The beneficiation experiments were designed based on the mineralogical and chemical characteristics of the Hantepe placer sands, as indicated during the characterization stage. Since the feed material consists of a mixture of quartz-dominated light gangue and heavy minerals such as ilmenite, rutile, zircon, allanite, monazite and magnetite, a two-step beneficiation strategy was adopted. This sequential approach enabled a systematic evaluation of the separation behavior of critical mineral phases and provided a basis for optimizing recovery pathways for Ti, Zr, and REE-bearing minerals.
3.2.1. Gravity Separation Tests
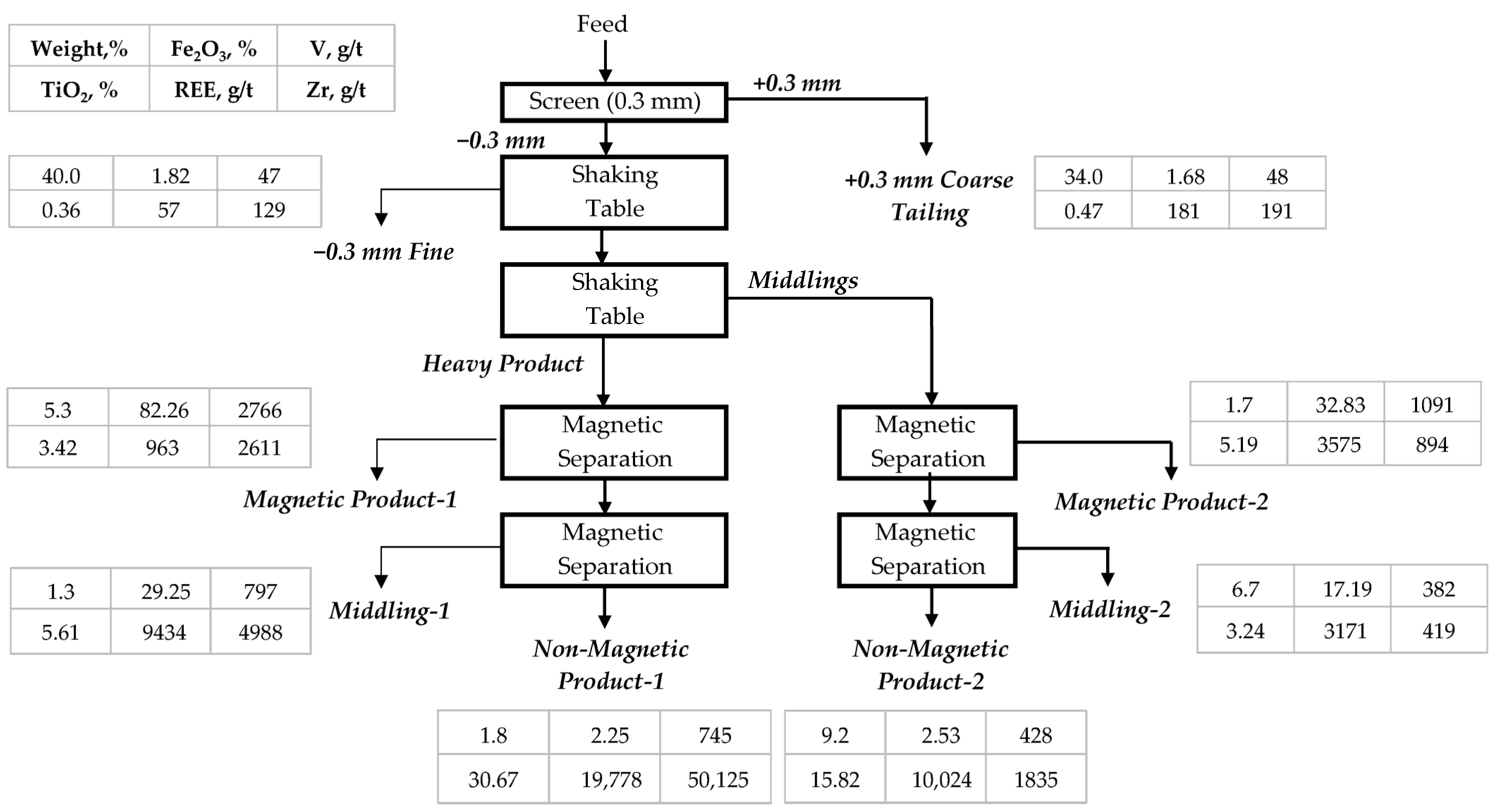
Shaking table tests were conducted on the Hantepe placer sands, classified into two size fractions: +0.3 mm (34% wt.) and −0.3 mm (66% wt.). In each fraction, a clean light product was initially removed, and the resulting bulk concentrate was subjected to further cleaning, ultimately producing a heavy concentrate, middlings, and a final light fraction. The chemical compositions of these products are presented in
Table 2 and
Table 3.
