <0.01 | 1.82 | 13.73 | <0.01 | |
| 40–63 | 33.34 | 21.19 | 2.85 | 1.23 | 5.38 | 0.52 | 0.48 | 0.21 | 13.77 | <0.01 | 1.67 | 19.35 | <0.01 | |
| <40 | 32.40 | 18.49 | 3.04 | 1.29 | 7.35 | 0.48 | 0.45 | 0.13 | 12.74 | <0.01 | 1.69 | 21.94 | <0.01 | |
| UCC | 3.59 | 66.6 | 15.4 | 5.04 | 2.48 | 2.8 | 3.27 | 0.64 | 0.15 |
| Particle Size (µm) | Analysis/EFs | REE | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||
| Units | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |
| LOD | 0.5 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.03 | 0.02 | 0.1 | 0.01 | 0.05 | 0.01 | 0.03 | 0.01 | 0.03 | 0.01 | |
| >100,000 | ICP-MS | 3 | 38 | 14 | 11 | 2 | 10 | 2 | 1 | 3 | 0 | 3 | 1 | 2 | 0 | 2 | 0 |
| Efs | 5 | 41 | 10 | 4 | 7 | 8 | 10 | 13 | 15 | 14 | 15 | 19 | 22 | 24 | 25 | 28 | |
| 10,000–100,000 | ICP-MS | 3 | 24 | 10 | 10 | 2 | 7 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 2 | 0 |
| EFs | 3 | 16 | 4 | 2 | 4 | 4 | 5 | 6 | 7 | 6 | 7 | 8 | 8 | 10 | 11 | 11 | |
| 3150–10,000 | ICP-MS | 5 | 26 | 12 | 13 | 2 | 9 | 2 | 0 | 2 | 0 | 2 | 1 | 2 | 0 | 1 | 0 |
| EFs | 3 | 11 | 3 | 2 | 3 | 3 | 3 | 3 | 5 | 4 | 5 | 6 | 6 | 7 | 7 | 8 | |
| 1000–3150 | ICP-MS | 5 | 38 | 17 | 20 | 3 | 14 | 3 | 1 | 3 | 0 | 3 | 1 | 2 | 0 | 2 | 0 |
| EFs | 3 | 17 | 5 | 3 | 4 | 5 | 5 | 7 | 7 | 6 | 7 | 9 | 9 | 10 | 10 | 13 | |
| 800–1000 | ICP-MS | 5 | 59 | 25 | 23 | 4 | 19 | 4 | 1 | 5 | 1 | 5 | 1 | 3 | 1 | 3 | 1 |
| EFs | 3 | 26 | 7 | 3 | 6 | 6 | 8 | 11 | 11 | 10 | 12 | 13 | 14 | 16 | 16 | 20 | |
| 630–800 | ICP-MS | 4 | 50 | 22 | 22 | 4 | 17 | 3 | 1 | 4 | 1 | 4 | 1 | 3 | 0 | 3 | 1 |
| EFs | 3 | 22 | 6 | 3 | 5 | 6 | 7 | 7 | 9 | 8 | 10 | 11 | 11 | 14 | 14 | 17 | |
| 400–630 | ICP-MS | 4 | 52 | 22 | 21 | 4 | 17 | 4 | 1 | 4 | 1 | 4 | 1 | 3 | 0 | 3 | 1 |
| EFs | 3 | 24 | 7 | 3 | 6 | 6 | 7 | 8 | 10 | 9 | 10 | 12 | 12 | 15 | 14 | 18 | |
| 315–400 | ICP-MS | 4 | 55 | 22 | 21 | 4 | 17 | 3 | 1 | 4 | 1 | 4 | 1 | 3 | 0 | 3 | 1 |
| EFs | 3 | 36 | 10 | 5 | 8 | 9 | 10 | 12 | 15 | 13 | 15 | 17 | 18 | 21 | 20 | 23 | |
| 160–315 | ICP-MS | 4 | 81 | 32 | 27 | 5 | 23 | 5 | 1 | 6 | 1 | 6 | 2 | 4 | 1 | 5 | 1 |
| EFs | 4 | 54 | 14 | 6 | 10 | 12 | 15 | 18 | 20 | 19 | 23 | 26 | 26 | 33 | 33 | 38 | |
| 125–160 | ICP-MS | 5 | 107 | 41 | 35 | 7 | 31 | 7 | 2 | 9 | 1 | 8 | 2 | 6 | 1 | 6 | 1 |