The results indicate clear mineralogical partitioning between size fractions and among shaking table products. In the coarse fraction (+0.3 mm), SiO2 is dominant (70.05%), reflecting the abundance of quartz and feldspar. Consequently, heavy minerals such as Fe–Ti oxides and REE-bearing phases are scarce, as confirmed by the relatively low concentrations of Fe2O3 (1.68%), TiO2 (0.47%), Zr (191 g/t), and REEs (e.g., Ce: 67 g/t, La: 41 g/t, Nd: 19 g/t, and Y: 29 g/t). Distribution data further demonstrate that the +0.3 mm fraction contributes less than 10% to the total REEs and Zr, highlighting its limited beneficiation potential and its gangue-dominated character.
In contrast, the fine fraction (−0.3 mm), which accounts for two-thirds of the bulk sample, exhibits significant enrichment in heavy mineral components. Fe2O3 (11.47%) and TiO2 (4.02%) concentrations are notably higher than in the coarse fraction, reflecting the presence of ilmenite, magnetite, and related Fe–Ti oxides. Similarly, Zr (1871 g/t) and REEs show pronounced enrichment, with Ce (1281 g/t), La (565 g/t), Nd (459 g/t), and Y (243 g/t) recording order of magnitude increases relative to the coarse size group. Importantly, distribution calculations reveal that the fine fraction hosts over 95% of the total Zr and REEs (e.g., 97% of Ce, 96% of La, 98% of Nd, 94% of Y), as well as more than 96% of Th and U, strongly indicating the preferential occurrence of zircon, apatite, monazite and, allanite within the finer particle range.
Within each size group, gravity concentration on the shaking table effectively separated light silicate gangue from denser mineral phases. For example, in the −0.3 mm fraction, the heavy and middlings products contributed over 80% of the total Fe2O3, 90% of TiO2, and over 95% of the total REE and radioactive element contents, demonstrating the high upgrading efficiency of the table for fine particles. Although the REE and radioactive element contents in the −0.3 mm heavy and middling products were relatively similar, they were processed separately in the following magnetic separation stage due to the significant differences in their Zr, Fe, and V contents. This approach allowed for the selective recovery of specific mineral phases and improved the overall efficiency of the beneficiation flowsheet.
The gravity separation tests confirm that the beneficiation potential of the Hantepe placer sands resides primarily in the −0.3 mm fraction, where heavy minerals are both concentrated and liberated sufficiently for efficient separation. The combination of compositional enrichment and distribution patterns establishes this fine fraction as the primary target for subsequent magnetic and flotation-based separation steps aimed at recovering ilmenite, zircon, and REE-bearing phases such as allanite, monazite as revealed in XRD analysis.
The enrichment ratios (ER) derived from the shaking table tests provide valuable insight into the beneficiation potential of the Hantepe placer sands. In the coarse fraction (+0.3 mm), although the heavy product accounts for only 2.5% of the feed, it exhibits remarkably high upgrading for several critical elements. For instance, Ce, La, Th, and U show enrichment ratios in the range of 11–12, while Nb and Y are concentrated by factors of about 8.0, and Zr and V by approximately 6.0 and 4.3, respectively. This indicates that the coarse fraction, despite its limited yield, acts as a selective carrier of REEs and associated high-value trace metals. By contrast, the fine fraction (−0.3 mm), yielding 8.4% heavy product, shows significant enrichment in traditional heavy minerals: Fe2O3 and V are upgraded by about fivefold, Zr by 6.2-fold, and TiO2 by 2.2-fold, reflecting the predominance of Fe–Ti oxides and zircon in the fine-grained matrix. Although REEs are also enriched in this fraction, the corresponding ratios are lower (2–3×) compared to the coarse fraction.