| EFs | 4 | 62 | 16 | 7 | 12 | 14 | 18 | 21 | 26 | 22 | 25 | 29 | 31 | 36 | 35 | 41 | |
| 80–125 | ICP-MS | 5 | 94 | 36 | 31 | 6 | 27 | 6 | 2 | 8 | 1 | 7 | 2 | 5 | 1 | 5 | 1 |
| EFs | 2 | 28 | 7 | 3 | 6 | 6 | 8 | 9 | 12 | 10 | 11 | 13 | 14 | 17 | 16 | 20 | |
| 63–80 | ICP-MS | 6 | 54 | 22 | 24 | 4 | 19 | 3 | 1 | 4 | 1 | 5 | 1 | 3 | 1 | 3 | 1 |
| EFs | 3 | 20 | 5 | 3 | 5 | 5 | 6 | 7 | 8 | 8 | 9 | 10 | 11 | 13 | 13 | 16 | |
| 40–63 | ICP-MS | 6 | 64 | 26 | 25 | 5 | 20 | 5 | 1 | 5 | 1 | 5 | 1 | 4 | 1 | 4 | 1 |
| EFs | 2 | 18 | 5 | 2 | 4 | 4 | 6 | 7 | 7 | 7 | 7 | 9 | 9 | 10 | 11 | 13 | |
| <40 | ICP-MS | 6 | 49 | 21 | 24 | 4 | 18 | 4 | 1 | 4 | 1 | 4 | 1 | 3 | 0 | 3 | 1 |
| EFs | 2 | 13 | 4 | 2 | 3 | 4 | 4 | 5 | 6 | 5 | 6 | 6 | 7 | 9 | 8 | 11 | |
| Particle Size (µm) | Analysis/EFs | Other Critical and Strategic Elements | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba | Cr | Cs | Ga | Hf | Nb | Rb | Sn | Sr | Ta | Th | U | V | W | Zr | ||
| Units | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |
| LOD | 0.5 | 5 | 0.01 | 0.1 | 0.05 | 0.1 | 0.2 | 0.5 | 0.1 | 0.1 | 0.05 | 0.05 | 5 | 0.5 | 1 | |
| >100,000 | ICP-MS | 71 | 241 | 0 | 1 | 1 | 1 | 4 | 21 | 502 | 0 | 1 | 92 | 80 | 22 | 48 |
| Efs | 3 | 59 | 1 | 2 | 5 | 2 | 1 | 224 | 35 | 3 | 2 | 772 | 19 | 262 | 6 | |
| 10,000–100,000 | ICP-MS | 75 | 277 | 0 | 2 | 1 | 1 | 6 | <0.5 | 304 | <0.1 | 1 | 48 | 103 | 35 | 42 |
| EFs | 2 | 42 | 1 | 1 | 3 | 1 | 1 | - | 13 | - | 2 | 248 | 15 | 255 | 3 | |
| 3150–10,000 | ICP-MS | 77 | 265 | 1 | 3 | 1 | 2 | 10 | 1 | 312 | <0.1 | 2 | 48 | 130 | 24 | 55 |
| EFs | 1 | 26 | 1 | 1 | 2 | 1 | 1 | 2 | 9 | - | 1 | 159 | 12 | 112 | 3 | |
| 1000–3150 | ICP-MS | 118 | 153 | 1 | 3 | 1 | 2 | 10 | <0.5 | 644 | 0 | 2 | 96 | 150 | 12 | 65 |
| EFs | 2 | 16 | 1 | 1 | 3 | 1 | 1 | - | 19 | 1 | 2 | 337 | 15 | 60 | 3 | |
| 800–1000 | ICP-MS | 119 | 196 | 1 | 3 | 2 | 2 | 10 | <0.5 | 718 | 0 | 2 | 115 | 162 | 6 | 94 |
| EFs | 2 | 20 | 1 | 1 | 4 | 2 | 1 | - | 21 | 1 | 2 | 400 | 16 | 27 | 5 | |
| 630–800 | ICP-MS | 122 | 176 | 1 | 3 | 2 | 2 | 11 | <0.5 | 791 | 0 | 2 | 122 | 155 | <0.5 | 80 |
| EFs | 2 | 18 | 1 | 2 | 3 | 2 | 1 | - | 23 | 2 | 2 | 414 | 15 | - | 4 | |
| 400–630 | ICP-MS | 119 | 144 | 1 | 3 | 2 | 2 | 10 | <0.5 | 806 | 0 | 2 | 128 | 153 | <0.5 | 73 |
| EFs | 2 | 15 | 1 | 2 | 3 | 1 | 1 | - | 25 | 1 | 2 | 461 | 15 | - | 4 | |
| 315–400 | ICP-MS | 116 | 125 | 1 | 3 | 1 | 2 | 9 | <0.5 | 800 | 0 | 2 | 132 | 143 | <0.5 | 51 |
| EFs | 3 | 19 | 2 | 2 | 3 | 2 | 2 | - | 34 | 2 | 2 | 666 | 20 | - | 4 | |
| 160–315 | ICP-MS | 131 | 127 | 0 | 2 | 1 | 1 | 7 | 1 | 1070 | 0 | 2 | 170 | 150 | <0.5 | 43 |
| EFs | 3 | 19 | 1 | 2 | 2 | 1 | 1 | 6 | 47 | 2 | 3 | 882 | 22 | - | 3 | |
| 125–160 | ICP-MS | 128 | 129 | 0 | 2 | 1 | 1 | 7 | 1 | 1090 | 0 | 4 | 174 | 152 | <0.5 | 45 |
| EFs | 2 | 17 | 1 | 2 | 2 | 1 | 1 | 6 | 42 | 1 | 4 | 783 | 19 | - | 3 | |
| 80–125 | ICP-MS | 120 | 125 | 0 | 2 | 1 | 1 | 7 | 1 | 918 | 0 | 3 | 149 | 141 | <0.5 | 39 |
| EFs | 1 | 8 | 1 | 1 | 1 | 1 | 1 | 2 | 18 | 1 | 2 | 343 | 9 | - | 1 | |
| 63–80 | ICP-MS | 144 | 190 | 1 | 4 | 8 | 4 | 16 | 1 | 596 | 0 | 3 | 107 | 192 | <0.5 | 339 |
| EFs | 2 | 16 | 1 | 2 | 11 | 2 | 1 | 3 | 14 | 3 | 2 | 300 | 15 | - | 13 | |
| 40–63 | ICP-MS | 130 | 175 | 1 | 3 | 3 | 2 | 13 | 1 | 687 | 0 | 3 | 119 | 173 | <0.5 | 123 |
| EFs | 1 | 11 | 1 | 1 | 3 | 1 | 1 | 3 | 12 | 2 | 2 | 255 | 10 | - | 4 | |
| <40 | ICP-MS | 125 | 212 | 1 | 4 | 5 | 3 | 16 | 3 | 533 | 0 | 3 | 95 | 227 | <0.5 | 210 |
| EFs | 1 | 13 | 1 | 1 | 5 | 2 | 1 | 8 | 9 | 1 | 2 | 193 | 13 | - | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidouri, M.; Ait-Khouia, Y.; Elghali, A.; El Ghorfi, M.; Benzaazoua, M.; Taha, Y. Phosphate Waste Rock Piles as a Secondary Resource: Insights into Composition and Strategic Element Potential. Minerals 2025, 15, 1319. https://doi.org/10.3390/min15121319
Haidouri M, Ait-Khouia Y, Elghali A, El Ghorfi M, Benzaazoua M, Taha Y. Phosphate Waste Rock Piles as a Secondary Resource: Insights into Composition and Strategic Element Potential. Minerals. 2025; 15(12):1319. https://doi.org/10.3390/min15121319
Chicago/Turabian StyleHaidouri, Mohamed, Yassine Ait-Khouia, Abdellatif Elghali, Mustapha El Ghorfi, Mostafa Benzaazoua, and Yassine Taha. 2025. "Phosphate Waste Rock Piles as a Secondary Resource: Insights into Composition and Strategic Element Potential" Minerals 15, no. 12: 1319. https://doi.org/10.3390/min15121319
APA StyleHaidouri, M., Ait-Khouia, Y., Elghali, A., El Ghorfi, M., Benzaazoua, M., & Taha, Y. (2025). Phosphate Waste Rock Piles as a Secondary Resource: Insights into Composition and Strategic Element Potential. Minerals, 15(12), 1319. https://doi.org/10.3390/min15121319