Overall, these results demonstrate a clear distinction: the +0.3 mm heavy product is particularly effective in concentrating REEs and radioactive elements, while the −0.3 mm heavy product serves as the principal source for Fe–Ti oxides and zircon. This dual beneficiation pathway emphasizes the importance of fraction-wise treatment to maximize recovery of both REE-bearing and traditional heavy mineral phases. As illustrated in
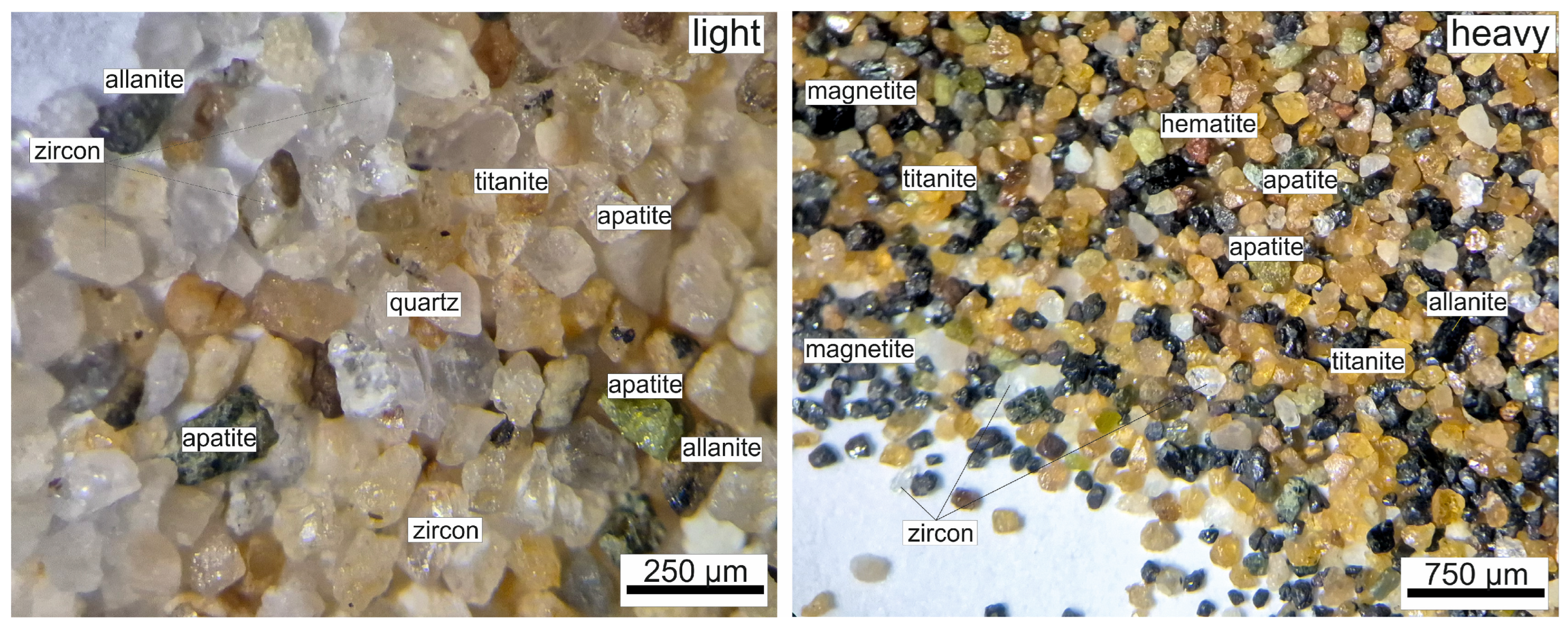
Figure 1, microscopic images of the −0.3 mm fraction products clearly demonstrate the effectiveness of gravity separation. In the heavy product, dark-colored heavy minerals predominate, with magnetite and titanite being particularly abundant, reflecting their high specific gravity and efficient recovery in the fine size range. Conversely, the light product is dominated by quartz and feldspar grains, with virtually no heavy minerals present. This distinct mineralogical partitioning within the fine fraction highlights the strong selectivity of the shaking table. Moreover, optical microscopy provided direct visual evidence of mineral liberation and textural associations, thereby supporting the chemical analyses and offering valuable insight into the beneficiation behavior of individual mineral phases within the fine fraction.
3.2.2. Magnetic Separation Tests
In the second stage of beneficiation, both the heavy and middlings products obtained from the shaking table were subjected to dry, disc-type magnetic separation to further fractionate minerals based on their magnetic susceptibilities. The procedure was performed sequentially using two magnetic field intensities. First, a low-intensity field of ≈3000 Gauss was applied to recover strongly magnetic minerals, primarily magnetite and other Fe-rich phases; the products obtained at this field strength are reported as magnetic products in
Table 4 and
Table 5. Subsequently, the non-magnetic portion from the first step was passed through a high-intensity field of ≈11,000 Gauss, enabling the separation of weakly paramagnetic minerals such as hematite, ilmenite, and titanite; the products obtained at this stage appear as middlings. The remaining non-magnetic fractions, enriched in zircon, rutile, apatite, and monazite, constitute the final non-magnetic products.
Table 4 and
Table 5 first summarize the chemical compositions and distribution patterns of magnetic and non-magnetic products obtained from the shaking table heavy fraction in the +0.3 mm and −0.3 mm size groups, respectively. Results for the middlings fraction are provided in the next section. Thus, the integration of gravity and magnetic separation created a stepwise beneficiation scheme that maximized the selective recovery of valuable heavy mineral and REE-bearing phases.
In the +0.3 mm size group, magnetic separation produced a moderate contrast between mineral fractions. The magnetic product was enriched in Fe2O3 (C: 20.46%, D: 44.8%), reflecting the recovery of magnetite-dominated grains, whereas the non-magnetic product consisted mainly of quartz–feldspar gangue, as indicated by its high SiO2 content (C: 70.69%, D: 79.1%). TiO2 and Zr both showed preferential reporting to the non-magnetic stream, with enrichment ratios (ER) of ~1.07 and ~1.28, respectively, consistent with the presence of titanite and zircon in the coarse non-magnetic fraction.
In the −0.3 mm size fraction, the partitioning of minerals became markedly more selective. The magnetic product exhibited strong Fe2O3 enrichment (C: 82.26%, D: 91.0%, ER ~1.45), confirming the dominance and finer liberation of magnetite and ilmenite. In contrast, TiO2 showed a dual distribution, with only 22.2% reporting to the magnetic stream and the majority (68.7%) recovered in the non-magnetic fraction, where TiO2 reached 30.67%, yielding a high ER of ~3.17. Zr behaved similarly, being strongly enriched in the fine non-magnetic stream (50,195 g/t; D: 81.8%; ER ~3.8). These trends indicate that the −0.3 mm fraction hosts both well-liberated magnetic Fe–Ti oxides and non-magnetic zircon and titanite, whereas middlings retain partially liberated grains.
Rare earth elements displayed a decoupled behaviour between coarse and fine fractions, reflecting different mineral carriers. In the +0.3 mm group, Ce (715 g/t), La (412 g/t), Nd (76 g/t), and Y (289 g/t) reported mainly to the non-magnetic product, but their ERs were near unity because enrichment resulted largely from the high mass of the non-magnetic stream rather than selective upgrading. The differing relative proportions of LREEs (Ce–La–Nd) and Y further indicate that multiple minerals contribute to REE inventories. In the −0.3 mm fraction, however, REEs were significantly concentrated in the non-magnetic product, with Ce (10,318 g/t; ER~3.3), La (2725 g/t; ER~1.9), Nd (3485 g/t; ER~3.8), and Y (1582 g/t; ER~3.6). This strongly suggests that fine-grained monazite (dominated by LREEs) and allanite (hosting both LREEs and Y) were efficiently recovered into the non-magnetic concentrates. The parallel enrichment of Th (1935 g/t) and U (594 g/t) supports this interpretation, given their typical association with monazite–allanite assemblages.
Collectively, these results demonstrate the complementary roles of gravity concentration and staged magnetic separation: the shaking table effectively pre-concentrated heavy minerals, whereas magnetic separation produced clear partitioning between ferromagnetic Fe–Ti oxides and non-magnetic Zr- and REE-bearing minerals. The substantially higher ERs obtained in the −0.3 mm size group confirm that fine-grained, well-liberated heavy minerals host the majority of Ti, Zr, REEs, Th, and U in the Hantepe placer sands, highlighting the importance of size classification before beneficiation.
The optical microscopy images of the magnetic and non-magnetic products obtained from separation are presented in
Figure 2. The magnetic fraction is predominantly composed of magnetite grains, which exhibit a distinct dark color and high reflectivity, consistent with their high magnetic susceptibility. In contrast, the non-magnetic fraction is enriched in accessory heavy minerals such as titanite, zircon, apatite, monazite and, allanite, which appear as transparent to reddish-brown grains under reflected light. The clear distinction between the fractions confirms the efficiency of magnetic separation in concentrating Fe–Ti oxides within the magnetic product, while non-magnetic but high-density minerals were successfully partitioned into the corresponding fraction.
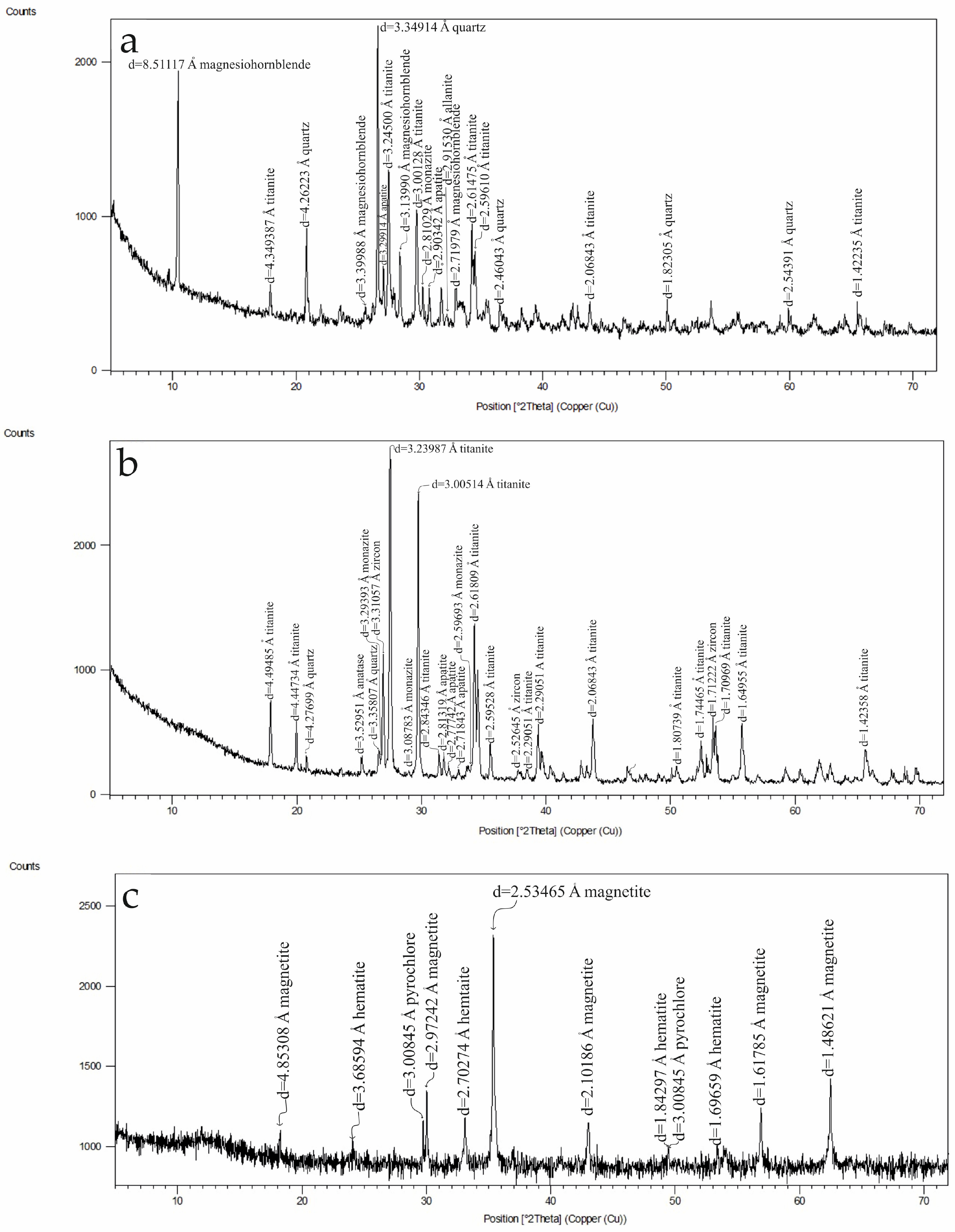
As shown in
Figure 3a, the XRD pattern of the −0.3 mm shaking-table middlings presents a broad but diagnostic set of low- to medium-intensity reflections, consistent with the mixed mineralogy of this intermediate product. The pattern is dominated by titanite and quartz, but multiple well-defined peaks in the 2θ ≈ 10–30° region correspond closely to the ICDD reference positions for allanite, supporting its presence as a major REE-bearing phase. The simultaneous appearance of Ca-, Fe-, and REE-related reflections is fully consistent with the elevated CaO, Fe
2O
3, Ce, La, Nd, and Th contents measured chemically. Importantly, several discrete peaks, notably at d ≈ 3.25 Å, 3.08 Å, and 2.60 Å, match the characteristic fingerprint of monazite providing independent evidence for a second REE–Th-bearing accessory mineral. This is in agreement with the enrichment of Th and U in this fraction and indicates that part of the radiogenic element budget is hosted in arsenate-type REE minerals. Minor reflections attributable to ferro-hornblende and Ti-oxide phases indicate limited contributions from amphibole alteration products and titanite-associated species. Collectively, the XRD pattern confirms that the middlings fraction contains a mixture of titanite, silicates, allanite, and gasparite, reinforcing the geochemical interpretation and demonstrating that this size class hosts the bulk of the REE-bearing accessory minerals in the Hantepe placer sands.
The non-magnetic fraction obtained at 11,000 Gauss (
Figure 3b) is dominated by titanite, which forms the principal Ti-bearing phase in this product. In addition to titanite, the pattern displays well-defined reflections attributable to zircon, fluorapatite, quartz, and minor Ti-oxide phases, confirming the coexistence of Zr-, Ca-, and P-bearing accessories. Notably, several diagnostic peaks, such as those at d ≈ 3.29 Å and 2.81 Å, correspond closely to reference positions for monazite indicating the presence of a Th- and REE-bearing phosphate phase within the non-magnetic concentrate. The combined occurrence of titanite together with zircon, fluorapatite, and monazite aligns with the chemical data showing co-enrichment of Ti, Ca, Zr, REEs, Th, and U in this product. These mineralogical features demonstrate that the material reporting to the non-magnetic fraction hosts a mixture of Ti-silicate, Zr-silicate, and REE–Th–phosphate minerals, and confirm that titanite, not ilmenite, is the dominant TiO
2-bearing phase in the Hantepe placer sands.
In contrast, the XRD pattern of the magnetic fraction obtained at 3000 Gauss (
Figure 3c) is dominated by magnetite, consistent with the strong magnetic response of this phase. In addition to magnetite, a minor iron-oxide phase is present, although its diffraction peaks are not sufficiently well resolved to confidently distinguish between hematite and goethite. The pattern also contains weak reflections attributable to pyrochlore and fluorapatite. These phases indicate that minerals with high magnetic susceptibility were effectively concentrated into the magnetic product. This interpretation is consistent with the chemical enrichment ratios: Fe
2O
3 increased from 8.1% in the feed to 20.5% in the +0.3 mm magnetic product and to over 80% in the finer −0.3 mm magnetic fraction, confirming the efficient recovery of iron oxides. Likewise, the substantial TiO
2 enrichment observed in the non-magnetic fractions (e.g., 30.7% in the −0.3 mm non-magnetic stream) aligns with the dominance of titanite suggested by the XRD Rietveld calculation results in
Table 6.
While the magnetic fractions represented the principal concentrates of Fe–Ti oxides, the non-magnetic fractions became enriched in titanite, zircon, and phosphate minerals such as monazite and apatite, thereby effectively partitioning the ore into technologically valuable products. Following the initial gravity separation, the −0.3 mm middling fraction obtained from the shaking table was subjected to magnetic separation to further upgrade the heavy mineral content. Unlike the coarser middlings, this fine-grained fraction exhibited significantly higher concentrations of Fe–Ti oxides and REE-bearing minerals, making it a suitable candidate for secondary beneficiation. The objective of this stage was to improve the overall recovery of valuable minerals and to establish a more efficient integrated flowsheet by reprocessing middlings products rather than discarding them as tailings. The results of chemical composition, metal distributions, and ERs for the obtained products are presented in
Table 7 and
Table 8.
Magnetic separation of the −0.3 mm middling fraction resulted in a distinct separation between magnetic Fe–Ti oxides and non-magnetic heavy mineral phases. The magnetic product (9.9 wt.%) was strongly enriched in Fe2O3 (C: 32.33%, ER: 2.9, D: 28.9%). This enrichment was accompanied by a notable decrease in SiO2 (C ≈ 5%, ER: 0.12, D: 1.2%), indicating efficient rejection of silicate gangue. However, TiO2 (C: 5.19%, ER: 0.52, D: 5.1%) showed only minor upgrading, reflecting the weak magnetic susceptibility of ilmenite and titanite minerals. The middlings product (38.1 wt.%) displayed intermediate grades of Fe2O3 (C: 17.19%, ER: 1.55, D: 59.2%) and TiO2 (C: 3.24%, ER: 0.32, D: 12.5%), suggesting partial liberation and incomplete separation of Fe–Ti oxides from associated gangue minerals. The non-magnetic product (52.0 wt.%) was notably rich in valuable non-magnetic heavy minerals, with TiO2 (C: 15.82%, ER: 1.58, D: 82.4%), Zr (C: 1835 g/t, ER: 1.52, D: 79.3%), and Nb (C: 928 g/t, ER: 1.66, D: 86.5%) representing the principal upgrading indicators. While V (C: 428 g/t, ER: 0.90, D: 46.8%) remained relatively evenly distributed, the dominance of Zr and Nb in this fraction reveals the preferential concentration of zircon, niobite, and possibly allanite-bearing phases in the non-magnetic stream.
The rare earth and actinide elements exhibited a parallel trend. The non-magnetic product contained Ce (C: 4816 g/t, ER: 1.46, D: 75.7%), La (C: 1186 g/t, ER: 1.35, D: 70.3%), Nd (C: 2078 g/t, ER: 1.66, D: 86.4%), Y (C: 1116 g/t, ER: 1.64, D: 85.5%), Th (C: 384 g/t, ER: 1.48, D: 77.0%), and U (C: 192 g/t, ER: 1.59, D: 82.2%), substantially higher than the magnetic product. Although the non-magnetic product accounts for just over half of the feed mass, its elevated ERs and high elemental distributions indicate true concentration rather than simple mass dilution.
Overall, magnetic separation of the −0.3 mm middlings fractions effectively recovered Fe-rich oxides into the magnetic fraction (Fe2O3 ER ≈ 2.9) while generating a non-magnetic concentrate enriched in TiO2, Zr, Nb, and REEs (ER ≈ 1.3–1.7). These findings confirm that reprocessing the middlings improves total recovery efficiency and enhances the economic potential of the flowsheet by producing separate Fe–oxide and REE–Zr–Nb-rich products.